Research on the Pathogenic Mechanism of Effector FvCfem7 in Fusarium verticillioides
Abstract
1. Introduction
1.1. Hemibiotrophic Pathogen Effectors
1.2. Cfem Effector
1.3. Host Pathogenesis-Related (PR) Protein Interactions with Effectors Affect Immunity
1.4. F. verticillioides–Host Interactions and Control Strategies
2. Materials and Methods
2.1. Bioinformatics and Phylogenetic Analyses
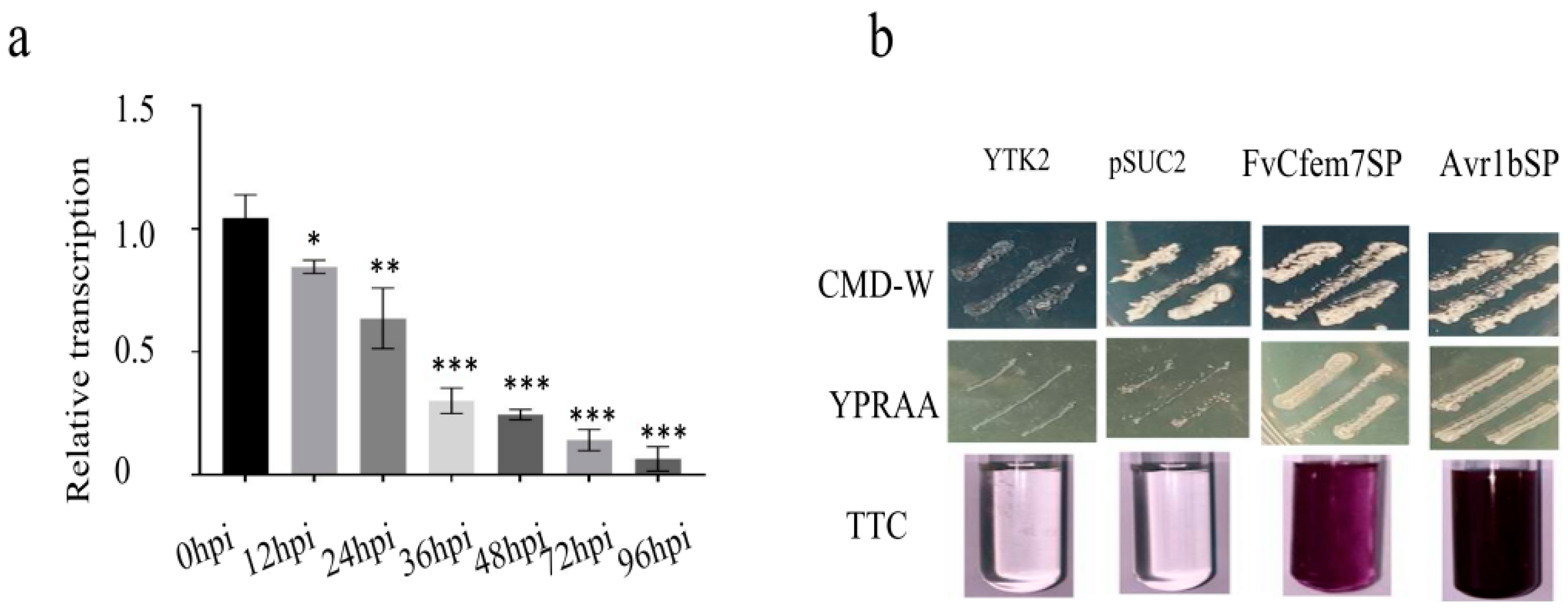
2.2. A Yeast Invertase Secretion Assay
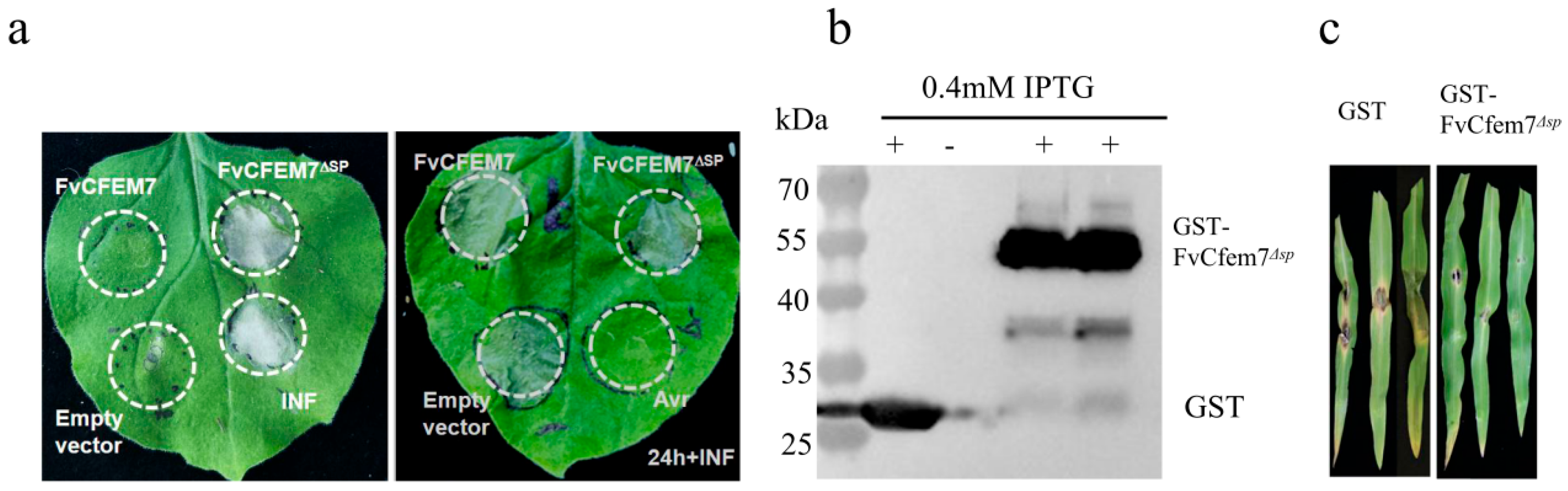
2.3. Transient Expression in Tobacco
2.4. Prokaryotic Heterologous Protein Expression and Disease Protection Assay
2.5. Strain and Growth Conditions
2.6. RNA Extraction and Quantitative PCR Analysis
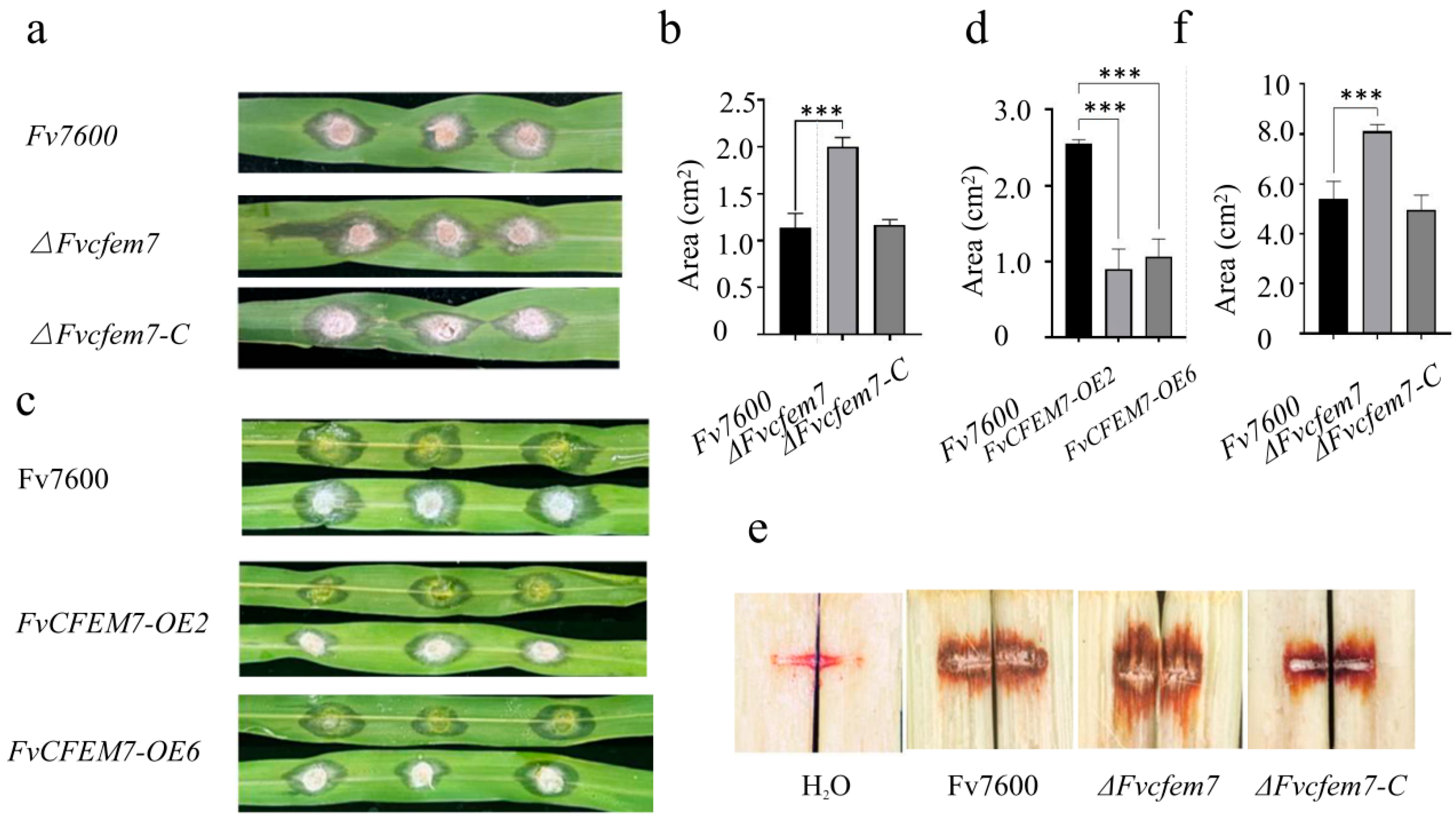
2.7. Generation of ∆Fvcfem7, ∆Fvcfem7-C, and FvCFEM7-OE Strains
2.8. Phylogenetic Analysis of Maize and Sugarcane
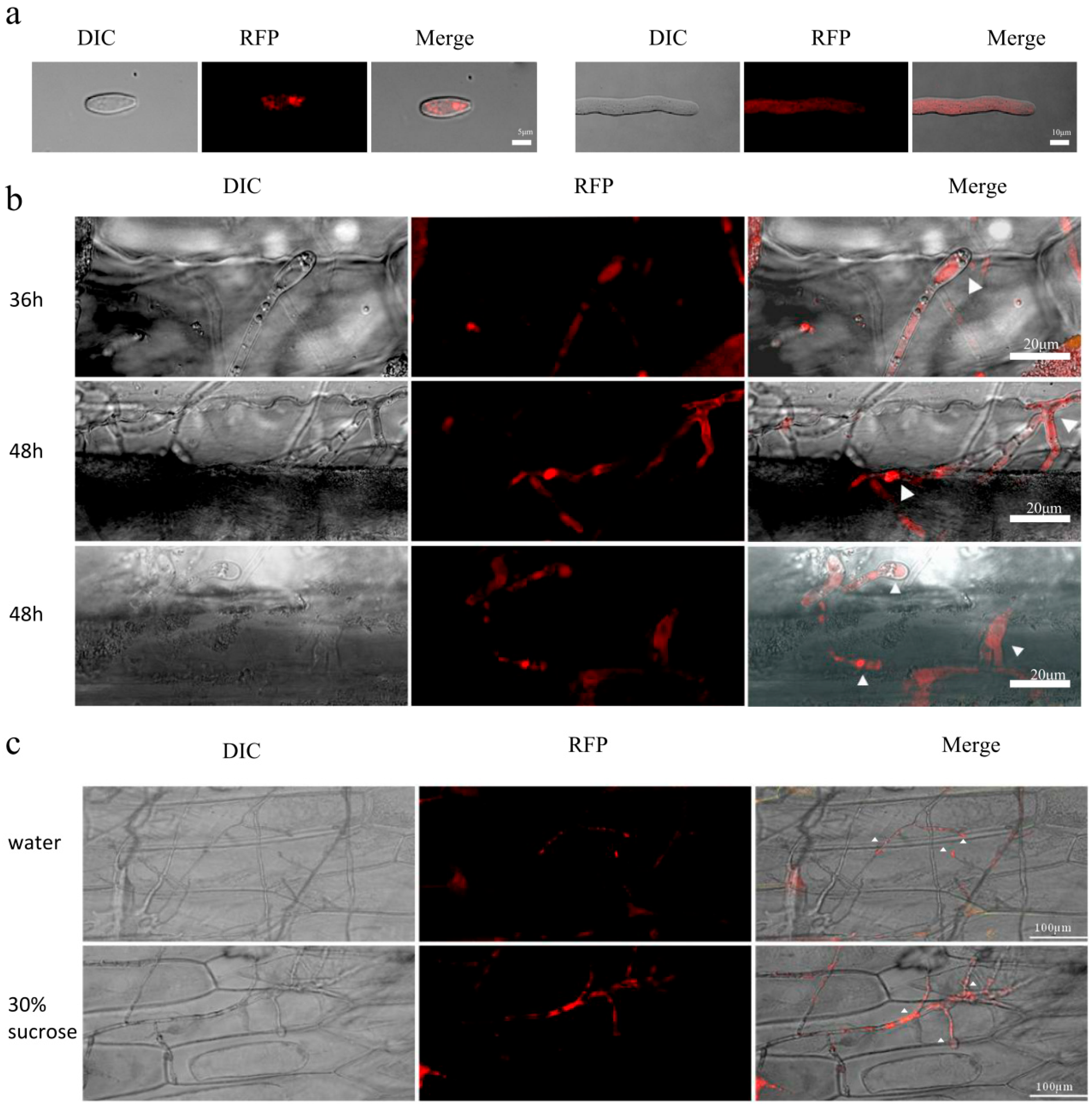
2.9. Subcellular Localization Observation
2.10. Yeast Two-Hybrid Library Screening and Assay
2.11. Statistical Analysis
3. Results
3.1. Fvcfem7 Serves as an Important Candidate Effector in F. verticillioides
3.2. FvCfem7 Negatively Regulates the Pathogenicity of F. verticillioides
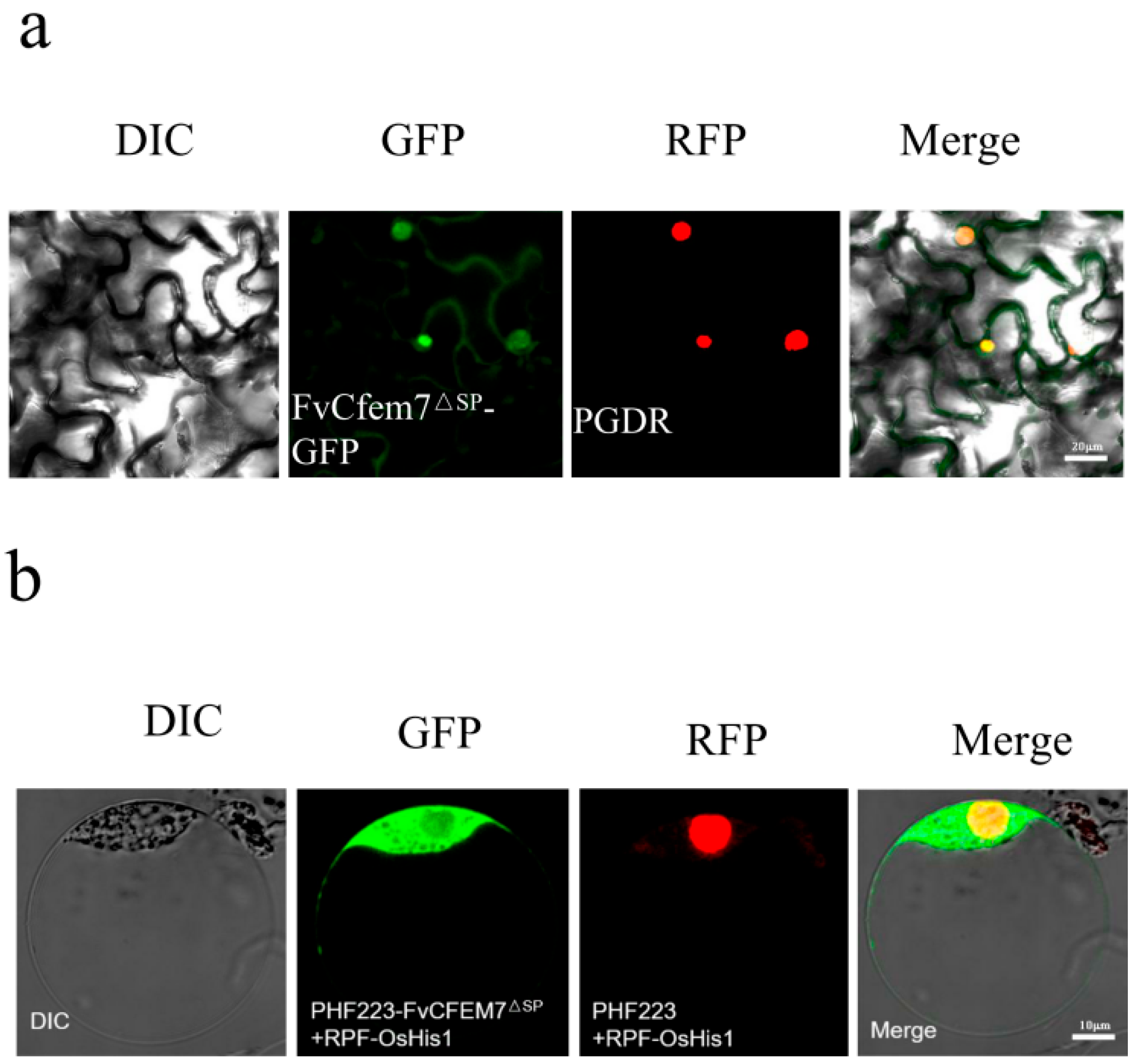
3.3. Differential Localization of Fvcfem7ΔSP In Vitro, During Hyphal Infection, and in Host Tissues
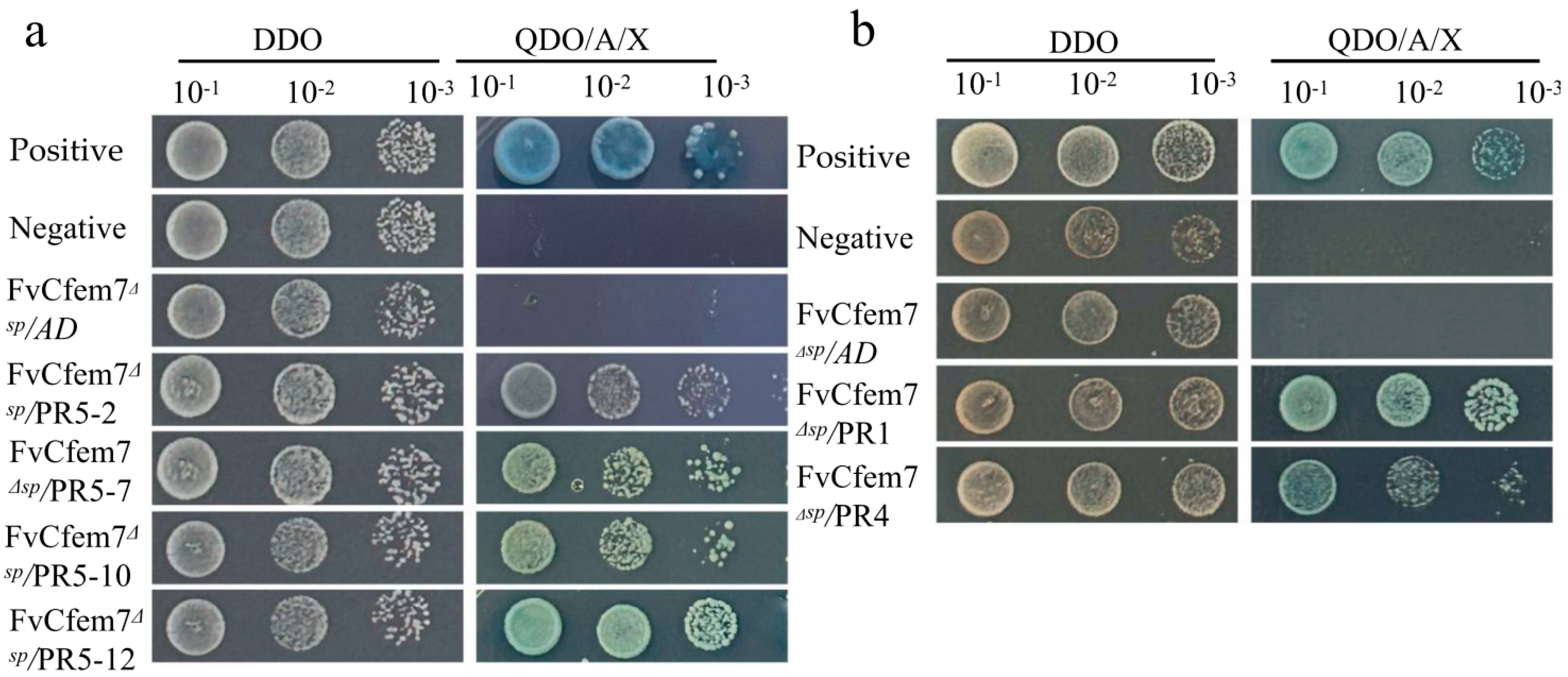
3.4. Fvcfem7ΔSP Interacts with PR Proteins
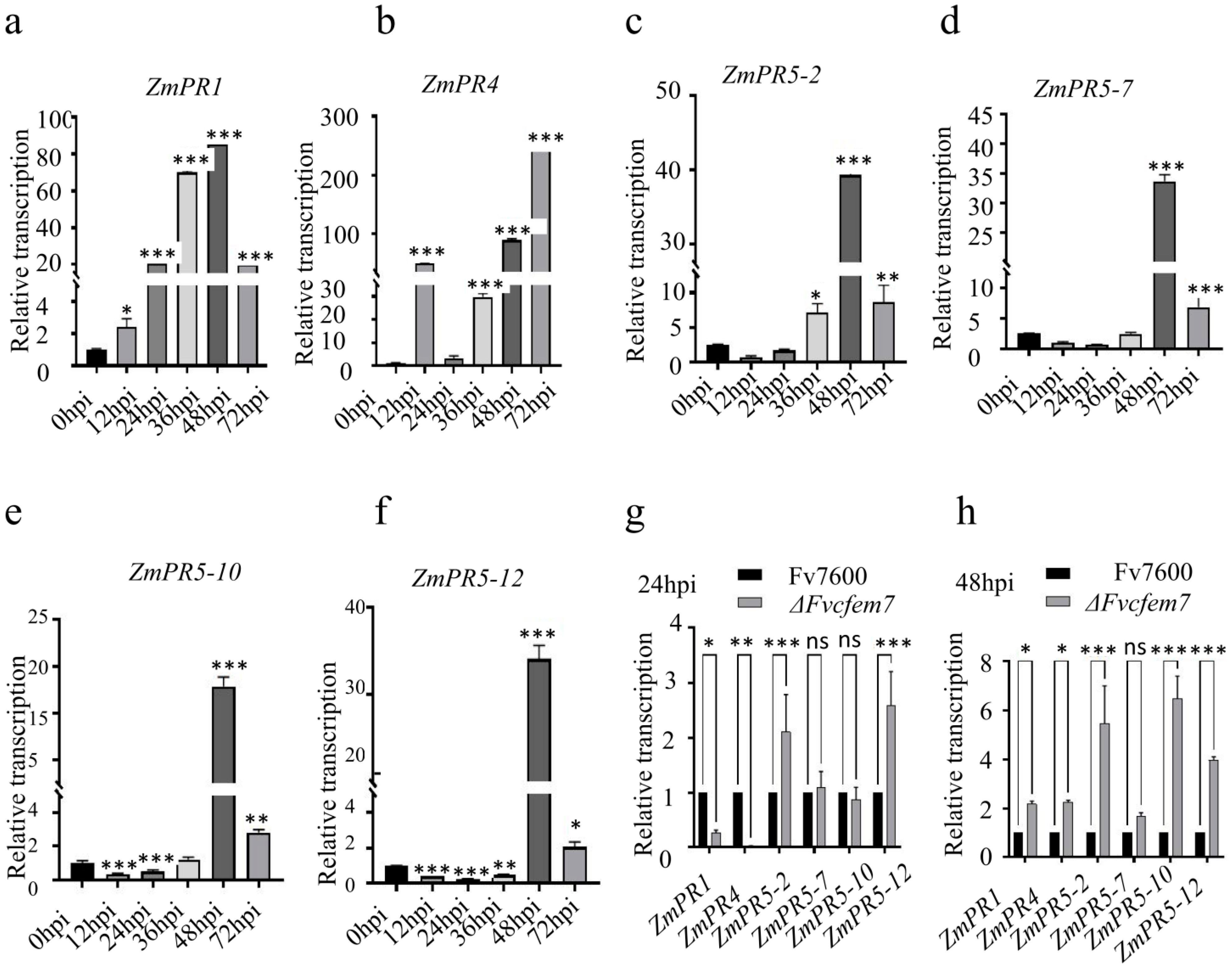
3.5. Effect of Fvcfem7 on the Expression of Interacting Maize PR Proteins
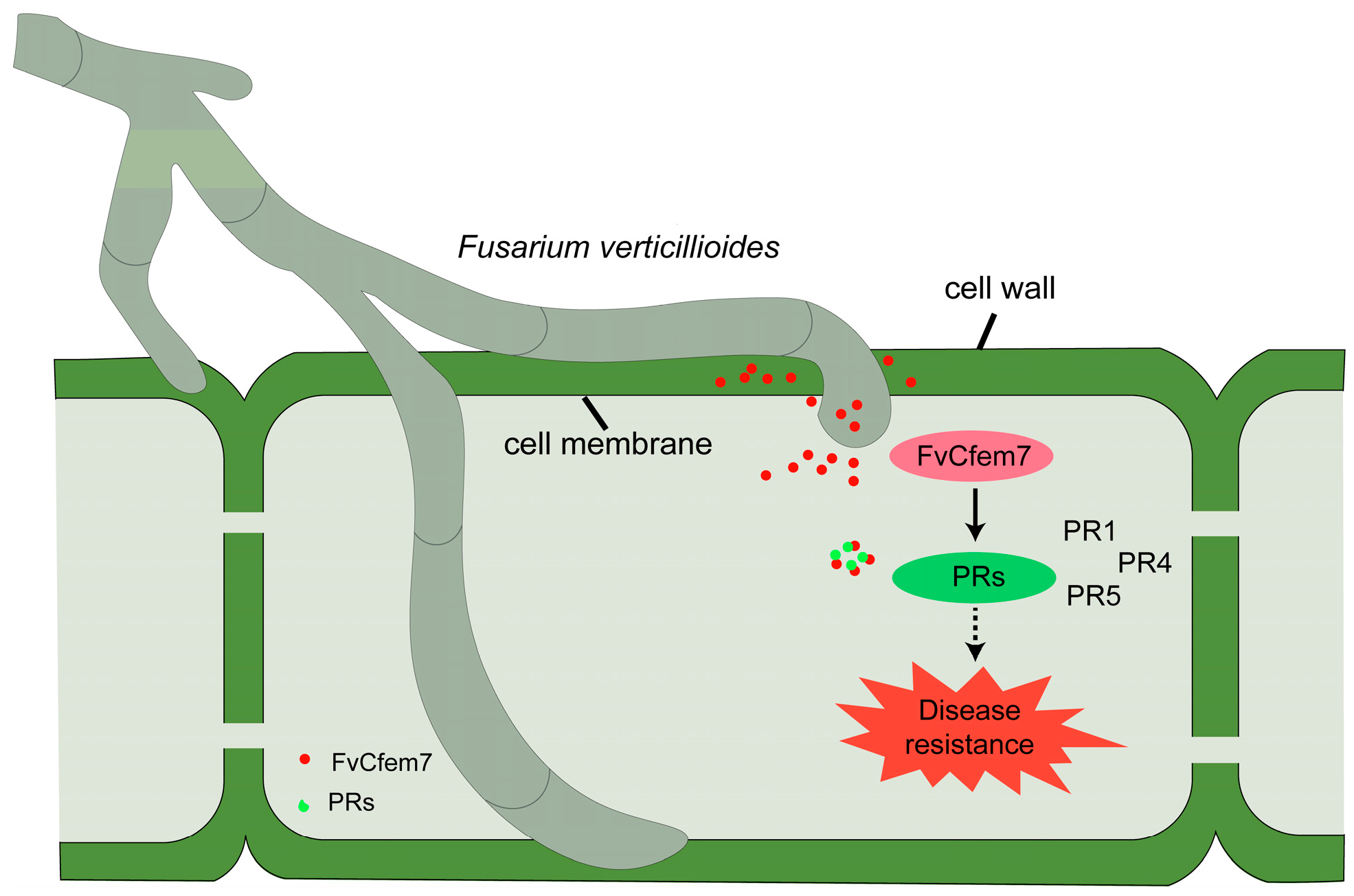
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hooven, H.W.v.D.; Burg, H.A.v.D.; Vossen, P.; Boeren, S.; de Wit, P.J.G.M.; Vervoort, J. Disulfide bond structure of the AVR9 elicitor of the fungal tomato pathogen Cladosporium fulvum: Evidence for a cystine knot. Biochemistry 2001, 40, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Burg, H.A.v.D.; Westerink, N.; Francoijs, K.-J.; Roth, R.; Woestenenk, E.; Boeren, S.; de Wit, P.J.G.M.; Joosten, M.H.A.J.; Vervoort, J. Natural disulfide bond-disrupted mutants of AVR4 of the tomato pathogen Cladosporium fulvum are sensitive to proteolysis, circumvent Cf-4-mediated resistance, but retain their chitin binding ability. J. Biol. Chem. 2003, 278, 27340–27346. [Google Scholar] [CrossRef]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O’Connell, R.J.; Narusaka, Y.; Takano, Y.; Kubo, Y.; Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef]
- Liu, L.; Xu, L.; Jia, Q.; Pan, R.; Oelmüller, R.; Zhang, W.; Wu, C. Arms race: Diverse effector proteins with conserved motifs. Plant Signal Behav. 2019, 14, 1557008. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Genome-wide analysis of small secreted cysteine-rich proteins identifies candidate effector proteins potentially involved in Fusarium graminearum-wheat interactions. Phytopathology 2016, 106, 166–176. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, I.; de Wit, P.J. Fungal effector proteins. Annu. Rev. Phytopathol. 2009, 47, 233–263. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef]
- Lowe, R.G.T.; Howlett, B.J. Indifferent, affectionate, or deceitful: Lifestyles and secretomes of fungi. PLoS Pathog. 2012, 8, e1002515. [Google Scholar] [CrossRef]
- Lahrmann, U.; Ding, Y.; Banhara, A.; Rath, M.; Hajirezaei, M.R.; Döhlemann, S.; von Wirén, N.; Parniske, M.; Zuccaro, A. Host-related metabolic cues affect colonization strategies of a root endophyte. Proc. Natl. Acad. Sci. USA 2013, 110, 13965–13970. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Kelkar, H.S.; Dean, R.A. An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem. Sci. 2003, 28, 118–121. [Google Scholar] [CrossRef]
- Zhang, Z.-N.; Wu, Q.-Y.; Zhang, G.-Z.; Zhu, Y.-Y.; Murphy, R.W.; Liu, Z.; Zou, C.-G. Systematic analyses reveal uniqueness and origin of the CFEM domain in fungi. Sci. Rep. 2015, 5, 13032. [Google Scholar] [CrossRef]
- Vaknin, Y.; Shadkchan, Y.; Levdansky, E.; Morozov, M.; Romano, J.; Osherov, N. The three Aspergillus fumigatus CFEM-domain GPI-anchored proteins (CfmA-C) affect cell-well stability but do not play a role in fungal virulence. Fungal Genet. Biol. 2014, 63, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Heard, S.; Brown, N.A.; Hammond-Kosack, K. An interspecies comparative analysis of the predicted secretomes of the necrotrophic plant pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS ONE 2015, 10, e0130534. [Google Scholar] [CrossRef]
- Ling, J.; Zeng, F.; Cao, Y.; Zhang, J.; Chen, G.; Mao, Z.; Yang, Y.; Xie, B. Identification of a class of CFEM proteins containing a new conserved motif in Fusarium oxyporum. Physiol. Mol. Plant Pathol. 2015, 89, 41–48. [Google Scholar] [CrossRef]
- Kou, Y.; Tan, Y.H.; Ramanujam, R.; Naqvi, N.I. Structure-function analyses of the Pth11 receptor reveal an important role for CFEM motif and redox regulation in rice blast. New Phytol. 2017, 214, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.; Yang, J.; Yang, X.; Zhang, M.; Zhao, Z.; Fan, Y.; Wang, C.; Wang, J. Bioinformatics and transcriptome. Analysis of CFEM proteins in Fusarium graminearum. J. Fungi 2021, 7, 871. [Google Scholar] [CrossRef]
- Li, H.; Ishfaq, S.; Liang, X.; Wang, R.; Wei, H.; Guo, W. A novel CFEM effector in Fusarium verticillioides required for virulence involved in plant immunity suppression and fungal cell wall integrity. Int. J. Mol. Sci. 2025, 26, 4369. [Google Scholar] [CrossRef]
- Moukadiri, I.; Armero, J.; Abad, A.; Sentandreu, R.; Zueco, J. Identification of a mannoprotein present in the inner layer of the cell wall of Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 2154–2162. [Google Scholar] [CrossRef]
- Kuznets, G.; Vigonsky, E.; Weissman, Z.; Lalli, D.; Gildor, T.; Kauffman, S.J.; Turano, P.; Becker, J.; Lewinson, O.; Kornitzer, D. A relay network of extracellular heme-binding proteins drives C. albicans iron acquisition from hemoglobin. PLoS Pathog. 2014, 10, e1004407. [Google Scholar] [CrossRef]
- Weissman, Z.; Kornitzer, D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol. Microbiol. 2004, 53, 1209–1220. [Google Scholar] [CrossRef]
- Nasser, L.; Weissman, Z.; Pinsky, M.; Amartely, H.; Dvir, H.; Kornitzer, D. Structural basis of haem-iron acquisition by fungal pathogens. Nat. Microbiol. 2016, 1, 16156. [Google Scholar] [CrossRef]
- Chen, C.; Pande, K.; French, S.D.; Tuch, B.B.; Noble, S.M. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 2011, 10, 118–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, C.; Magee, D.; Cox, R.A. Coccidioides immitis antigen 2: Analysis of gene and protein. Gene 1996, 181, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wei, W.; Wu, Y.; Zhou, Y.; Peng, F.; Zhang, S.; Chen, P.; Xu, X. BcCFEM1, a CFEM domain-containing protein with putative gpi-anchored site, is involved in pathogenicity, conidial production, and stress tolerance in Botrytis cinerea. Front. Microbiol. 2017, 8, 1807. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.-D.; Jing, Z.-Y.; Zhang, K.; Tan, Q.-Q.; Wang, G.-L.; Liu, W.-D. Bioinformatic analysis and functional characterization of the CFEM proteins in maize anthracnose fungus Colletotrichum graminicola. J. Integr. Agric. 2020, 19, 541–550. [Google Scholar] [CrossRef]
- Choi, W.; Dean, R.A. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 1997, 9, 1973–1983. [Google Scholar] [CrossRef]
- DeZwaan, T.M.; Carroll, A.M.; Valent, B.; Sweigard, J.A. Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell 1999, 11, 2013–2030. [Google Scholar] [CrossRef]
- Sabnam, N.; Barman, S.R. WISH, a novel CFEM GPCR is indispensable for surface sensing, asexual and pathogenic differentiation in rice blast fungus. Fungal Genet. Biol. 2017, 105, 37–51. [Google Scholar] [CrossRef]
- Zuo, N.; Bai, W.-Z.; Wei, W.-Q.; Yuan, T.-L.; Zhang, D.; Wang, Y.-Z.; Tang, W.-H. Fungal CFEM effectors negatively regulate a maize wall-associated kinase by interacting with its alternatively spliced variant to dampen resistance. Cell Rep. 2022, 41, 111877. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, D.-D.; Song, J.; Li, J.-J.; Wang, J.; Li, R.; Klosterman, S.J.; Kong, Z.-Q.; Lin, F.-Z.; Dai, X.-F.; et al. Verticillium dahliae CFEM proteins manipulate host immunity and differentially contribute to virulence. BMC Biol. 2022, 20, 55. [Google Scholar] [CrossRef]
- Punja, Z.K.; Zhang, Y.Y. Plant chitinases and their roles in resistance to fungal diseases. J. Nematol. 1993, 25, 526–540. [Google Scholar] [PubMed]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad, K. Plant chitinases. Plant J. 1993, 3, 31–40. [Google Scholar] [CrossRef]
- Qian, H.; Xiao, Z.; Cheng, L.; Geng, R.; Ma, Y.; Bi, Y.; Liang, W.; Yang, A. A novel secreted protein of Fusarium oxysporum promotes infection by inhibiting PR-5 protein in plant. Plant Cell Environ. 2025, 48, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Faris, J.D.; Sherwood, R.; Friesen, T.L.; Edwards, M.C. A dimeric PR-1-type pathogenesis-related protein interacts with ToxA and potentially mediates ToxA-induced necrosis in sensitive wheat. Mol. Plant Pathol. 2014, 15, 650–663. [Google Scholar] [CrossRef]
- Sung, Y.; Outram, M.A.; Breen, S.; Wang, C.; Dagvadorj, B.; Winterberg, B.; Kobe, B.; Williams, S.J.; Solomon, P.S. PR1-mediated defence via C-terminal peptide release is targeted by a fungal pathogen effector. New Phytol. 2021, 229, 3467–3480. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Winterberg, B.; Kobe, B.; Solomon, P.S. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 2016, 88, 13–25. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Gong, Y.; Xie, J.; Fu, Y.; Jiang, D.; Li, G.; Collinge, D.B.; Chen, W.; Cheng, J. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 2018, 217, 739–755. [Google Scholar] [CrossRef]
- Luo, X.; Tian, T.; Feng, L.; Yang, X.; Li, L.; Tan, X.; Wu, W.; Li, Z.; Treves, H.; Serneels, F.; et al. Pathogenesis-related protein 1 suppresses oomycete pathogen by targeting against AMPK kinase complex. J. Adv. Res. 2023, 43, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Dehne, H.W.; Steiner, U. Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum, and F. verticillioides. Fungal Biol. 2016, 120, 1094–1104. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, H.; Zheng, D.; Xin, X.; Zhang, X.; Wang, Q.; Wu, F.; Xu, J.; Lu, Y. Identification of Fusarium verticillioides resistance alleles in three maize populations with teosinte gene introgression. Front. Plant Sci. 2022, 13, 942397. [Google Scholar] [CrossRef]
- Bai, H.; Si, H.; Zang, J.; Pang, X.; Yu, L.; Cao, H.; Xing, J.; Zhang, K.; Dong, J. Comparative proteomic analysis of the defense response to Gibberella stalk rot in maize and reveals that ZmWRKY83 is involved in plant disease resistance. Front. Plant Sci. 2021, 12, 694973. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.H.; Langin, T.T.; Kroj, T.T.; Cockram, J.; Oliver, R.; Kema, G.; Valade, R.; Praud, S.; Laurent, V.; Duchalais, L. Wheat effector assisted breeding for resistance to fungal pathogens (WEAB). In Proceedings of the (JJC)-11emes Rencontres de Phytopathologie-Mycologie, Societe Française de Phytopathologie (SFP), Aussois, France, 1 January 2016; Volume 49. [Google Scholar]
- Zhang, H.; Kim, M.S.; Huang, J.; Yan, H.; Yang, T.; Song, L.; Yu, W.; Shim, W.B. Transcriptome analysis of maize pathogen Fusarium verticillioides revealed FvLcp1, a secreted protein with type-D fungal LysM and chitin-binding domains, that plays important roles in pathogenesis and mycotoxin production. Microbiol. Res. 2022, 265, 127195. [Google Scholar] [CrossRef] [PubMed]
- Naumann, T.A.; Wicklow, D.T.; Price, N.P.J. Identification of a chitinase-modifying protein from Fusarium verticillioides: Truncation of a host resistance protein by a fungalysin metalloprotease. J. Biol. Chem. 2011, 286, 35358–35366. [Google Scholar] [CrossRef]
- Wen, G.; Lu, X.; Liang, J.; Liu, Y.; Zhang, X.; Lu, G.; Wang, Z.; Yu, W. The Global Transcription Factor FvCon7 Plays a Role in the Morphology, FB1 Toxin Production, and Pathogenesis of Fusarium verticillioides. Plants 2025, 14, 2725. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Wu, H.; Zhang, B.; Liu, C.; Gao, Y.; Guo, H.; Zhao, J. Hyphopodium-specific signaling is required for plant Iinfection by Verticillium dahliae. J. Fungi. 2023, 9, 484. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Van der Hoorn, R.A.; Terauchi, R.; Kamoun, S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 2009, 22, 115–122. [Google Scholar] [CrossRef]
- Dos Santos, C.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Flor, H.H. The complementary systems in flax and flax rust. Adv. Genet. 1956, 8, 29–54. [Google Scholar]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Shi, T.; Yang, J.; Shi, W.; Gao, X.; Chen, D.; Xu, X.; Xu, J.-R.; Talbot, N.J.; Peng, Y.-L. N-glycosylation of effector proteins by an α-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity. Plant Cell 2014, 26, 1360–1376. [Google Scholar] [CrossRef]
- Shang, S.; Liu, G.; Zhang, S.; Liang, X.; Zhang, R.; Sun, G. A fungal CFEM-containing effector targets NPR1 regulator NIMIN2 to suppress plant immunity. Plant Biotechnol. J. 2024, 22, 82–97. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.; Wang, W.; Li, Y.; Guo, Y.; Zhang, D.; Ma, X.; Song, W.; Zhao, J.; Xu, M. A transposon-directed epigenetic change in ZmCCT underlies quantitative resistance to Gibberella stalk rot in maize. New Phytol. 2017, 215, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Okmen, B.; Doehlemann, G. Inside plant: Biotrophic strategies to modulate host immunity and metabolism. Curr. Opin. Plant Biol. 2014, 20, 19–25. [Google Scholar] [CrossRef]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-Glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Héloir, M.-C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of elicitors in grapevine: From MAMP and DAMP perception to induced resistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Abramovitch, R.B.; Martin, G.B. Strategies used by bacterial pathogens to suppress plant defenses. Curr. Opin. Plant Biol. 2004, 7, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.S.; McCormack, M.E.; Argueso, C.T.; Pajerowska-Mukhtar, K.M. Pathogen tactics to manipulate plant cell death. Curr. Biol. 2016, 26, R608–R619. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Liu, Y.; Li, X.; Lin, S.; Ke, L.; Wen, G.; Lu, G.; Wang, Z.; Yu, W. Research on the Pathogenic Mechanism of Effector FvCfem7 in Fusarium verticillioides. Agronomy 2025, 15, 2706. https://doi.org/10.3390/agronomy15122706
Wang M, Liu Y, Li X, Lin S, Ke L, Wen G, Lu G, Wang Z, Yu W. Research on the Pathogenic Mechanism of Effector FvCfem7 in Fusarium verticillioides. Agronomy. 2025; 15(12):2706. https://doi.org/10.3390/agronomy15122706
Chicago/Turabian StyleWang, Meiduo, Yi Liu, Xinyi Li, Shiqing Lin, Lifan Ke, Gaolong Wen, Guodong Lu, Zonghua Wang, and Wenying Yu. 2025. "Research on the Pathogenic Mechanism of Effector FvCfem7 in Fusarium verticillioides" Agronomy 15, no. 12: 2706. https://doi.org/10.3390/agronomy15122706
APA StyleWang, M., Liu, Y., Li, X., Lin, S., Ke, L., Wen, G., Lu, G., Wang, Z., & Yu, W. (2025). Research on the Pathogenic Mechanism of Effector FvCfem7 in Fusarium verticillioides. Agronomy, 15(12), 2706. https://doi.org/10.3390/agronomy15122706






