Overexpression of CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Reinforcing Antioxidant Defense and Activating the ICE-CBF-COR Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Generation of Transgenic Melon Lines
2.2. Cold Stress Treatment and Sampling
2.3. Measurement of Phenotypic Indicators
2.4. Measurement of Physiological Indicators
2.5. Transcriptome Analysis
2.6. Analysis of Quantitative Real-Time PCR
2.7. Data Statistical Analysis and Graphical Presentation
3. Results
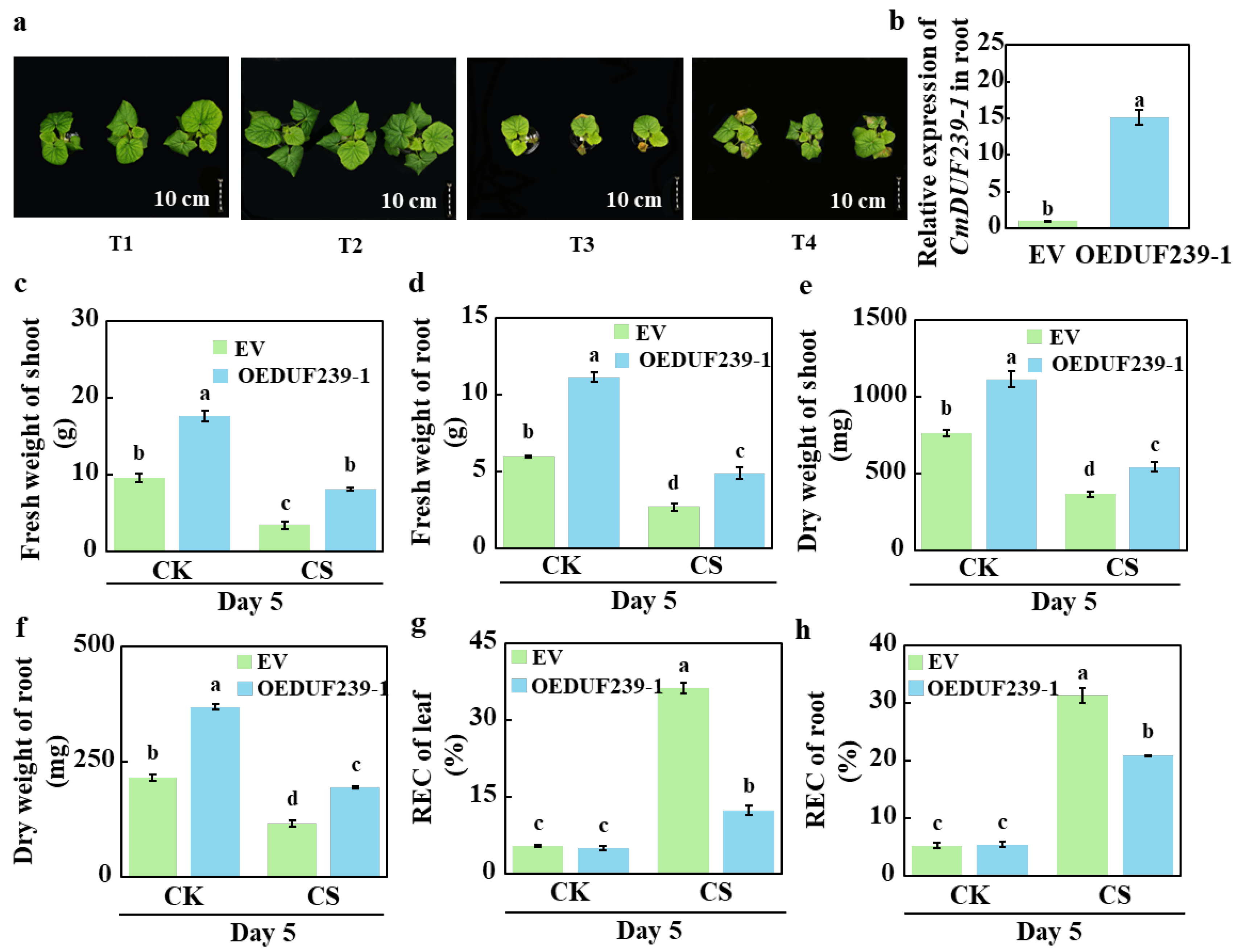
3.1. Effects of CmDUF239-1 Overexpression on the Phenotype and Membrane Stability of Melon Seedlings Under Cold Stress
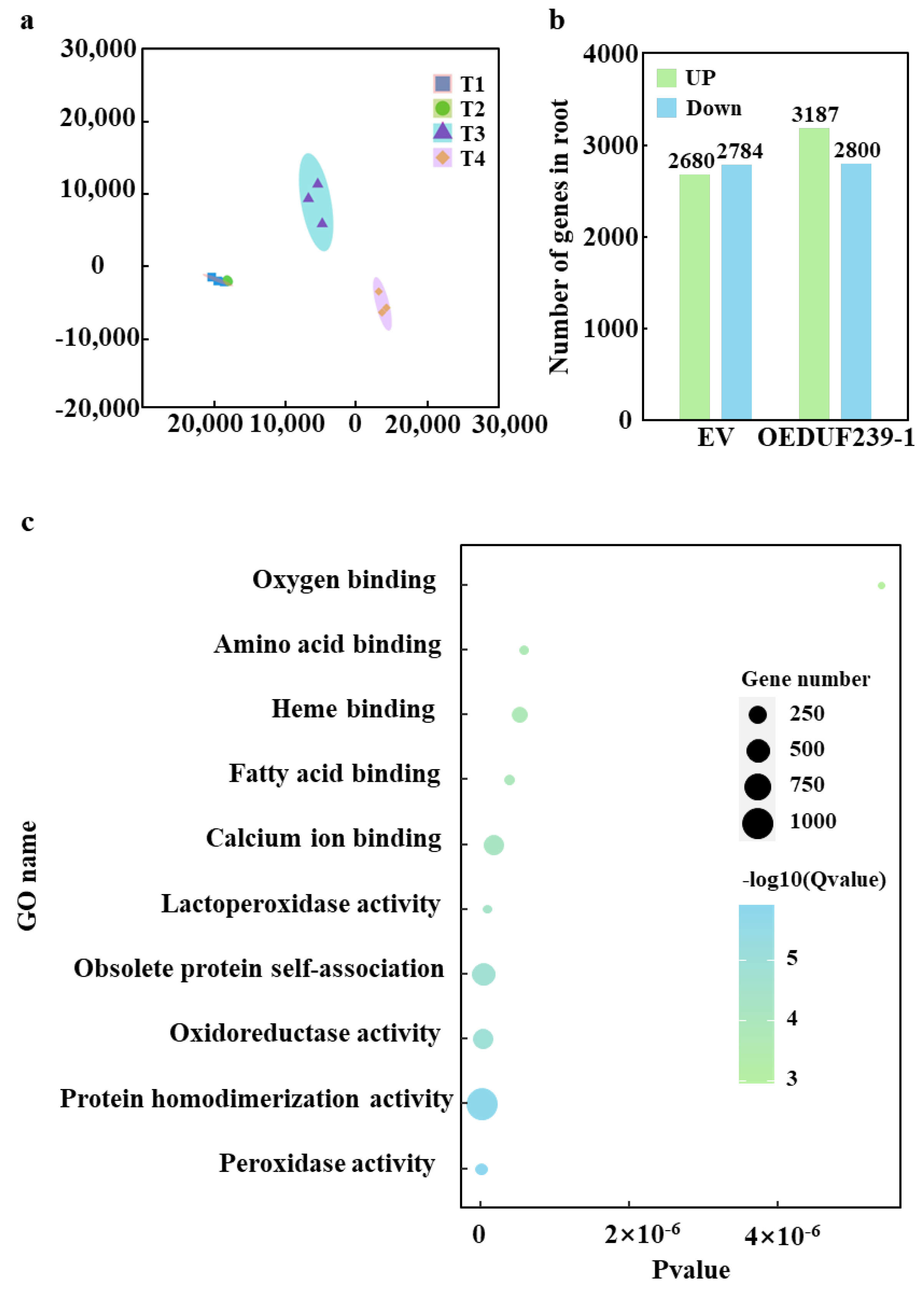
3.2. Differential Gene Analysis
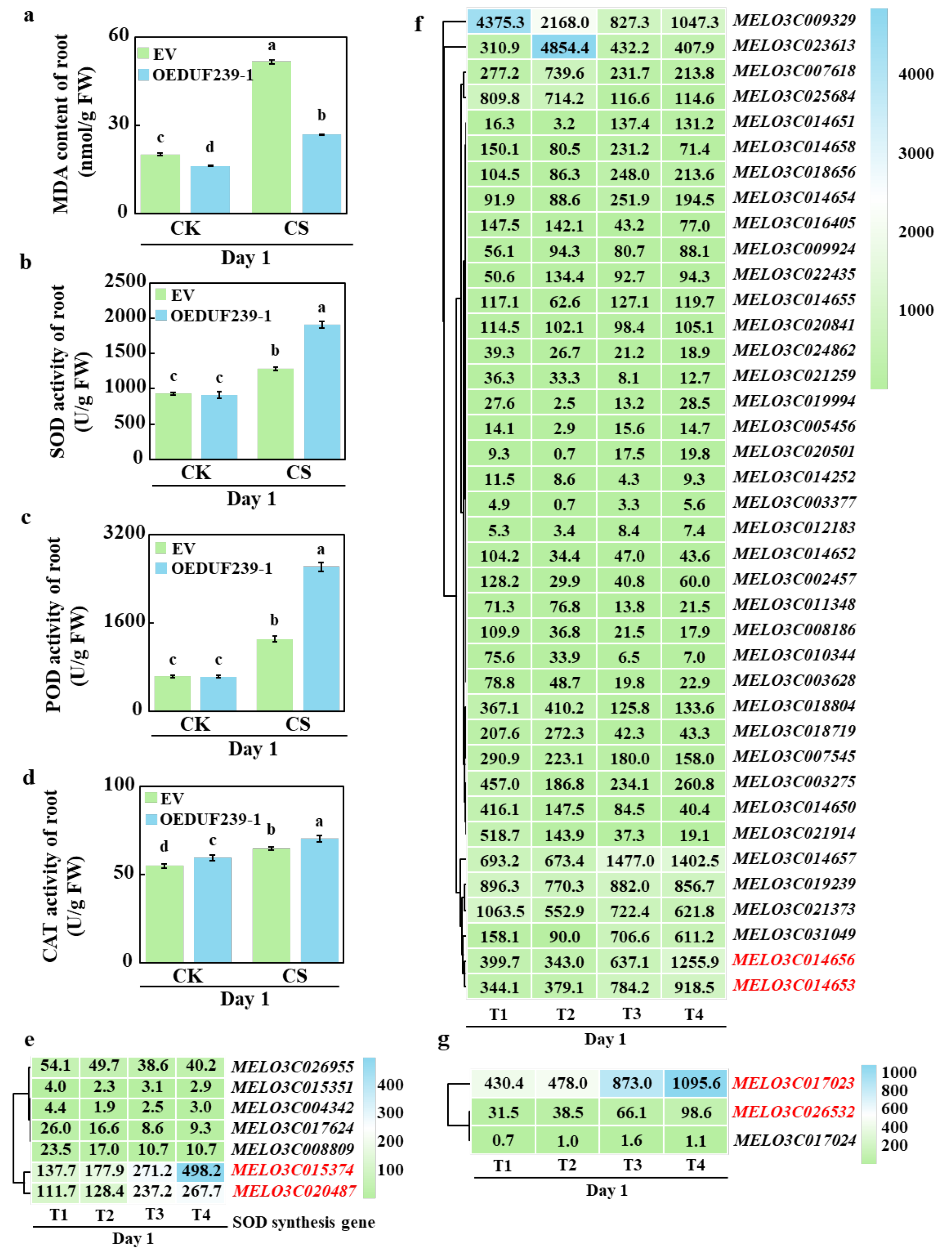
3.3. The Effect of Overexpressing CmDUF239-1 on Antioxidant Enzyme Activity in Roots
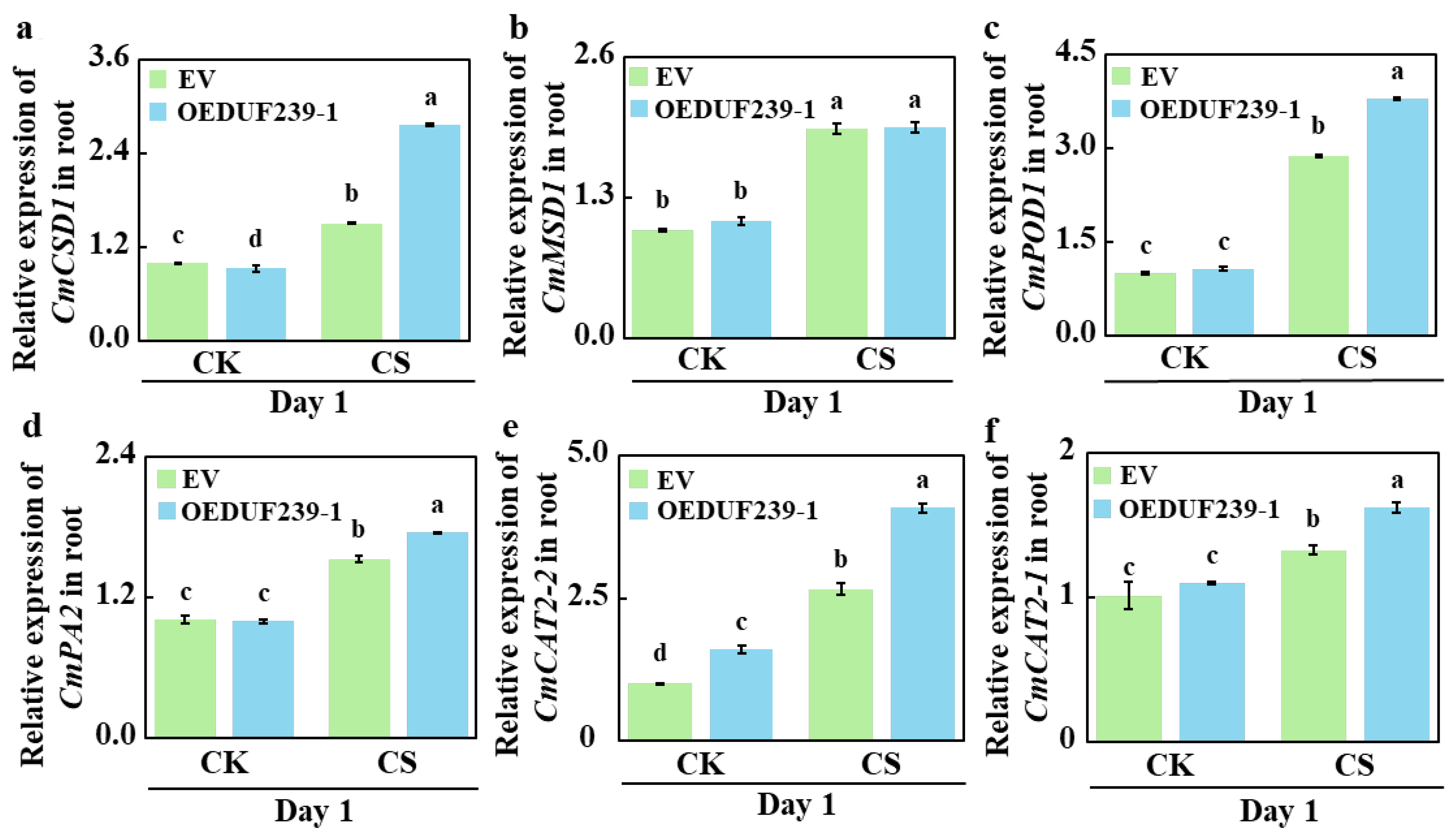
3.4. CmDUF239-1 Overexpression Upregulates the Expression of Antioxidant Enzyme-Related Genes Under Cold Stress
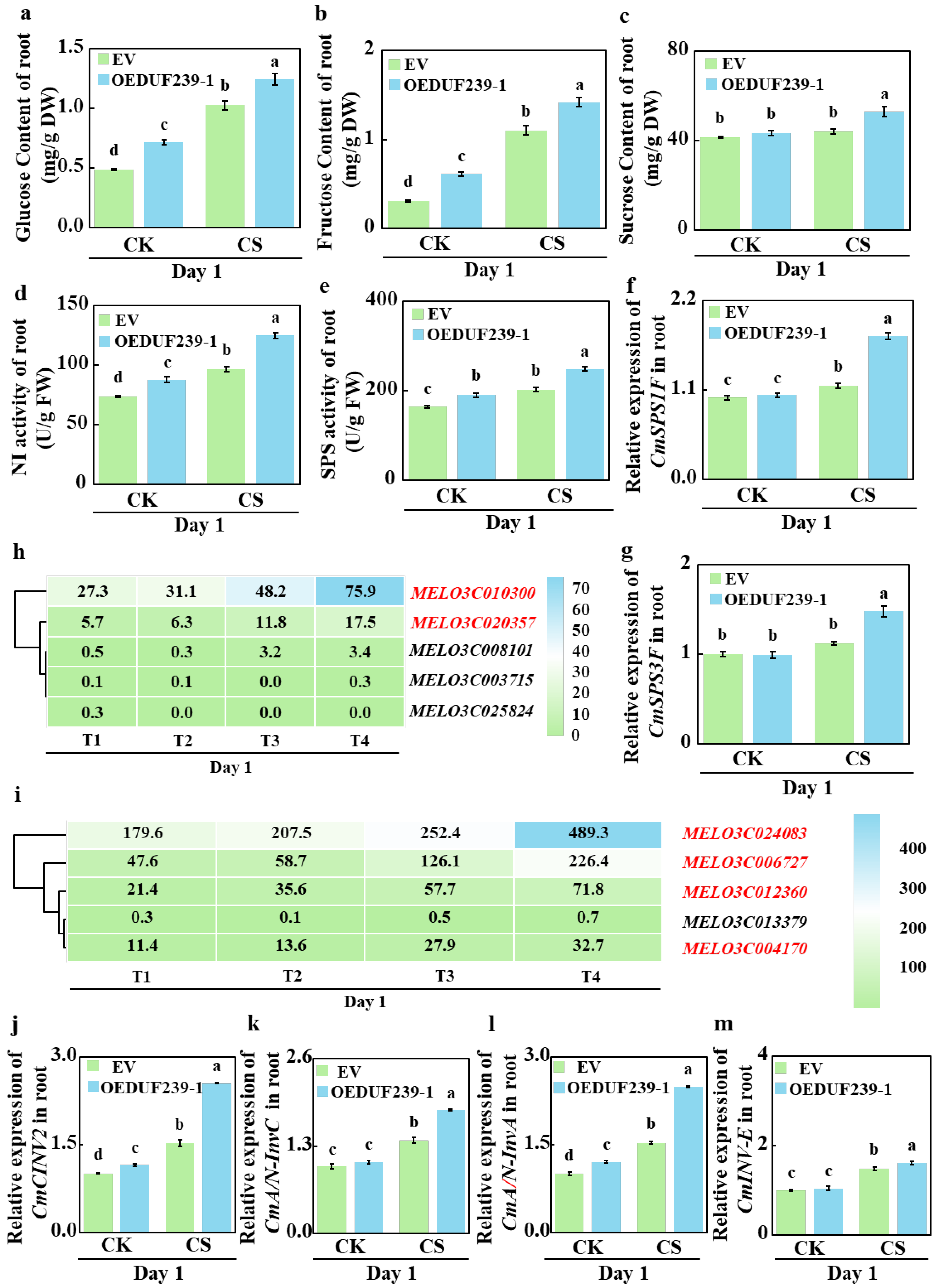
3.5. CmDUF239-1 Overexpression Regulates Soluble Sugar Content, Related Enzyme Activities and the Expression of Sugar Metabolism-Related Genes
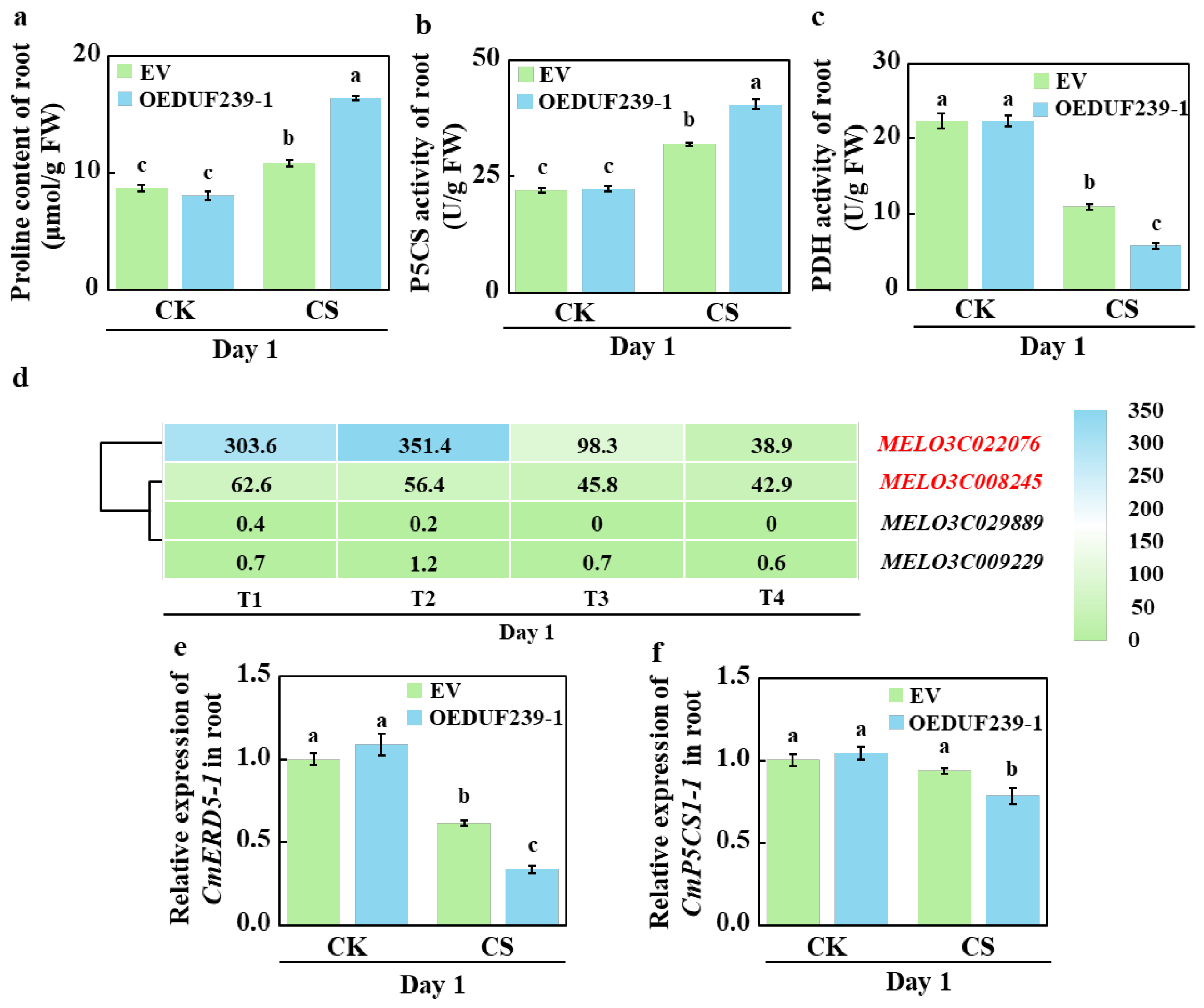
3.6. CmDUF239-1 Differentially Regulates Proline Metabolism at the Physiological and Transcriptional Levels
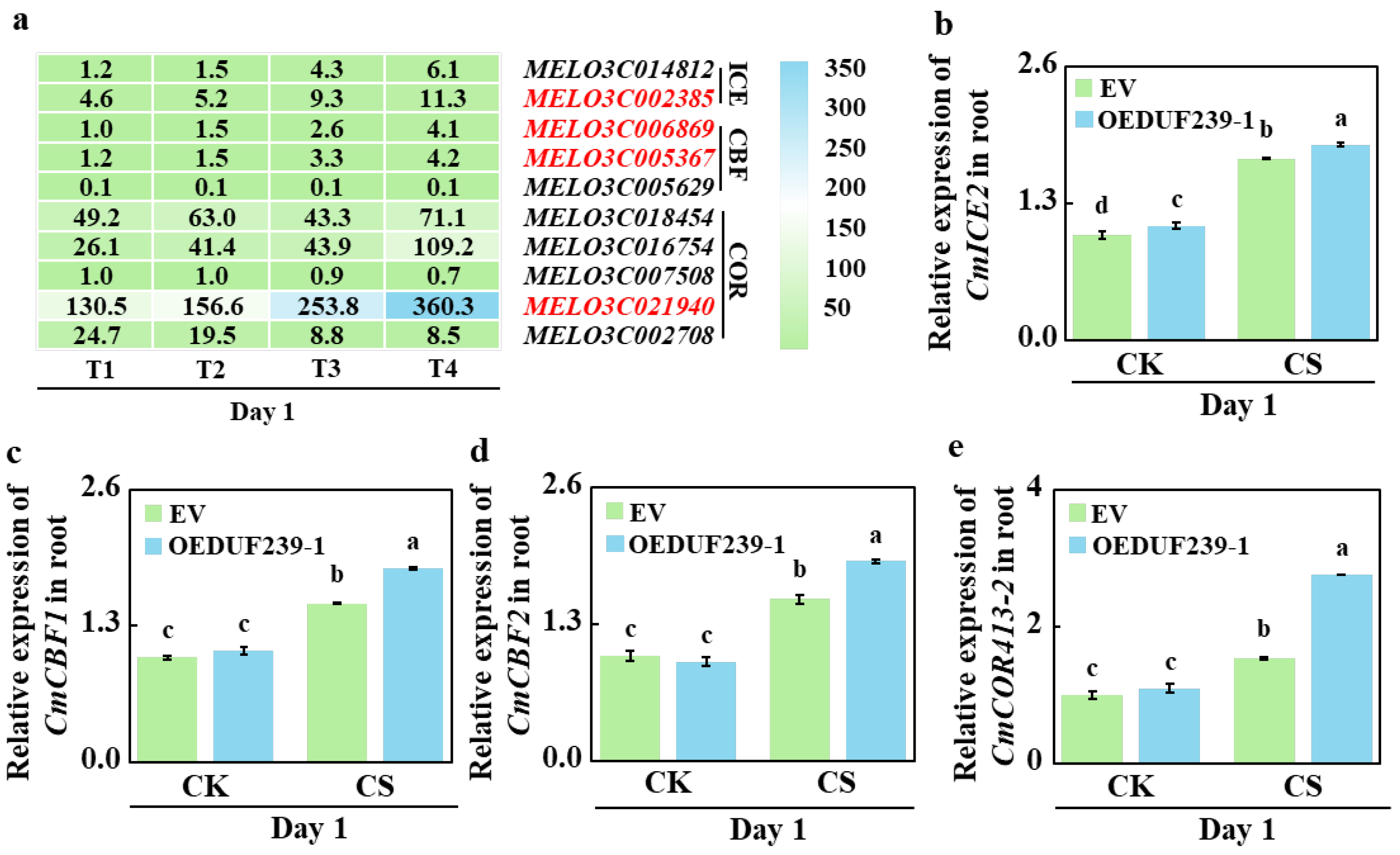
3.7. Overexpression of CmDUF239-1 Amplifies the Transcriptional Response of the ICE-CBF-COR Pathway
4. Discussion
4.1. Positive Regulation of Cold Tolerance in Melon by CmDUF239-1
4.2. CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Increasing Antioxidant
Enzyme Activity
4.3. CmDUF239-1 Modulates Sugar Metabolism and Proline Regulation to Maintain Cellular Homeostasis Under Cold Stress
4.4. CmDUF239-1 Activates the Central ICE-CBF-COR Cold Response Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| PRO | proline |
| SOD | superoxide dismutase |
| POD | peroxidase |
| CAT | catalase |
| NI | neutral invertase |
| SPS | sucrose phosphate synthase |
| P5CS | Δ1-pyrroline-5-carboxylate synthetase |
| PDH | proline dehydrogenase |
| GO | Gene Ontology |
References
- Zhu, J.; Dong, C.H.; Zhu, J.K. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr. Opin. Plant Biol. 2007, 10, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhang, X.; Song, G.; Lv, Q.; Li, M.; Fu, D.; Zhang, Z.; Gao, L.; Zhang, S.; Yang, X.; et al. Genetic variation in the aquaporin TONOPLAST INTRINSIC PROTEIN 4;3 modulates maize cold tolerance. Plant Biotechnol. J. 2024, 22, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R.; Davis, S.J. Ambient thermometers in plants: From physiological outputs towards mechanisms of thermal sensing. Curr. Biol. 2010, 20, R1086–1092. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhou, L.; Ullah, F.; Zou, J.; Zeng, X. Molecular and Physiological Responses of Plants that Enhance Cold Tolerance. Int. J. Mol. Sci. 2025, 26, 1157. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Khanna, K.; Bhardwaj, R.; Alam, P.; Reiter, R.J.; Ahmad, P. Phytomelatonin: A master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023, 196, 260–269. [Google Scholar] [CrossRef]
- Geng, J.; Wei, T.; Wang, Y.; Huang, X.; Liu, J.-H. Overexpression of PtrbHLH, a basic helix-loop-helix transcription factor from Poncirus trifoliata, confers enhanced cold tolerance in pummelo (Citrus grandis) by modulation of H2O2 level via regulating a CAT gene. Tree Physiol. 2019, 39, 2045–2054. [Google Scholar] [CrossRef]
- Marta, B.; Szafranska, K.; Posmyk, M.M. Exogenous Melatonin Improves Antioxidant Defense in Cucumber Seeds (Cucumis sativus L.) Germinated under Chilling Stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-X.; Xu, N.-W.; Yang, M.; Li, X.-L.; Han, J.-L.; Lin, X.-H.; Yang, Q.; Lv, G.-H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 37248–37265. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, G.K.; Saxena, S. Plant growth-promoting endophyte Nigrospora oryzae mitigates abiotic stress in rice (Oryza sativa L.). FEMS Microbiol. Ecol. 2023, 99, fiad094. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, H.; Li, H.; Su, R.; Khan, N.U.; Li, J.; Han, S.; Zhao, W.; Ye, W.; Gao, S.; et al. QTL mapping by GWAS and functional analysis of OsbZIP72 for cold tolerance at rice seedling stage. Crop J. 2024, 12, 1697–1708. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Li, M.; Yang, H.; Fu, D.; Lv, J.; Ding, Y.; Gong, Z.; Shi, Y.; Yang, S. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021, 63, 1874–1887. [Google Scholar] [CrossRef]
- Li, M.; Duan, X.; Gao, G.; Liu, T.; Qi, H. Running title: ABA pathway meets CBF pathway at CmADC. Hortic. Res. 2022, 9, uhac002. [Google Scholar] [CrossRef]
- Chen, T.; Chen, J.H.; Zhang, W.; Yang, G.; Yu, L.J.; Li, D.M.; Li, B.; Sheng, H.M.; Zhang, H.; An, L.Z. BYPASS1-LIKE, A DUF793 Family Protein, Participates in Freezing Tolerance via the CBF Pathway in Arabidopsis. Front. Plant Sci. 2019, 10, 807. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Adhikari, A.; Lee, I.J.; Loake, G.J.; Yun, B.W. A Novel DUF569 Gene Is a Positive Regulator of the Drought Stress Response in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 5316. [Google Scholar] [CrossRef]
- Chang, Z.; Tian, X.; Niu, X.; Bai, M.; Bai, W.; Wang, R.; Li, G.; Yang, Q. AtPADRE13 Negatively Regulates Salt Stress Tolerance in Arabidopsis thaliana. Plants 2025, 14, 1514. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Xiong, T.; Wang, X.J.; Chen, Y.R.; Wang, J.L.; Guo, C.L.; Ye, Z.Y. Lineage-specific gene duplication and expansion of DUF1216 gene family in Brassicaceae. PLoS ONE 2024, 19, e0302292. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Nong, X.; Zhang, Y.; Tang, T.; Chen, Y.; Shen, Q.; Yan, C.; Lu, B. Characterization and in silico analysis of the domain unknown function DUF568-containing gene family in rice (Oryza sativa L.). BMC Genom. 2023, 24, 544. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Sevanthi, A.M.; Raman, K.V.; Jiwani, G.; Solanke, A.U.; Mandal, P.K.; Mohapatra, T. Overexpression of a DUF740 family gene (LOC_Os04g59420) imparts enhanced climate resilience through multiple stress tolerance in rice. Front. Plant Sci. 2022, 13, 947312. [Google Scholar] [CrossRef] [PubMed]
- Ying, S. Genome-Wide Identification and Transcriptional Analysis of Arabidopsis DUF506 Gene Family. Int. J. Mol. Sci. 2021, 22, 11442. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-q.; Lu, G.-q.; Wang, L.-h.; Wang, C.-l.; Guo, H.-m.; Li, Y.-f.; Cheng, H.-m. Overexpression of AmDUF1517 enhanced tolerance to salinity, drought, and cold stress in transgenic cotton. J. Integr. Agric. 2018, 17, 2204–2214. [Google Scholar] [CrossRef]
- Gu, L.; Cheng, H. Isolation, molecular cloning and characterization of a cold-responsive gene, AmDUF1517, from Ammopiptanthus mongolicus. Plant Cell Tissue Organ. Cult. 2014, 117, 201–211. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Z.; Meng, L.; Li, Y.; Peng, Y. CmDUF239-1 Improves the Salt Tolerance of Grafted Melon by Enhancing Antioxidant Capacity and Na+/K+ Homeostasis. Plants 2025, 14, 2670. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Z.; Liu, Y.; Peng, Y.; Liu, C. Root-Specific Overexpression of the CmDUF239-1 Gene Enhances Heat Tolerance in Melon Seedlings by Upregulating Antioxidant Enzymes Activities, Proline Content, and Expression of Heat Shock Protein-Related Genes. Horticulturae 2025, 11, 1198. [Google Scholar] [CrossRef]
- Han, Y.A.-O.; Zhang, H.; Zhang, Y.; Dong, L.; Li, H.; Tian, J.; Lu, K.; Liu, T.; Qi, H. CmCBF4 crosstalk with H2O2 signal involved in trehalose-promoted cold tolerance of melon seedlings. Plant J. Cell Mol. Biol. 2025, 124, e70520. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Y.; Yuan, L.; Zhang, Y.; Liu, J.; Zhou, F.; Wang, Q.; Hu, X. Genome-Wide Identification, Characterization, and Expression Analysis Related to Low-Temperature Stress of the CmGLP Gene Family in Cucumis melo L. Int. J. Mol. Sci. 2022, 23, 8190. [Google Scholar] [CrossRef]
- Han, Y.A.-O.; Luo, F.A.-O.; Liang, A.A.-O.; Xu, D.A.-O.; Zhang, H.A.-O.; Liu, T.A.-O.; Qi, H. Aquaporin CmPIP2;3 links H2O2 signal and antioxidation to modulate trehalose-induced cold tolerance in melon seedlings. Plant Physiol. 2024, 197, kiae477. [Google Scholar] [CrossRef]
- Peng, Y.; Cao, H.; Cui, L.; Wang, Y.; Wei, L.; Geng, S.; Yang, L.; Huang, Y.; Bie, Z. CmoNAC1 in pumpkin rootstocks improves salt tolerance of grafted cucumbers by binding to the promoters of CmoRBOHD1, CmoNCED6, CmoAKT1;2 and CmoHKT1;1 to regulate H2O2, ABA signaling and K+/Na+ homeostasis. Hortic. Res. 2023, 10, uhad157. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, T.; Han, J.; Wang, J.; Xiao, H.; Shang, F. Exogenous glucose irrigation alleviates cold stress by regulating soluble sugars, ABA and photosynthesis in melon seedlings. Environ. Exp. Bot. 2023, 212, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.A.-O.; Zhang, Y.A.-O.; Niu, Q. Physiological and Transcriptomic Analysis Reveals the Responses and Difference to High Temperature and Humidity Stress in Two Melon Genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Liao, J.; Cao, S.; Li, M.; Lv, T.; Qi, H. CmLOX10 positively regulates drought tolerance through jasmonic acid -mediated stomatal closure in oriental melon (Cucumis melo var. makuwa Makino). Sci. Rep. 2020, 10, 17452. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.M.; Chen, Y.Z.; Liang, Y.L.; Xu, M.Y.; Xu, X.M. Cadmium-induced membrane lipid peroxidation and changes in antioxidant enzyme activities and peroxidase isoforms in Jerusalem artichoke seedlings. J. Plant Physiol. Mol. Biol. 2007, 33, 301–308. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.H.; Kim, E.G.; Kim, N.; Kim, K.M. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef]
- Tian, H.; Ding, S.; Zhang, D.; Wang, J.; Hu, M.; Yang, K.; Hao, Y.; Qiao, N.; Du, W.; Li, R.; et al. Sodium Bicarbonate Tolerance During Seedling Stages of Maize (Zea mays L.) Lines. Food Energy Secur. 2024, 13, e70013. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, R.; Yao, X.; Chen, Y. Effects of Cadmium Stress on Antioxidant Enzyme Activities and Photosynthesis in Melon Seedlings. Chin. Agric. Sci. Bull. 2015, 31, 82–88. [Google Scholar]
- Huang, Q.; Liu, Y.; Qin, X.; Zhao, L.; Liang, X.; Xu, Y. Selenite mitigates cadmium-induced oxidative stress and affects Cd uptake in rice seedlings under different water management systems. Ecotoxicol. Environ. Saf. 2019, 168, 486–494. [Google Scholar] [CrossRef]
- Liang, S.A.-O.; Abeer, H.; Fathi Abd Allah, E.; Wu, Q.S. Transcriptomic analysis reveals potential roles of polyamine and proline metabolism in waterlogged peach roots inoculated with Funneliformis mosseae and Serendipita indica. Tree Physiol. 2025, 45, tpaf013. [Google Scholar] [CrossRef]
- Fan, L.; Wu, X.; Tian, Z.; Jia, K.; Pan, Y.; Li, J.; Gao, H. Comparative proteomic analysis of gamma-aminobutyric acid responses in hypoxia-treated and untreated melon roots. Phytochemistry 2015, 116, 28–37. [Google Scholar] [CrossRef]
- Niedziela, G.; Szabelska-Beręsewicz, A.; Zyprych-Walczak, J.; Graczyk, M. Application of edgeR and DESeq2 methods in plant experiments based on RNA-seq technology. Biom. Lett. 2022, 59, 127–139. [Google Scholar] [CrossRef]
- Li, D.A.-O.; Zand, M.S.; Dye, T.D.; Goniewicz, M.L.; Rahman, I.; Xie, Z. An evaluation of RNA-seq differential analysis methods. PLoS ONE 2022, 17, e0264246. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhu, H.; Shi, Y.; Li, Y.; Miao, Y.; Yu, X.; Zhang, Y.; Li, Y. Antisense Overexpression of Gγ Subunit CsGG3.1-2 Reduces Soluble Sugar Content and Chilling Tolerance in Cucumber. Horticulturae 2023, 9, 240. [Google Scholar] [CrossRef]
- Zogopoulos, V.A.-O.; Malatras, A.A.-O.; Michalopoulos, I.A.-O. Special Issue on Differential Gene Expression and Coexpression. Biology 2023, 12, 1226. [Google Scholar] [CrossRef]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, P.; Gao, W.; Long, Y.; Wang, Y.; Geng, S.; Su, X.; Jiao, Y.; Chen, Q.; Qu, Y. Genome-wide identification of the DUF668 gene family in cotton and expression profiling analysis of GhDUF668 in Gossypium hirsutum under adverse stress. BMC Genom. 2021, 22, 395. [Google Scholar] [CrossRef]
- Liu, K.; Li, M.; Zhang, B.; Yin, X.; Xia, X.; Wang, M.; Cui, Y. Poaceae Orthologs of Rice OsSGL, DUF1645 Domain-Containing Genes, Positively Regulate Drought Tolerance, Grain Length and Weight in Rice. Rice Sci. 2022, 29, 257–267. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Hu, Y.; Zhong, Q.; Yin, J.; Zhu, Y. Mining the Roles of Cucumber DUF966 Genes in Fruit Development and Stress Response. Plants 2022, 11, 2497. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Y.; Li, X. Overexpression of OsDUF6 increases salt stress tolerance in rice. BMC Plant Biol. 2024, 24, 216. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chen, B.; Wang, J.; Ding, F.; Wang, P.; Zhang, X.; Hou, J.; Luo, R.; Li, X.; et al. Identification of candidate genes that regulate the trade-off between seedling cold tolerance and fruit quality in melon (Cucumis melo L.). Hortic. Res. 2023, 10, uhad093. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.X.; Liu, Y.; Chen, Z.Y.; Guo, J.; Chen, J.; Zhou, Y.B.; Chen, M.; Ma, Y.Z.; Xu, Z.S.; Cui, X.Y. Genome-Wide Analysis of the DUF4228 Family in Soybean and Functional Identification of GmDUF4228 -70 in Response to Drought and Salt Stresses. Front. Plant Sci. 2021, 12, 628299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.; Chen, H.; Chen, M.; Zhao, X.; Mao, C.; Kong, Y.; Yang, J.; Jia, X.; Ye, X.; et al. Overexpression of OsDUF846.2 enhances the sensitivity of rice to salt and heat stresses. BMC Plant Biol. 2025, 25, 1359. [Google Scholar] [CrossRef]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Quan, X.; Shan, Q.; Wang, W.; Yin, N.; Wang, S.; Wang, Z.; He, W. Comprehensive analysis of cucumber C-repeat/dehydration-responsive element binding factor family genes and their potential roles in cold tolerance of cucumber. BMC Plant Biol. 2022, 22, 270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tan, Z.; Liu, Y.; Wu, X.; Zhu, J.; Peng, Y. Overexpression of CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Reinforcing Antioxidant Defense and Activating the ICE-CBF-COR Pathway. Agronomy 2025, 15, 2725. https://doi.org/10.3390/agronomy15122725
Li Y, Tan Z, Liu Y, Wu X, Zhu J, Peng Y. Overexpression of CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Reinforcing Antioxidant Defense and Activating the ICE-CBF-COR Pathway. Agronomy. 2025; 15(12):2725. https://doi.org/10.3390/agronomy15122725
Chicago/Turabian StyleLi, Yang, Zhanming Tan, Yanjun Liu, Xiaoye Wu, Jin Zhu, and Yuquan Peng. 2025. "Overexpression of CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Reinforcing Antioxidant Defense and Activating the ICE-CBF-COR Pathway" Agronomy 15, no. 12: 2725. https://doi.org/10.3390/agronomy15122725
APA StyleLi, Y., Tan, Z., Liu, Y., Wu, X., Zhu, J., & Peng, Y. (2025). Overexpression of CmDUF239-1 Enhances Cold Tolerance in Melon Seedlings by Reinforcing Antioxidant Defense and Activating the ICE-CBF-COR Pathway. Agronomy, 15(12), 2725. https://doi.org/10.3390/agronomy15122725






