Assessing the Carbon Balance and Its Drivers for Banana Cultivation in Hainan Island, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sampling and Data Collection
- (1)
- Data on carbon emissions from agricultural inputs were obtained from household surveys conducted between 2018 and 2020. Twenty-two growers of MA and thirty of MB were selected, and their production practices were continuously monitored for two years, covering a total plantation area of approximately 450 ha. All selected farmers had more than two years of banana-growing experience and cultivated more than 3 ha. The questionnaire collected information on banana variety, planting area and density, yield, fertilizer and pesticide use, bagging, electricity consumption for irrigation, and diesel use for machinery.
- (2)
- Soil respiration was continuously monitored from October 2019 to December 2020, during the second and third banana cropping cycles. In the initial phase, soil texture and organic matter sampling analyses were conducted at candidate sites. Study plots with minimal soil property variations were selected. Three monitoring points were randomly assigned to each plot to ensure representative observations, with soil rings installed at each location. A 60 cm deep trench was dug around the soil ring, and a double-layer thick plastic film was placed and backfilled to isolate the plant roots around it, and the soil heterotrophic respiration was monitored. Measurements were conducted monthly using a Li-8100A soil respiration monitoring system, with continuous 48 h monitoring at 1 h intervals, and each plot was measured three times.
- (3)
- Measuring banana biomass. During the ripening and harvest stage, fifty representative plants were selected from each of the eight near-average fertilizer-applied plantations for MB and MA. Morphological parameters such as plant height, pseudostem diameter, and leaf length were recorded and averaged. One representative plant matching the average values of these parameters was selected from each plantation. The entire plant was harvested. Aboveground organs (pseudostems, leaves, fruits) were weighed separately, while underground parts were excavated, washed, and weighed. Samples from each organ were returned to the laboratory. Water content was determined by oven drying at 60 °C until constant weight. Dried samples were ground into powder and sieved (0.25 mm). Carbon content was determined via potassium dichromate–sulfuric acid oxidation. The amount of carbon sequestered in the dry biomass of each plant was quantified and extrapolated to the plantation scale based on planting density.
- (4)
- Measuring soil carbon sequestration. Soil organic carbon (SOC) dynamics were assessed over a two-year period. Specifically, in September 2018 and October 2020, soil samples were collected from three distinct 15 m × 15 m plots within each banana plantation. Using a 50 mm diameter auger, five individual samples were taken from the 0–30 cm depth profile (0–10 cm, 10–20 cm, and 20–30 cm layers) within each plot. Samples from the same layer were combined to form a composite sample. In total, 18 composite samples were obtained (2 plantations × 3 plots × 3 soil layers). These samples were air-dried, passed through a 2 mm sieve, and then ground to pass through a 100-mesh sieve for SOC analysis. SOC content was measured using the potassium dichromate oxidation–spectrophotometric method, and bulk density was determined using the ring-knife method [28]. The annual soil carbon sequestration rate in the banana plantation was calculated based on the SOC content measured in September 2018 and October 2020.
2.4. Calculations of Indices
2.4.1. System Boundaries
2.4.2. Carbon Emissions and Footprint
2.4.3. Carbon Fixation and Measurement Methods
2.5. Statistical Analysis
3. Results and Analysis
3.1. Carbon Emission and Carbon Footprint of Banana Plantations
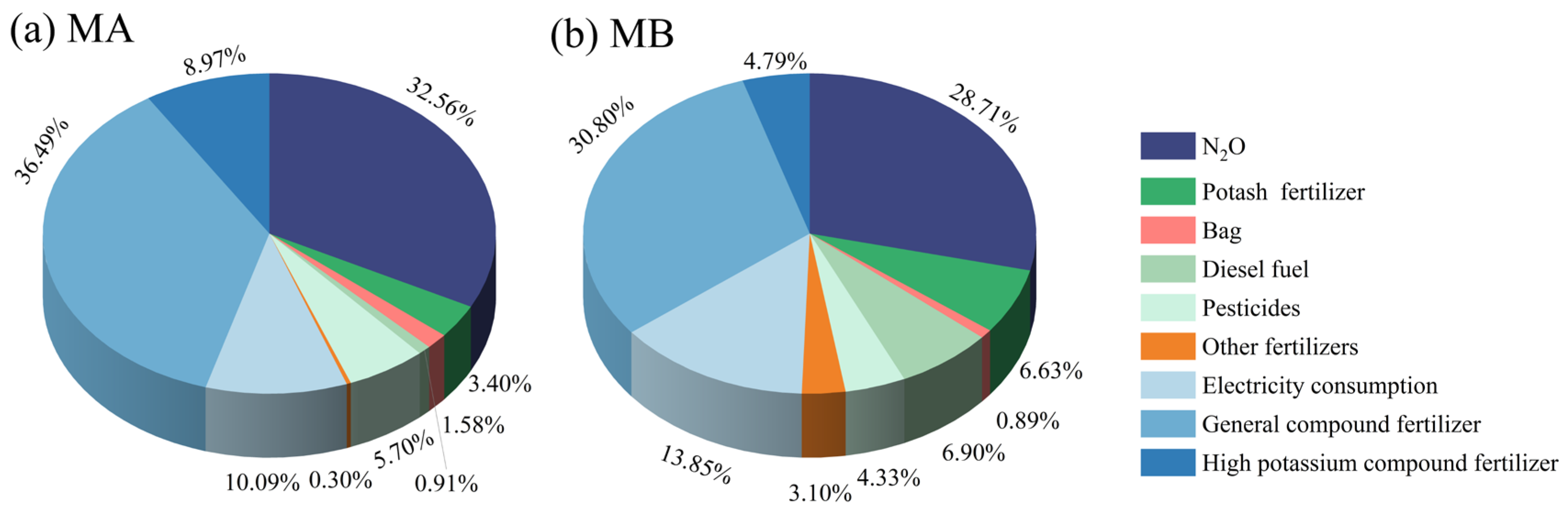
3.1.1. Carbon Emissions of Agricultural Material
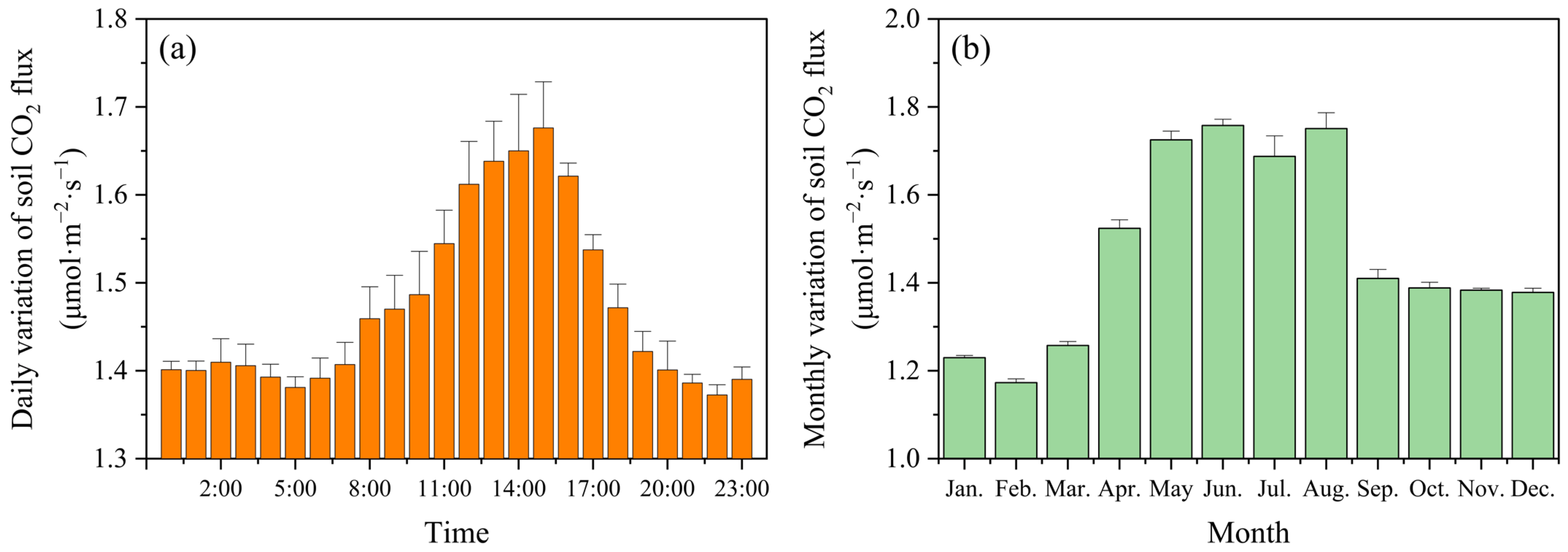
3.1.2. Carbon Emissions of Soil Respiration
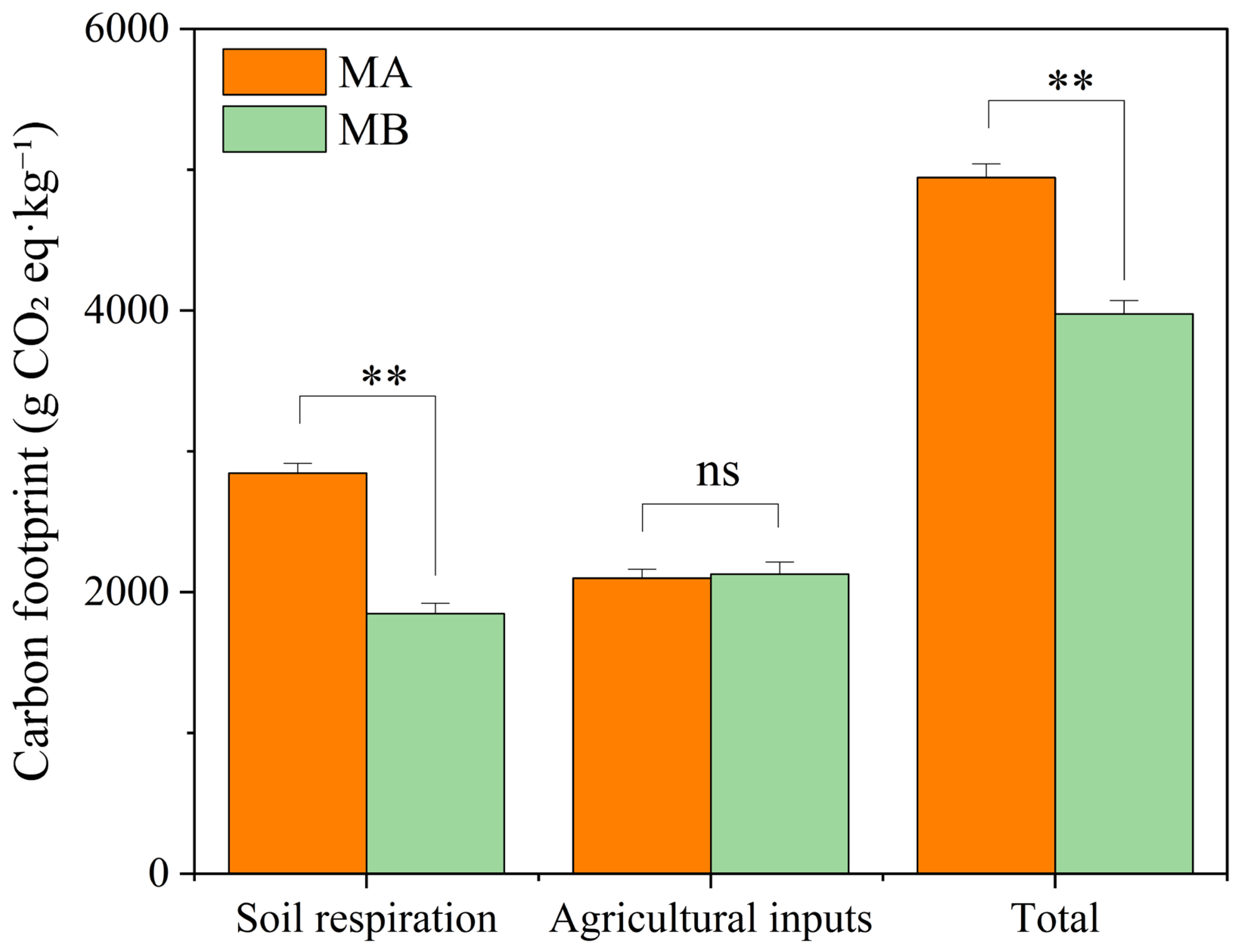
3.1.3. Carbon Footprint of Banana Plantations
3.2. Carbon Fixation of Banana Plantation Ecosystem
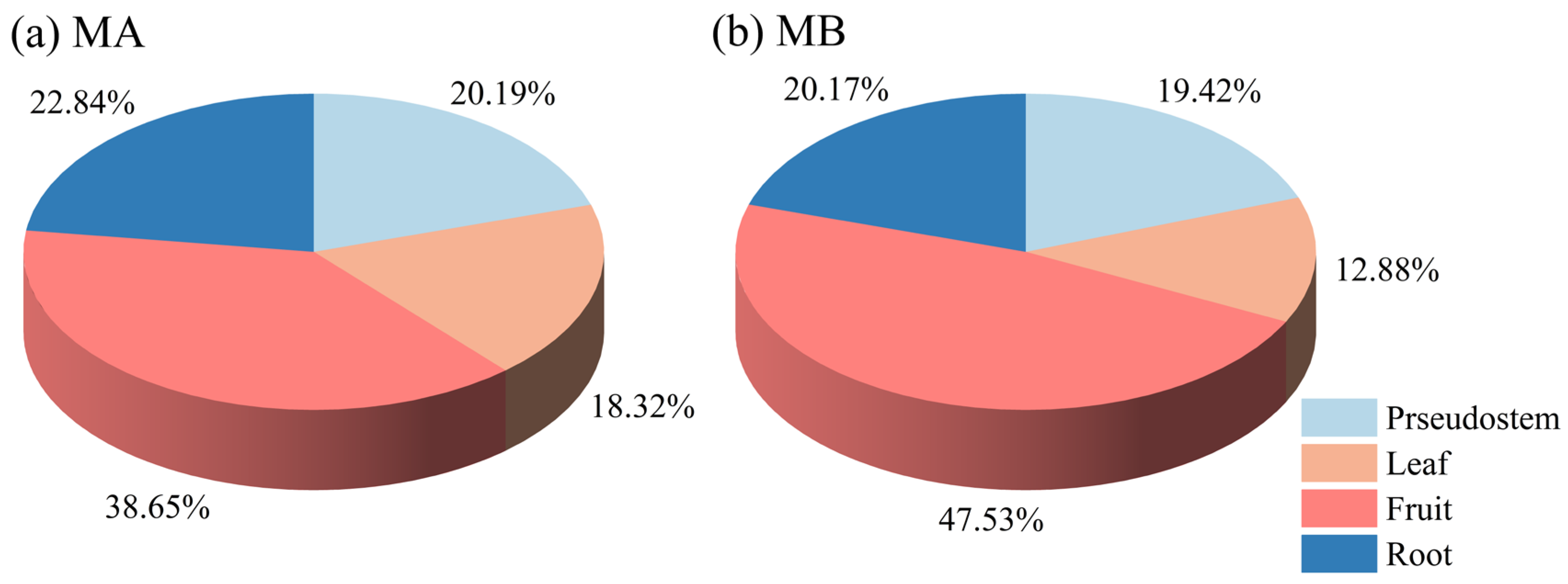
3.2.1. Plant Carbon Fixation of Banana Plantations
3.2.2. Soil Carbon Sequestration of Banana Plantations
3.3. Carbon Balance of Banana Plantations
3.4. Sensitivity and Uncertainty Analysis
4. Discussion
4.1. Sources and Drivers of Agricultural Carbon Emissions in Banana Cultivation Systems
| Species | Study Area | Carbon Emissions Per Unit Area kg CO2 eq·ha−1 | Carbon Emissions Per Unit of Production kg CO2 eq·kg−1 | Source |
|---|---|---|---|---|
| Myrica rubra fruit | Huaihua, Hunan, China | - | 0.19 | [41] |
| Banana | Ecuadorian | - | 1.94 | [42] |
| Wheat-corn rotation | Gaomi, Shandong, China | 8960 | 0.53 | [43] |
| maize | Xinjiang, China | 7823 | 0.35 | [46] |
| wheat, | Xinjiang, China | 6475 | 0.41 | [46] |
| cotton | Xinjiang, China | 10,577 | 1.91 | [46] |
4.2. Factors Affecting Carbon Fixation in Banana Plantation Ecosystems
4.3. Scientific Management and Development of Agricultural Land
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.C. Researcher Zhou Hongchun from the Development Research Center of the Carbon Peaking and Carbon Neutrality Goals Leading the Comprehensive Green Transformation in China’s Economy and Society: An Interview withe State Council. J. Poyang Lake 2022, 1, 5–14+125. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups i, ii and iii to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Beillouin, D.; Demenois, J.; Cardinael, R.; Berre, D.; Corbeels, M.; Fallot, A.; Boyer, A.; Feder, F. A global database of land management, land-use change and climate change effects on soil organic carbon. Sci. Data 2022, 9, 228. [Google Scholar] [CrossRef]
- Mandal, A.; Majumder, A.; Dhaliwal, S.S.; Toor, A.S.; Mani, P.K.; Naresh, R.K.; Gupta, R.K.; Mitran, T. Impact of agricultural management practices on soil carbon sequestration and its monitoring through simulation models and remote sensing techniques: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1–49. [Google Scholar] [CrossRef]
- Sha, Z.; Bai, Y.; Li, R.; Lan, H.; Zhang, X.; Li, J.; Liu, X.; Chang, S.; Xie, Y. The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management. Commun. Earth Environ. 2022, 3, 8. [Google Scholar] [CrossRef]
- Kamyab, H.; SaberiKamarposhti, M.; Hashim, H.; Yusuf, M. Carbon dynamics in agricultural greenhouse gas emissions and removals: A comprehensive review. Carbon Lett. 2024, 34, 265–289. [Google Scholar] [CrossRef]
- Madrid-Solórzano, J.M.; García-Alcaraz, J.L.; Martínez Cámara, E.; Blanco Fernández, J.; Jiménez Macías, E. Sustainable Industrial Sotol Production in Mexico—A Life Cycle Assessment. Agriculture 2022, 12, 2159. [Google Scholar] [CrossRef]
- Ozlu, E.; Arriaga, F.J.; Bilen, S.; Gozukara, G.; Babur, E. Carbon Footprint Management by Agricultural Practices. Biology 2022, 11, 1453. [Google Scholar] [CrossRef]
- Wang, J.; Chang, G.; Liu, H.; Zhang, Y.; Li, H.; Hu, K. Carbon balance analysis of agricultural production systems in oasis areas. Sci. Rep. 2024, 14, 16698. [Google Scholar] [CrossRef]
- Suwan, C.; Somjai, T. Carbon Footprint Analysis of the Cultivated Banana Cultivation in Prachinburi Province, Thailand. E3S Web Conf. 2022, 355, 02002. [Google Scholar] [CrossRef]
- Luo, D.; Xu, G.; Luo, J.; Cui, X.; Shang, S.; Qian, H. Integrated Carbon Footprint and Economic Performance of Five Types of Dominant Cropping Systems in China’s Semiarid Zone. Sustainability 2022, 14, 5844. [Google Scholar] [CrossRef]
- Bartzas, G.; Doula, M.; Komnitsas, K. Life Cycle Assessment of Key Mediterranean Agricultural Products at the Farm Level Using GHG Measurements. Agriculture 2025, 15, 1494. [Google Scholar] [CrossRef]
- Sun, T.; Feng, X.; Lal, R.; Cao, T.; Guo, J.; Deng, A.; Zheng, C.; Zhang, J.; Song, Z.; Zhang, W. Crop diversification practice faces a tradeoff between increasing productivity and reducing carbon footprints. Agric. Ecosyst. Environ. 2021, 321, 107614. [Google Scholar] [CrossRef]
- Mehta, H.; Kumar, P.; Banyal, V.; Sharma, N.; Kumar, N. Driving Sustainability in Fruit-Based Cropping Systems: Intercropping Impacts on Growth, Soil Health, Microbial Dynamics and Yield Stability. Physiol. Mol. Plant Pathol. 2025, 139, 102774. [Google Scholar] [CrossRef]
- Yang, C.; Wang, A.; Zhu, Z.; Lin, S.; Bi, Y.; Du, X. Impact of Extensive Management System on Soil Properties and Carbon Sequestration under an Age Chronosequence of Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 497, 119535. [Google Scholar] [CrossRef]
- López-Blanco, E.; Väisänen, M.; Salmon, E.; Jones, C.P.; Schmidt, N.M.; Marttila, H.; Lohila, A.; Juutinen, S.; Scheller, J.; Christensen, T.R. The Net Ecosystem Carbon Balance (NECB) at Catchment Scales in the Arctic. Front. Environ. Sci. 2025, 13, 1544586. [Google Scholar] [CrossRef]
- Abdelkader, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R. Life Cycle Assessment of the Cultivation Processes for the Main Vegetable Crops in Southern Egypt. Agronomy 2022, 12, 1527. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating Root and Soil Microbial Contributions to Soil Respiration: A Review of Methods and Observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Shi, Z.; Vargas, R.; Todd-Brown, K. Twenty years of progress, challenges, and opportunities in soil respiration research. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007637. [Google Scholar] [CrossRef]
- Jansson, C.; Faiola, C.; Wingler, A.; Zhu, X.-G.; Kravchenko, A.; De Graaff, M.-A.; Ogden, A.J.; Handakumbura, P.P.; Werner, C.; Beckles, D.M. Crops for Carbon Farming. Front. Plant Sci. 2021, 12, 636709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Z.; McCarl, B.A.; Fei, C. Enhancing Agricultural Soil Carbon Sequestration: A Review with Some Research Needs. Climate 2024, 12, 151. [Google Scholar] [CrossRef]
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; De Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; Van Den Hoogen, J.; Araza, A.; Herold, M. Integrated Global Assessment of the Natural Forest Carbon Potential. Nature 2023, 624, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Li, Y.; Wang, X.; He, X.; Xu, M.; Cai, A. Carbon Gain in Upper but Loss in Deeper Cropland Soils across China over the Last Four Decades. Proc. Natl. Acad. Sci. USA 2025, 122, e2422371122. [Google Scholar] [CrossRef]
- Gelaye, Y.; Getahun, S. A review of the carbon sequestration potential of fruit trees and their implications for climate change mitigation: The case of Ethiopia. Cogent Food Agric. 2024, 10, 2294544. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, X.; Yang, J.; Yang, Y.; Jv, H.; Li, R.; Jia, Z.; Ruan, Y. Selection of rhizosphere communities of diverse rotation crops reveals unique core microbiome associated with reduced banana Fusarium wilt disease. New Phytol. 2023, 238, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Suppen-Reynaga, N.; Guerrero, A.; Dominguez, E.; Sacayón, E.; Solano, A. Life cycle assessment of bananas, melons, and watermelons from Costa Rica. Clean. Circ. Bioecon. 2024, 9, 100120. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Zhang, C.; Ju, X. Nitrogen and Phosphorus Surplus and Soil Nitrate Nitrogen Accumulation in Typical Rice-Vegetable Rotation and Banana Garden in Hainan. Sci. Agric. Sin. 2023, 56, 2954–2965. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 265–267. [Google Scholar]
- Wróbel-Jędrzejewska, M.; Włodarczyk, E. Comparison of Carbon Footprint Analysis Methods in Grain Processing—Studies Using Flour Production as an Example. Agriculture 2023, 14, 14. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, M. Temporospatial Variation of Partial Carbon Source/Sink of Farmland Ecosystem in Coastal China. J. Ecol. Rural Environ. 2007, 23, 1–6. [Google Scholar]
- Chen, S.; Lu, F.; Wang, X.K. Estimation of greenhouse gases emission factors for China’s nitrogen, phosphate, and potash fertilizers. Acta Ecol. Sin. 2015, 35, 6371–6383. [Google Scholar]
- Zhang, G.; Lu, F.; Huang, Z.G.; Chen, S.; Wang, X. Estimations of application dosage and greenhouse gas emission of chemical pesticides in staple crops in China. Chin. J. Appl. Ecol. 2016, 27, 2875–2883. [Google Scholar]
- Ministry Policy Paper. China Low Carbon Yearbook—2013 China Regional Grid Baseline Emission Factors, 1st ed.; Metallurgical Industry Press: Beijing, China, 2014; pp. 189–191. [Google Scholar]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Zhou, T.; Gao, M.; Xie, D.; Wei, C. Research on Carbon Source/Sink and Carbon Footprint of Cropland Ecosystem in Chongqing. J. Southwest Agric. Univ. 2014, 36, 96–102. [Google Scholar]
- Chen, C.; Qiu, R. Estimation of Energy Consumption and Carbon Emissions of China’s Pulp & Paper Industry. Chin. Pulp Paper 2014, 32, 50–55. [Google Scholar]
- Department of Climate Change. National Development and Reform Commission. In Guidelines for the Preparation of Provincial Greenhouse Gas Inventories (for Trial Implementation); State Council Executive Meeting: Beijing, China, 2011. [Google Scholar]
- Izett, R.W.; Fennel, K.; Stoer, A.C.; Nicholson, D.P. Reviews and Syntheses: Expanding the Global Coverage of Gross Primary Production and Net Community Production Measurements Using Biogeochemical-Argo Floats. Biogeosciences 2024, 21, 13–47. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.K.; Lu, F.; Duan, X.N.; OuYang, Z.Y. Soil carbon sequestration and its potential by cropland ecosystems in China. Acta Ecol. Sin. 2008, 28, 612–619. [Google Scholar]
- Jiang, R.; Xu, Q.; Li, J.Y.; Dai, L.X.; Ao, D.C.; Dou, Z.; Gao, H. Sensitivity and Uncertainty Analysis of Carbon Footprint Evaluation: A Case Study of Rice–Crayfish Coculture in China. Chin. J. Eco-Agric. 2022, 30, 1577–1587. [Google Scholar]
- He, J.; Xiong, Z.; Deng, L.; Liao, Q.; Liu, D.; Dai, Q. Carbon Footprint Assessment of Fresh Myrica Rubra Fruit Based on LCA. Chin. J. Eco-Agric. 2025, 33, 413–423. [Google Scholar]
- Veliz, K.; Chico-Santamarta, L.; Ramirez, A.D. The Environmental Profile of Ecuadorian Export Banana: A Life Cycle Assessment. Foods 2022, 11, 3288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Jiang, D.; Zou, X. Life Cycle Assessment on Carbon Footprint of Winter Wheat -Summer Maize Cropping System Based on Survey Data of Gaomi in Shandong Province, China. J. Agric. Resour. Environ. 2017, 34, 473–482. [Google Scholar]
- Wang, S.; Zhou, S. The dynamic relation between fertilization, precipitation and the diffusion of agricultural non-point pollution in Hainan Island. Front. Environ. Sci. 2024, 12, 1419912. [Google Scholar] [CrossRef]
- da Silva, R.B.; Antunes, T.; Rosa, J.S.; Packer, A.P.; Bento, C.B.; do Carmo, J.B.; de Melo Silva, F.A. CO2, CH4 and N2O Emissions after Fertilizer Application in Banana Plantations Located in the Brazilian Atlantic Forest. Soil Use Manag. 2022, 38, 1597–1613. [Google Scholar] [CrossRef]
- Yang, L.; Yue, K.; Zhang, L. Carbon Footprint of Major Crop Production under the Goal of’ double Carbon’ in Xinjiang, China. Chin. J. Appl. Ecol. 2025, 36, 1147–1158. [Google Scholar]
- Meng, X.; Meng, F.; Chen, P.; Hou, D.; Zheng, E.; Xu, T. A meta-analysis of conservation tillage management effects on soil organic carbon sequestration and soil greenhouse gas flux. Sci. Total Environ. 2024, 954, 176315. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, Z.; He, H.; Lin, B.; He, C.; Dang, Y.; Zhao, X.; Zhang, H. Improving soil organic carbon sequestration through conservation tillage incorporating legume-based crop rotations. Soil Till. Res. 2026, 255, 106792. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Qiu, G.; Yu, H.; Liu, F.; Wang, G.; Duan, Y. Conservation tillage facilitates the accumulation of soil organic carbon fractions by affecting the microbial community in an eolian sandy soil. Front. Microbiol. 2024, 15, 1394179. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, L.; Zhang, J.W.; Li, W.; Jiang, Y.; Wang, S.; Ding, Y.; Liu, Z.; Li, G. Low N Apparent Surplus with Higher Rice Yield under Long-Term Fertilizer Postponing in the Rice-Wheat Cropping System. Crop J. 2022, 10, 1178–1186. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, X.; Zhang, X.; Shi, H.; Chen, J.; Xu, R.; Chen, Y.; Ying, J.; Wu, Y.; Zhou, Y. Effects of Fertilizer Application Intensity on Carbon Accumulation and Greenhouse Gas Emissions in Moso Bamboo Forest–Polygonatum Cyrtonema Hua Agroforestry Systems. Plants 2024, 13, 1941. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, S.; Liu, H.; Zhao, Z.; Wei, S.; Sun, S. Effects of Nitrogen Reduction Combined with Organic Fertilizer on Growth and Nitrogen Fate in Banana at Seedling Stage. Environ. Res. 2022, 214, 113826. [Google Scholar] [CrossRef]
- Leno, N.; Sudharmaidevi, C.R. Physicochemical and Nutrient Release Characteristics of a Thermochemical Organic Fertilizer Produced from Degradable Solid Waste and Its Effect on Productivity of Banana. Commun. Soil Sci. Plant Anal. 2021, 52, 2562–2577. [Google Scholar] [CrossRef]
- Meya, A.I.; Swennen, R.; Ndakidemi, P.A.; Mtei, K.M.; Merckx, R. Better nitrogen fertilizer management improved Mchare banana productivity and profitability in northern highlands, Tanzania. Agronomy 2023, 13, 1418. [Google Scholar] [CrossRef]
- Behera, S.S.; Ojha, C.S.P.; Prasad, K.H.; Dash, S.S. Yield, water, and carbon footprint of rainfed rice production under the lens of mid-century climate change: A case study in the eastern coastal agro-climatic zone, Odisha, India. Environ. Monit. Assess. 2023, 195, 544. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, H.; Wang, C.; Li, R.; Zhao, Z.; Guo, B.; Yao, L.; Gao, X. The Carbon Footprint and Influencing Factors of the Main Grain Crops in the North China Plain. Agronomy 2024, 14, 1720. [Google Scholar] [CrossRef]
- Cammarata, M.; Tadiello, T.; Scuderi, A.; Millar, N.; Basso, B. Regenerative practices can lead to carbon-negative orange groves in Sicily. J. Agric. Food Res. 2024, 19, 101615. [Google Scholar] [CrossRef]
- Ye, W.; Wang, C.; Zhao, C.; Zheng, X. Spatial and temporal patterns of the carbon footprint of tropical farmland ecosystems on Hainan Island in the last 20 years. Chin. J. Agric. Resour. Reg. Plan. 2021, 42, 114–126. [Google Scholar]
- Madane, D.A.; Singh, M.C.; Sharma, P.; Mane, M. Water and carbon footprint assessment of onion crop cultivated under differential irrigation scenarios. Arab. J. Geosci. 2023, 16, 419. [Google Scholar] [CrossRef]
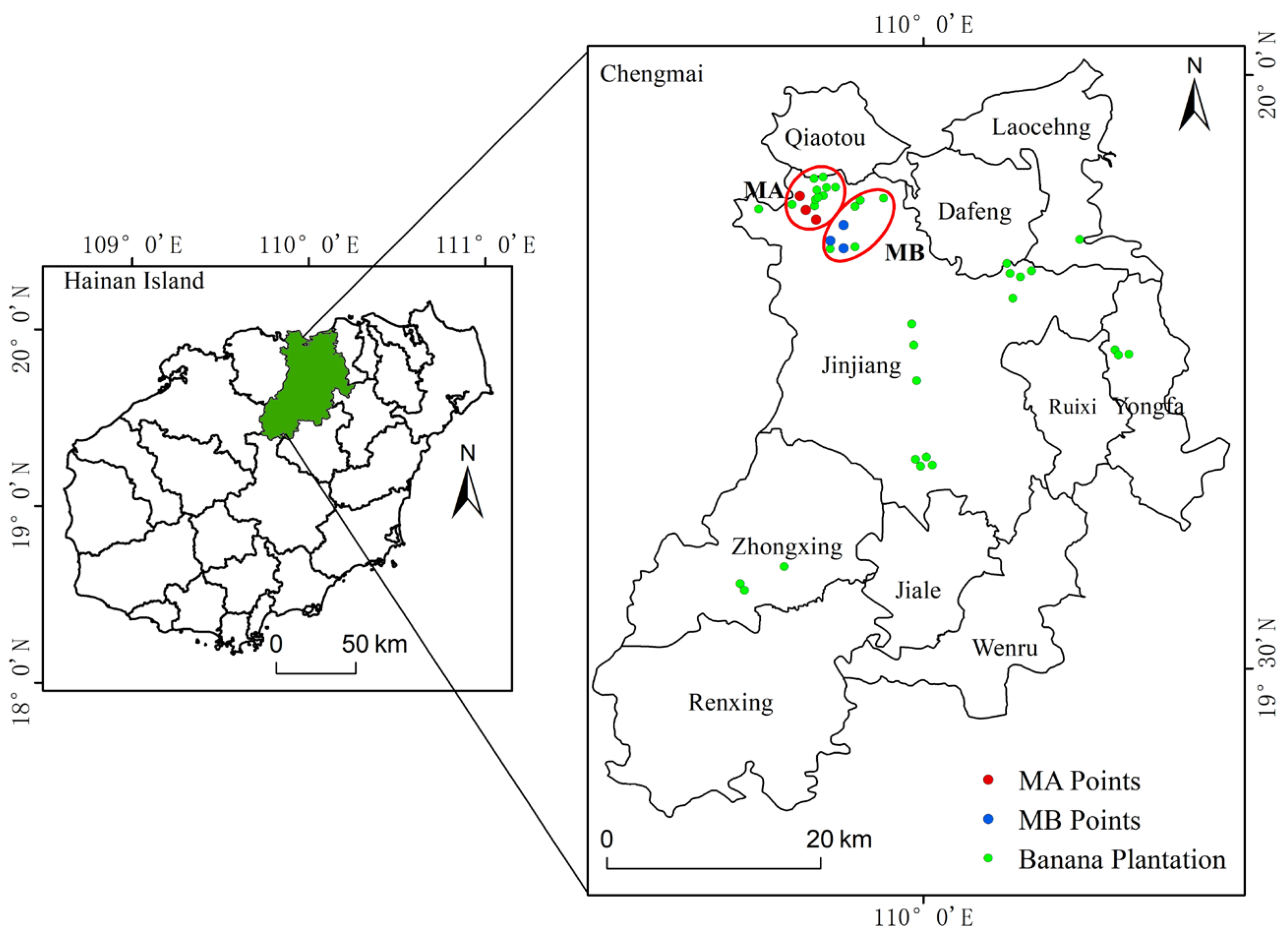
| Parameter | Musa paradisiaca AA (MA) | M. AAA Cavendish var. Brazil (MB) |
|---|---|---|
| Planting density (plants/ha) | ~3000 | ~2700 |
| Spacing (plant × row) | 1.2–1.5 m × 2.0–2.5 m | 1.5–2.0 m × 2.0–2.5 m |
| Pseudostem height | 2.5–3.2 m | 2.5–3.2 m |
| Basal circumference (at 20 cm) | 40–50 cm | 50–65 cm |
| Yield/plant | 5–10 kg | 18–35 kg |
| Basal fertilizer | Organic manure + compound fertilizer | Organic manure + compound fertilizer |
| Topdressing (seedling stage) | 1–2 times/month | 1–2 times/month |
| Topdressing (growth stage) | 2–3 times/month | 3–4 times/month |
| Irrigation | Drip irrigation | Drip irrigation |
| Carbon Source | Emission Factors | Data Sources |
|---|---|---|
| Urea | 2.041 kg CE/kg | [31] |
| Calcium superphosphate | 0.195 kg CE/kg | [31] |
| Potassium chloride | 0.168 kg CE/kg | [31] |
| Potassium sulfate | 0.409 kg CE/kg | [31] |
| Ternary compound fertilizer | 0.939 kg CE/kg | [31] |
| High potassium low phosphorus compound fertilizer | 0.836 kg CE/kg | [31] |
| Bactericide | 12.78 kg CE/kg | [32] |
| Insecticide | 10.0 kg CE/kg | [32] |
| Herbicide | 7.91 kg CE/kg | [32] |
| Preservative | 12.78 kg CE/kg | [32] |
| Agricultural Electricity | 0.917 kg CO2 eq/kW·h | [33] |
| Diesel fuel | 3.933 kg CO2 eq/kg | [34] |
| Agricultural film | 5.18 kg CO2 eq/kg | [35] |
| Paper bags | 1.5 kg CO2 eq/kg | [36] |
| Item | Specie | Pseudostem | Leaf | Fruit | Root | Whole Plant |
|---|---|---|---|---|---|---|
| Water Content (Fresh Weight) (%) | MA | 94.6 a | 86.0 b | 77.9 b | 89.2 a | 88.96 a |
| MB | 94.9 a | 88.1 a | 81.4 a | 89.9 a | 89.64 a | |
| Carbon Content (Dry Weight) (%) | MA | 38.1 a | 41.0 a | 40.9 a | 36.4 a | 39.23 a |
| MB | 36.4 b | 40.6 a | 40.4 a | 36.2 a | 38.66 a |
| Time | Site | Depth (cm) | SOC Content (g·kg−1) | SOC Storage (t C·ha−1) |
|---|---|---|---|---|
| September 2018 | Inter-plant | 0–10 | 14.30 ± 0.29 Aa | 16.87 ± 0.51 Bb |
| 10–20 | 12.70 ± 0.38 Ba | 14.99 ± 0.60 Ba | ||
| 20–30 | 10.50 ± 0.21 Ba | 12.39 ± 0.25 Bb | ||
| Inter-row | 0–10 | 14.70 ± 0.59 Ba | 18.23 ± 0.91 Ba | |
| 10–20 | 12.20 ± 0.24 Ba | 15.13 ± 0.30 Ba | ||
| 20–30 | 11.00 ± 0.44 Ba | 13.64 ± 0.27 Ba | ||
| October 2020 | Inter-plant | 0–10 | 14.80 ± 0.30 Ab | 17.46 ± 0.35 Ab |
| 10–20 | 14.00 ± 0.28 Ab | 16.52 ± 0.50 Ab | ||
| 20–30 | 13.30 ± 0.27 Aa | 15.69 ± 0.31 Ab | ||
| Inter-row | 0–10 | 16.80 ± 0.34 Aa | 20.16 ± 0.40 Aa | |
| 10–20 | 16.20 ± 0.32 Aa | 19.44 ± 0.39 Aa | ||
| 20–30 | 13.90 ± 0.56 Aa | 16.68 ± 1.00 Aa | ||
| ANOVA results (p values) | ||||
| Soil depth (D) | <0.001 ** | <0.001 ** | ||
| Sampling Point (P) | <0.001 ** | <0.001 ** | ||
| Sampling Time (T) | <0.001 ** | <0.001 ** | ||
| D × P | 0.119 ns | 0.139 ns | ||
| D × T | <0.001 ** | <0.001 ** | ||
| P × T | <0.001 ** | 0.001 ** | ||
| D × P × T | 0.001 ** | 0.008 ** | ||
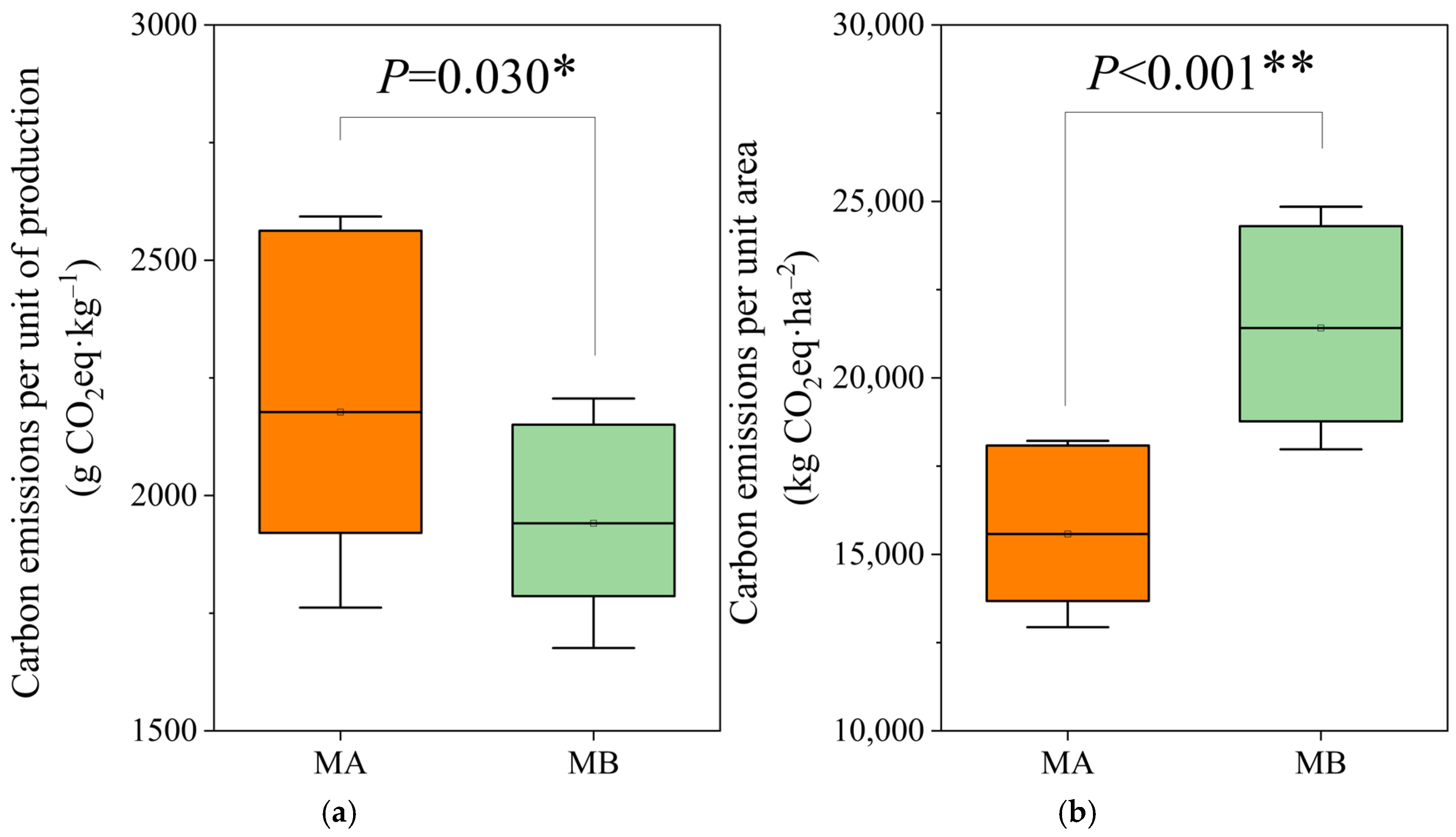
| Parameter | MA (Musa paradisiaca AA) | MB (M. AAA Cavendish var. Brazil) |
|---|---|---|
| Carbon emissions per unit of production | 2177.27 ± 415.63 g CO2 eq·kg−1 | 1940.98 ± 265.06 g CO2 eq·kg−1 |
| Carbon emissions per unit area | 15,575.66 ± 2640.11 kg CO2 eq ha−1 | 21,411.60 ± 3437.15 kg CO2 eq ha−1 |
| Carbon footprint | 4944.511 g CO2 eq·kg−1 | 3975.871 g CO2 eq·kg−1 |
| Carbon Fixation | 37,092.82 kg CO2 eq | 30,451,982.50 kg CO2 eq |
| Net Carbon Fixation per Area | 15,197.96 kg CO2 eq·ha−1 | 21,652.88 kg CO2 eq·ha−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Kuang, C.; Ye, W.; Mei, M.; Zhao, C. Assessing the Carbon Balance and Its Drivers for Banana Cultivation in Hainan Island, China. Agronomy 2025, 15, 2676. https://doi.org/10.3390/agronomy15122676
Shi X, Kuang C, Ye W, Mei M, Zhao C. Assessing the Carbon Balance and Its Drivers for Banana Cultivation in Hainan Island, China. Agronomy. 2025; 15(12):2676. https://doi.org/10.3390/agronomy15122676
Chicago/Turabian StyleShi, Xuesong, Changgeng Kuang, Wenwei Ye, Minhua Mei, and Congju Zhao. 2025. "Assessing the Carbon Balance and Its Drivers for Banana Cultivation in Hainan Island, China" Agronomy 15, no. 12: 2676. https://doi.org/10.3390/agronomy15122676
APA StyleShi, X., Kuang, C., Ye, W., Mei, M., & Zhao, C. (2025). Assessing the Carbon Balance and Its Drivers for Banana Cultivation in Hainan Island, China. Agronomy, 15(12), 2676. https://doi.org/10.3390/agronomy15122676





