Iron–Zinc Synergy Blocks Cadmium Translocation in Rice: Minimizing Grain Contamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characterization
2.2. Chemical Reagents and Seed Germination
2.3. Pretreatment Iron and Zinc Absorption Experiments
2.4. Field Experiments
2.5. Sampling and Measurements
2.6. Statistical Analysis
3. Results
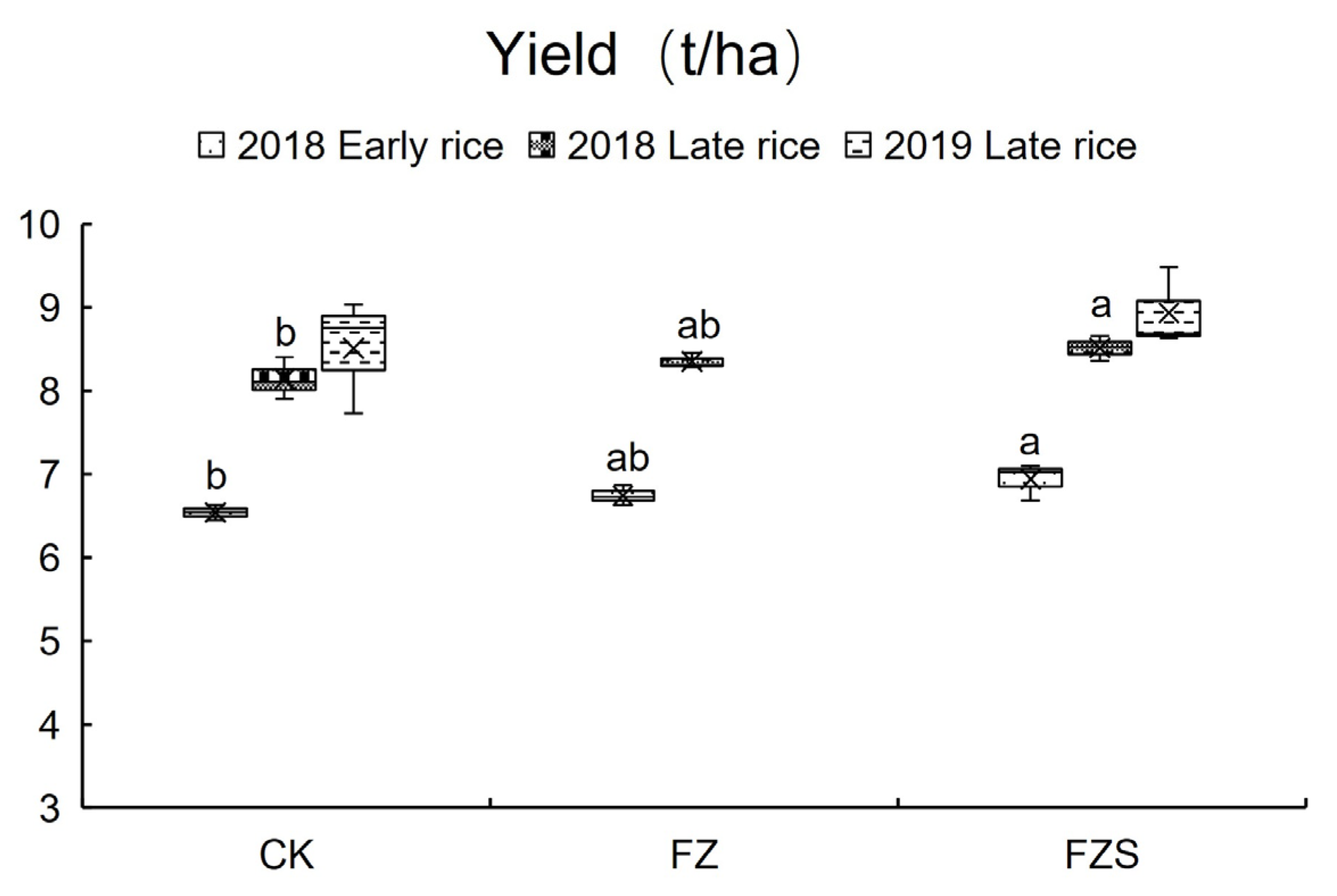
3.1. Rice Yields
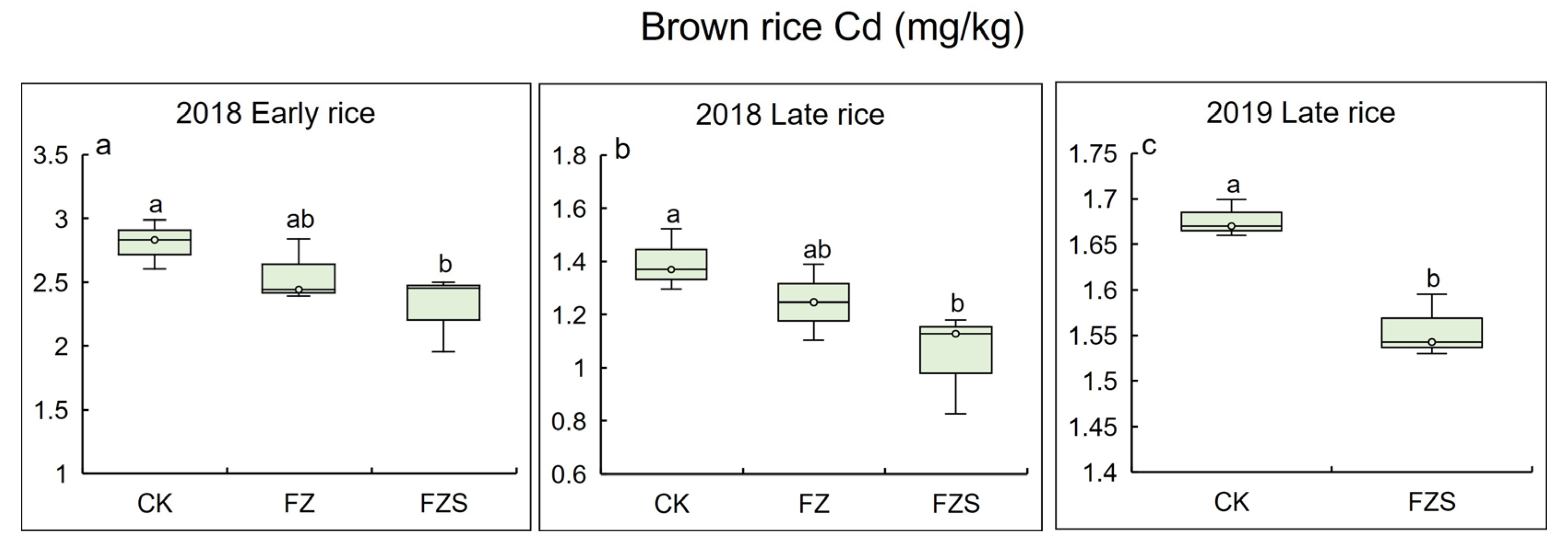
3.2. Cd Concentrations in Brown Rice over Three Growing Seasons
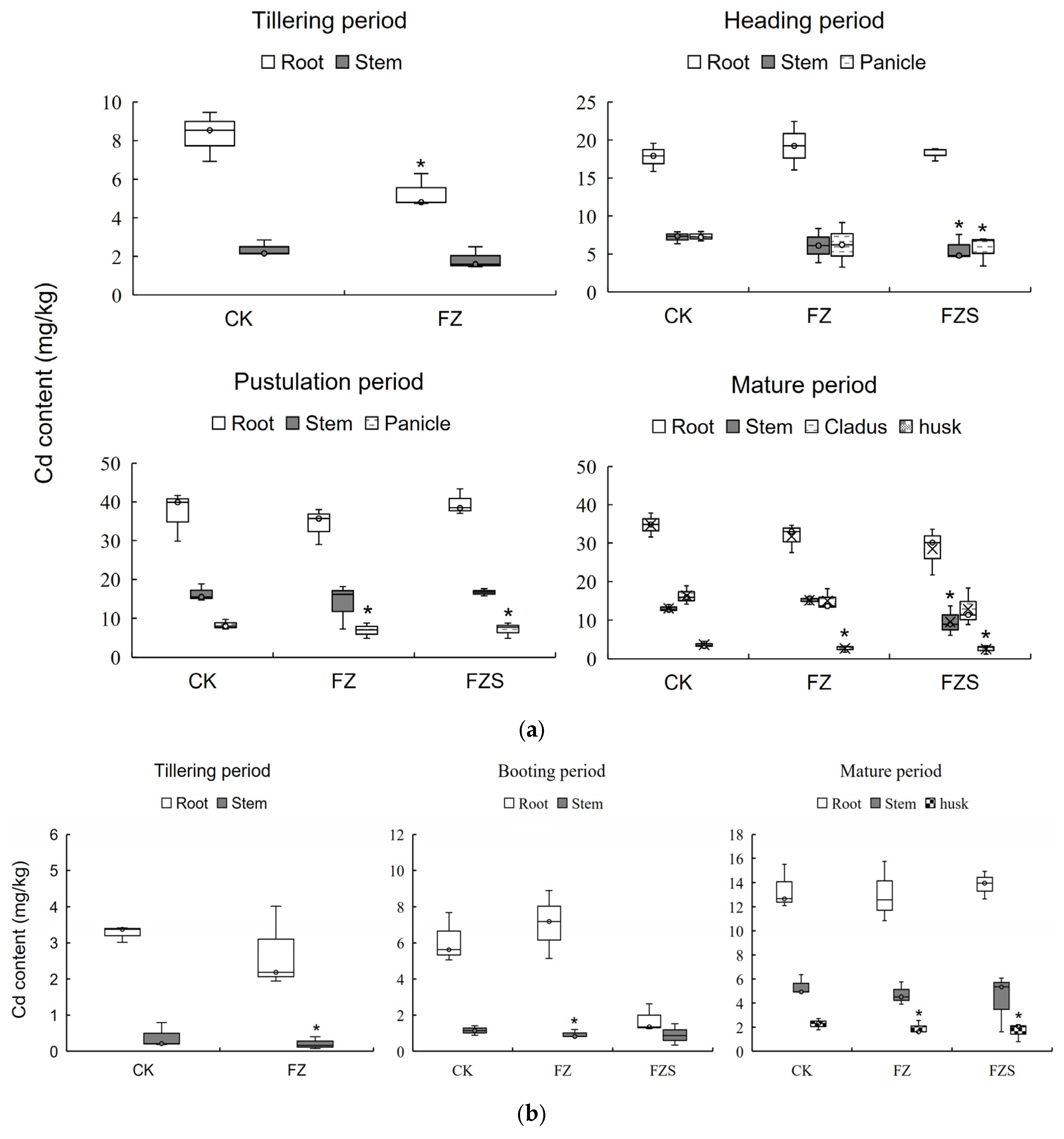
3.3. Cd Accumulation and Distribution in Root–Stem–Panicle Across Growth Stages
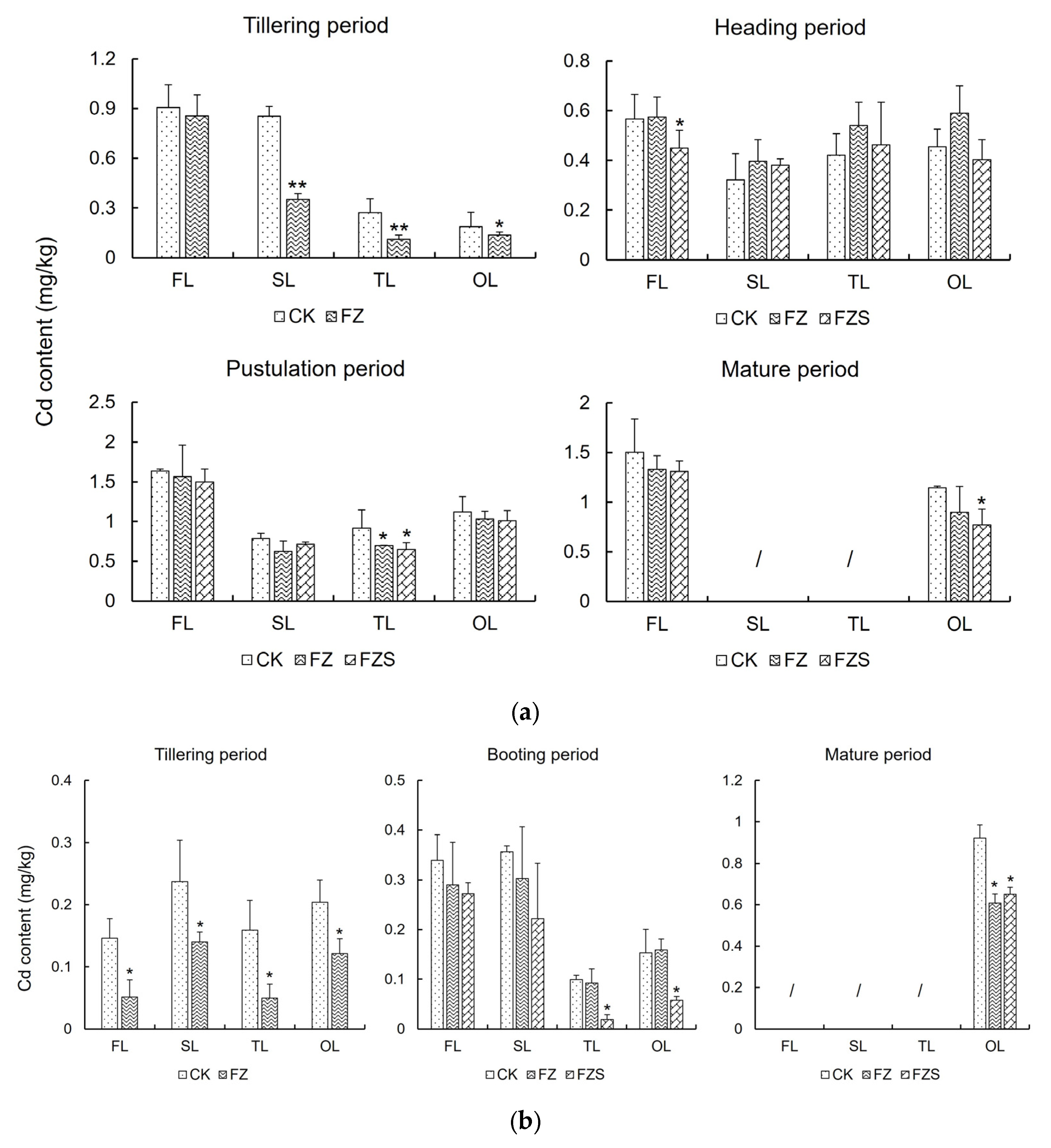
3.4. Cd Dynamics in Rice Leaves Across Growth Stages
3.5. Cd Transport Coefficients in Rice Organ Systems Throughout the Growth Cycle
3.6. Analysis of Potential Correlations in Cd Accumulation over Rice Developmental Phases
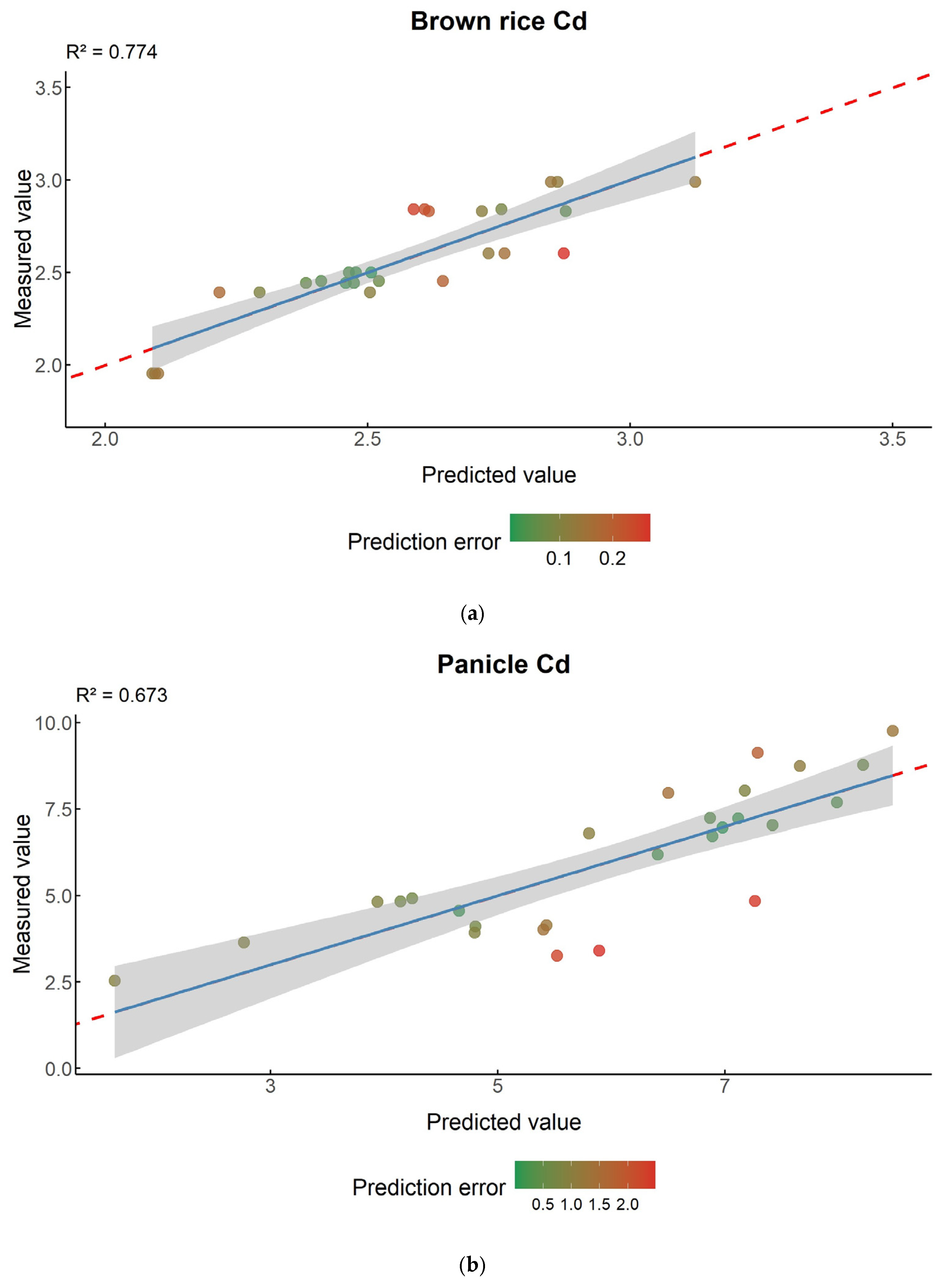
3.7. Predicting Brown Rice Cd Contamination Risk Across Life Cycle via Multivariate Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiggenhauser, M.; Aucour, A.-M.; Bureau, S.; Campillo, S.; Telouk, P.; Romani, M.; Ma, J.F.; Landrot, G.; Sarret, G. Cadmium transfer in contaminated soil-rice systems: Insights from solid-state speciation analysis and stable isotope fractionation. Environ. Pollut. 2021, 269, 115934. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.F.; Ji, X.H.; Liu, X.L.; Xie, Y.-H.; Xiao, S.Y.; Tian, F.X.; Xue, T.; Liu, S.-H. Influence of landform, soil properties, soil Cd pollution and rainfall on the spatial variation of Cd in rice: Contribution and pathway models based on big data. Sci. Total Environ. 2024, 912, 168687. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Y.; Zhao, F.J.; Wang, P. The relative contributions of root uptake and remobilization to the loading of Cd and As into rice grains: Implications in simultaneously controlling grain Cd and As accumulation using a segmented water management strategy. Environ. Pollut. 2022, 293, 118497. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Su, S.; Zhu, Q.; Zhang, N.; Yang, Z.; Zeng, X. Simultaneous mitigation of Cd and As availability in soil-rice continuum via the addition of an Fe-based desulfurization material. Sci. Total Environ. 2022, 812, 152603. [Google Scholar] [CrossRef]
- Lu, T.; Ge, W.; Li, A.; Deng, S.; Min, T.; Qiu, G. Endogenous silicon-activated rice husk biochar prepared for the remediation of cadmium-contaminated soils: Performance and mechanism. Environ. Pollut. 2024, 362, 125030. [Google Scholar] [CrossRef]
- Yuan, S.; Cui, C.; Han, Y.; Chen, P.; Tu, N.; Yi, Z. Silicon Calcium Fertilizer Application and Foliar Spraying with Silicon Fertilizer Decreases Cadmium Uptake and Translocation in Rice Grown in Polluted Soil. Agronomy 2023, 13, 1170. [Google Scholar] [CrossRef]
- Li, S.; He, Z.; Qiu, W.; Yu, M.; Wu, L.; Han, X.; Zhuo, R. SpCTP3 from the hyperaccumulator Sedum plumbizincicola positively regulates cadmium tolerance by interacting with SpMDH1. J. Hazard. Mater. 2024, 472, 134517. [Google Scholar] [CrossRef]
- Li, X.; He, Z.; Li, Y.; Yao, Y.; Han, X.; Xu, J.; Zheng, C.; Zhuo, R.; Qiu, W. The Sedum plumbizincicola defensin gene, SpPDF, conferred cadmium accumulation via its chelation. Ecotoxicol. Environ. Saf. 2025, 294, 118095. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Wei, S.; Zhan, J.; Kong, G.; Wang, B.; Robinson, B.H.; Skuza, L.; Xue, J.; Dai, H. Differences in cadmium accumulation and physiological characteristics of three different ecotypes of the hyperaccumulator Solanum nigrum L. survived in northern, central and southern China. Sci. Total Environ. 2025, 989, 179885. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Can, H.; Dogan, I. Phytoremediation using genetically engineered plants to remove metals: A review. Environ. Chem. Lett. 2021, 19, 669–698. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of Cadmium Accumulation in Plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Yan, Y.F.; Choi, D.H.; Soon, K.D.; Woo, L.B. Absorption, translocation, and remobilization of cadmium supplied at different growth stages of rice. J. Crop Sci. Biotechnol. 2010, 13, 113–119. [Google Scholar] [CrossRef]
- Hu, C.; Yan, B.; Liu, Y.; Gong, C.; Zhao, M.; Qiu, R.; Tang, Y. Differential Effects of Senescence on the Phloem Exports of Cadmium and Zinc from Leaves to Grains in Rice during Grain Filling. Plants 2023, 12, 1902. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, W.; Yang, W.T.; Gu, J.F.; Gao, Z.X.; Chen, L.W.; Du, W.Q.; Zhang, P.; Peng, P.Q.; Liao, B.H. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages. Ecotoxicol. Environ. Saf. 2018, 152, 91–97. [Google Scholar] [CrossRef]
- Liu, Y.T.; Yan, B.F.; Cai, X.; Zheng, H.X.; Qiu, R.L.; Tang, Y.T. Foliar-applied zinc promotes cadmium allocation from leaf surfaces to grains in rice. J. Environ. Sci. 2025, 151, 582–593. [Google Scholar] [CrossRef]
- Ning, M.; Liu, S.J.; Deng, F.L.; Huang, L.Y.; Li, H.; Che, J.; Yamaji, N.; Hu, F.Y.; Lei, G.J. A vacuolar transporter plays important roles in zinc and cadmium accumulation in rice grain. New Phytol. 2023, 239, 1919–1934. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Y.; Shao, C.; Du, J.; Cui, C.; Ren, W.; Xiao, J.; Wang, X. CGF1 and CGF2 jointly participate in iron homeostasis in chloroplasts. Plant Physiol. Biochem. 2025, 226, 110073. [Google Scholar] [CrossRef]
- Hui, X.; Luo, L.; Chen, Y.; Palta, J.A.; Wang, Z. Zinc agronomic biofortification in wheat and its drivers: A global meta-analysis. Nat. Commun. 2025, 16, 3913. [Google Scholar] [CrossRef]
- Walker, E.L.; Connolly, E.L. Time to pump iron: Iron-deficiency-signaling mechanisms of higher plants. Curr. Opin. Plant Biol. 2008, 11, 530–535. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Chen, X.; Yang, Y.; Wang, S.; Li, Y.; Hu, H.; Ge, Y.; Cheng, W. Effects of pH, Fe, and Cd on the uptake of Fe2+ and Cd2+ by rice. Environ. Sci. Pollut. Res. Int. 2013, 20, 8947–8954. [Google Scholar] [CrossRef]
- Zhong, S.; Li, X.; Fang, L.; Bai, J.; Gao, R.; Huang, Y.; Huang, Y.; Liu, Y.; Liu, C.; Yin, H.; et al. Multifunctional Roles of Zinc in Cadmium Transport in Soil-Rice Systems: Novel Insights from Stable Isotope Fractionation and Gene Expression. Environ. Sci. Technol. 2024, 58, 12467–12476. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Bai, B.; Xie, Y.; Yao, D.; Chen, Z.; Zhang, D.; Liu, Y.; Xiao, Y.; Yu, Y.; Wu, J. Spatial ionomics provides new insights into the accumulation and transport of mineral ions in rice (Oryza sativa L.) under Cadmium stress. Environ. Exp. Bot. 2023, 208, 105267. [Google Scholar] [CrossRef]
- Guan, D.; Wu, J.; Xie, Y.; Xie, J.; Huang, X.; Ji, X. Double Prevention of Cadmium Uptake by Iron and Zinc in Rice Seedling-A Hypotonic Study. J. Soil. Sci. Plant Nutr. 2024, 24, 318–330. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, D.Y.; Li, B.; Ma, T.C.; Xu, C.; Zhu, Q.H.; Zhu, H.H.; Zhang, Q. Effects of Iron Intensity-regulated Root Microbial Community Structure and Function on Cadmium Accumulation in Rice. Huan Jing Ke Xue 2022, 43, 4313–4321. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Wang, H.; Yu, Z.; Han, T.; Liu, K.; Liu, L.; Huang, J.; Wen, S.; Zhang, H. Distribution characteristics of effective medium and micronutrient element contents in paddy soils of China. Trans. Chin. Soc. Agric. Eng. 2020, 36, 62–70. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Peng, H.; Deng, K.; Shi, Y.; Liu, S.; Jian, Z.; Li, C.; Ji, X.; Li, S. Alleviation of Cd-polluted paddy soils through Si fertilizer application and its effects on the soil microbial community. Sci. Total Environ. 2023, 855, 158735. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, X.; Xie, Y.; Guan, D.; Peng, H.; Zhu, J.; Tian, F. Efects of quicklime application at diferent rice growing stage on the cadmium contents in rice grain. J. Agro-Environ. Sci. 2017, 35, 1867–1872. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, J.L.; Zhang, F.S. Role of iron plaque in Cd uptake by and translocation within rice (Oryza sativa L.) seedlings grown in solution culture. Environ. Exp. Bot. 2007, 59, 314–320. [Google Scholar] [CrossRef]
- Judesse Soviguidi, D.R.; Ping, H.; Pan, B.; Lei, R.; Liang, G. Oryza sativa iron regulated transporter 1 (OsIRT1) and OsIRT2 are involved in ferrous iron uptake in rice. Plant Physiol. Biochem. 2025, 226, 110059. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, D.; Wang, M.; Zhong, S.; Huang, Y.; Li, F.; Li, X.; Xing, B. Regulation of iron and cadmium uptake in rice roots by iron(iii) oxide nanoparticles: Insights from iron plaque formation, gene expression, and nanoparticle accumulation. Environ. Sci. Nano 2022, 9, 4093–4103. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, Q.; Zhou, H.; Zhou, X.; Yang, W.T.; Huang, G.M.; Gu, J.F.; Liao, B.H. Exogenous Fe(II) enhanced iron plaque formation influences the adsorption and immobilization of Cd in rice root. J. Sci. Food Agric. 2025, 106, 317–327. [Google Scholar] [CrossRef]
- Guan, D.; Wu, J.M.; Sun, S.H.; Ji, X.H.; Huang, X.; Liu, S.H.; Xie, Y.H. Iron and zinc-mediated regulation of cadmium transport in the rice (Oryza sativa L.) stem-leaf-grain pathway. Ecotoxicol. Environ. Saf. 2025, 307, 119430. [Google Scholar] [CrossRef]
- Guan, M.Y.; Xia, Y.C.; Zhang, W.X.; Chen, M.X.; Cao, Z.Z. A Review of Reducing Cadmium Pollution in the Rice-Soil System in China. Foods 2025, 14, 1747. [Google Scholar] [CrossRef]
- Luo, J.S.; Tang, J.; He, Y.; Liu, D.; Yang, Y.; Zhang, Z. Overexpression of vacuolar transporters OsVIT1 and OsVIT2 reduces cadmium accumulation in rice. Plant Mol. Biol. 2024, 114, 14. [Google Scholar] [CrossRef]
- Rabelo, F.H.S.; Daneluzzi, G.S.; dos Santos, F.H.; Colzato, M.; Montanha, G.S.; Nakamura, L.R.; de Carvalho, H.W.P.; Lavres, J.; Alleoni, L.R.F. Role of nodes in accumulation and distribution of cadmium and its relationship with nutrient distribution and photosynthesis in the growth and regrowth of Brachiaria decumbens. Environ. Exp. Bot. 2022, 195, 104794. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yin, X.M.; Gao, K.H.; Ge, Y.; Cheng, W.D. Non-protein thiols and glutathione S-transferase alleviate Cd stress and reduce root-to-shoot translocation of Cd in rice. J. Plant Nutr. Soil. Sci. 2013, 176, 626–633. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Okamura, K.; Miyazaki, M.; Phan, T.; Yuasa, T.; Iwaya-Inoue, M. Expression of rice sucrose transporter gene OsSUT1 in sink and source organs shaded during grain filling may affect grain yield and quality. Environ. Exp. Bot. 2014, 97, 49–54. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Liu, P.; Sun, L.; Zhang, Y.; Tan, Y.J.; Zhu, Y.X.; Peng, C.; Wang, J.R.; Yan, H.L.; Mao, D.H.; Liang, G.H.; et al. The metal tolerance protein OsMTP11 facilitates cadmium sequestration in the vacuoles of leaf vascular cells for restricting its translocation into rice grains. Mol. Plant 2024, 17, 1733–1752. [Google Scholar] [CrossRef]
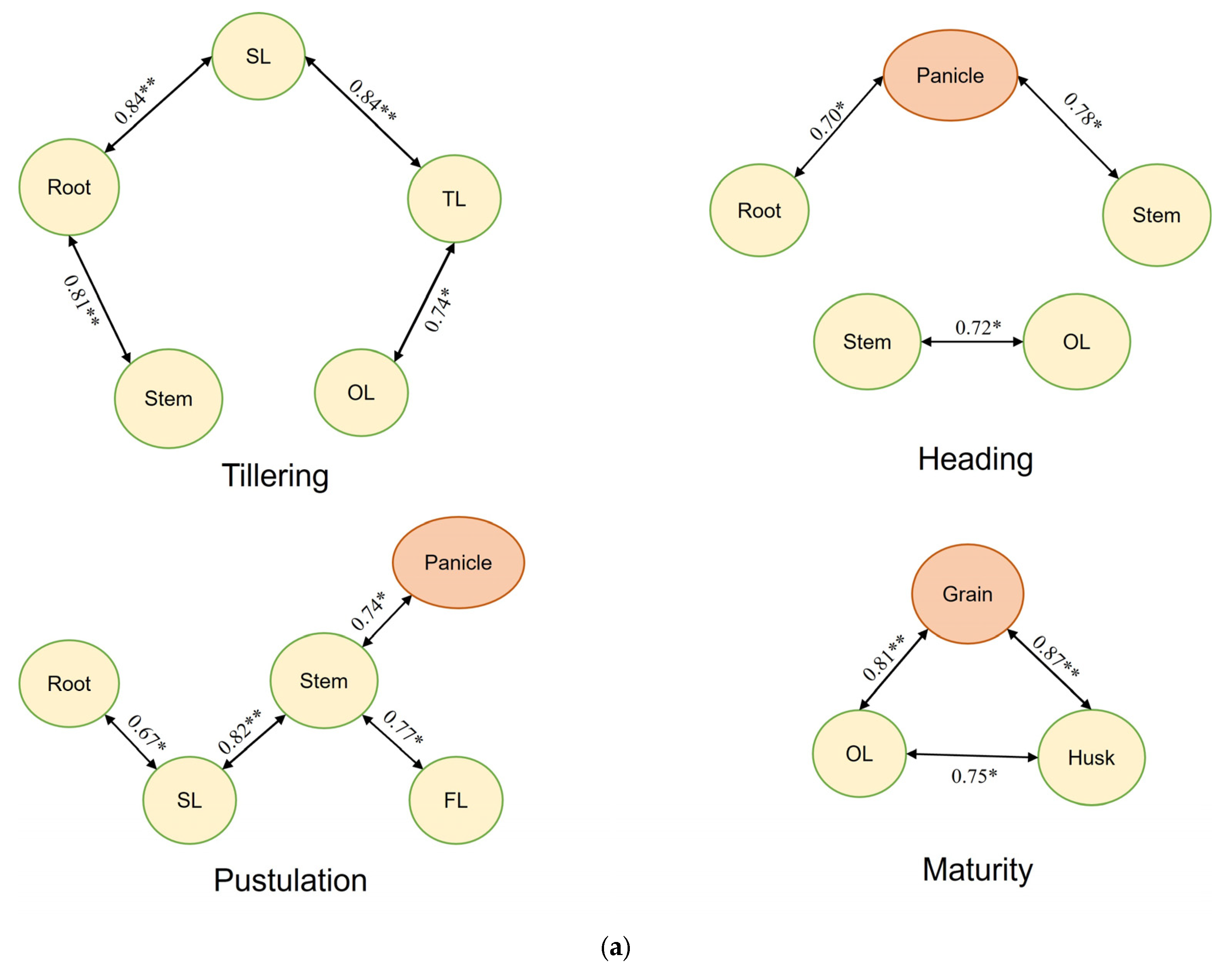
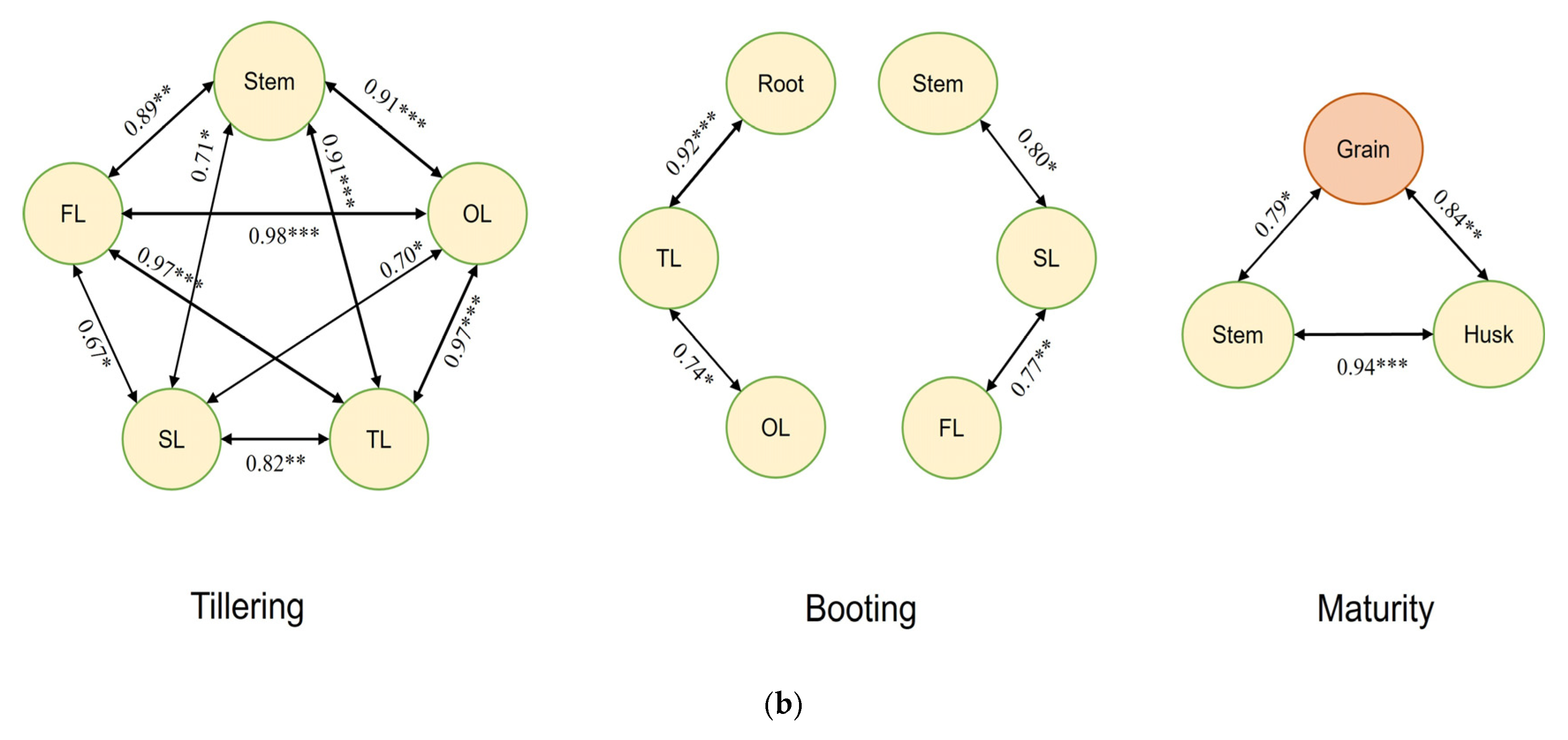
| (a) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Stages | Root/Soil | Stem/Root | OL/Stem | Panicle (Cladus)/Stem | TL/OL | SL/TL | FL/SL | Husk/Cladus | Brown Rice/Husk | |||||
| Tillering stage | CK | 8.4 | 0.28 | 0.078 | / | 1.5 | 3.2 | 1.1 | / | / | ||||
| FZ | 5.3 | 0.35 | 0.074 | / | 0.81 | 3.2 | 2.4 | / | / | |||||
| Heading stage | CK | 17.9 | 0.40 | 0.063 | 1.0 | 0.93 | 0.76 | 1.8 | / | / | ||||
| FZ | 19.4 | 0.31 | 0.097 | 1.0 | 0.92 | 0.73 | 1.5 | / | / | |||||
| FZS | 18.4 | 0.30 | 0.071 | 1.0 | 1.2 | 0.82 | 1.2 | / | / | |||||
| Pustulation stage | CK | 37.4 | 0.44 | 0.069 | 0.51 | 0.82 | 0.86 | 2.1 | / | / | ||||
| FZ | 34.5 | 0.40 | 0.074 | 0.49 | 0.68 | 0.89 | 2.5 | / | / | |||||
| FZS | 39.9 | 0.42 | 0.061 | 0.42 | 0.64 | 1.1 | 2.1 | / | / | |||||
| Maturity | CK | 35.0 | 0.37 | 0.088 | 1.2 | / | / | / | 0.22 | 0.76 | ||||
| FZ | 32.0 | 0.47 | 0.060 | 0.99 | / | / | / | 0.17 | 0.95 | |||||
| FZS | 28.7 | 0.33 | 0.081 | 1.3 | / | / | / | 0.19 | 0.91 | |||||
| (b) | ||||||||||||||
| Growth Stages | Root/Soil | Stem/Root | OL/Stem | TL/OL | SL/TL | FL/SL | Husk/Cladus | Brown Rice/Husk | ||||||
| Tillering stage | CK | 3.3 | 0.40 | 0.51 | 0.78 | 1.5 | 0.61 | / | / | |||||
| FZ | 2.7 | 0.21 | 0.57 | 0.41 | 2.8 | 0.36 | / | / | ||||||
| Booting stage | CK | 6.2 | 1.2 | 0.13 | 0.64 | 3.6 | 0.95 | / | / | |||||
| FZ | 7.1 | 0.95 | 0.16 | 0.58 | 3.3 | 0.96 | / | / | ||||||
| FZS | 1.8 | 0.91 | 0.064 | 0.32 | 11.7 | 1.2 | / | / | ||||||
| Maturity | CK | 13.5 | 5.4 | 0.17 | / | / | / | 0.42 | 0.61 | |||||
| FZ | 13.2 | 4.8 | 0.12 | / | / | / | 0.40 | 0.64 | ||||||
| FZS | 14.0 | 4.4 | 0.15 | / | / | / | 0.38 | 0.61 | ||||||
| Type of Organ | Multiple Regression Models | R Square | P |
|---|---|---|---|
| Brown rice | Cd brown rice = 0.276 × OL + 0.122 × P + 0.0088 × T − 0.254 × FL − 0.061 × FZ − 0.016 × Root + 1.801 | 0.774 | 1.44 × 10−5 |
| Panicle | Cd panicle = 0.393 × Stem − 0.073T | 0.673 | 1.49 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Xie, Y.; Ji, S.; Wu, J.; Zhu, F.; Ji, X.; Guan, D. Iron–Zinc Synergy Blocks Cadmium Translocation in Rice: Minimizing Grain Contamination. Agronomy 2025, 15, 2740. https://doi.org/10.3390/agronomy15122740
Sun S, Xie Y, Ji S, Wu J, Zhu F, Ji X, Guan D. Iron–Zinc Synergy Blocks Cadmium Translocation in Rice: Minimizing Grain Contamination. Agronomy. 2025; 15(12):2740. https://doi.org/10.3390/agronomy15122740
Chicago/Turabian StyleSun, Shaohui, Yunhe Xie, Shengying Ji, Jiamei Wu, Feiying Zhu, Xionghui Ji, and Di Guan. 2025. "Iron–Zinc Synergy Blocks Cadmium Translocation in Rice: Minimizing Grain Contamination" Agronomy 15, no. 12: 2740. https://doi.org/10.3390/agronomy15122740
APA StyleSun, S., Xie, Y., Ji, S., Wu, J., Zhu, F., Ji, X., & Guan, D. (2025). Iron–Zinc Synergy Blocks Cadmium Translocation in Rice: Minimizing Grain Contamination. Agronomy, 15(12), 2740. https://doi.org/10.3390/agronomy15122740







