Improved Estimation and Graphical Representation of the Reliability Measures of the SNP Marker Method for Crop Variety Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Sources
2.2. Statistical Analysis Method
2.2.1. Formulas for Calculating SNP Locus Similarity
2.2.2. Formulas for Detection Precision and Uncertainty Statistics
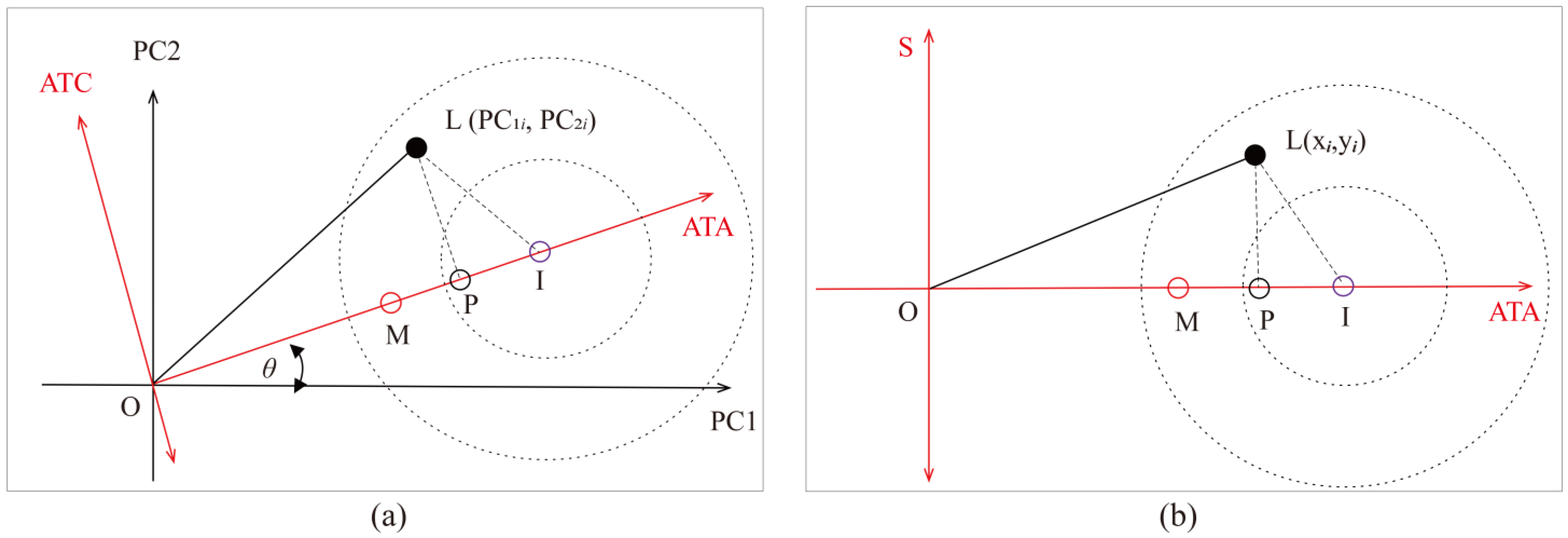
2.2.3. Proposed LLG Biplot Method for Graphical Analysis of Detection Trueness, Precision and Accuracy
3. Results
3.1. Variance Analysis of the Trueness of SNP Molecular Marker Detection for Five Major Crop Varieties
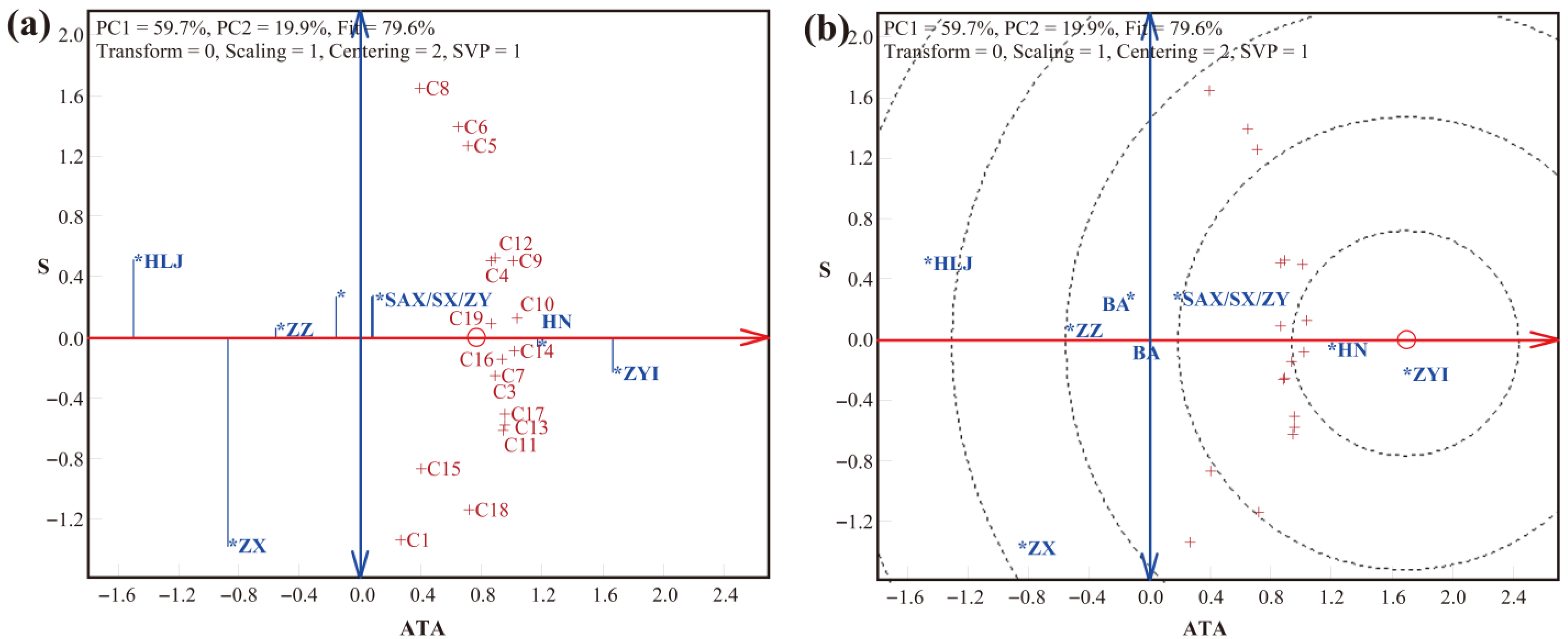
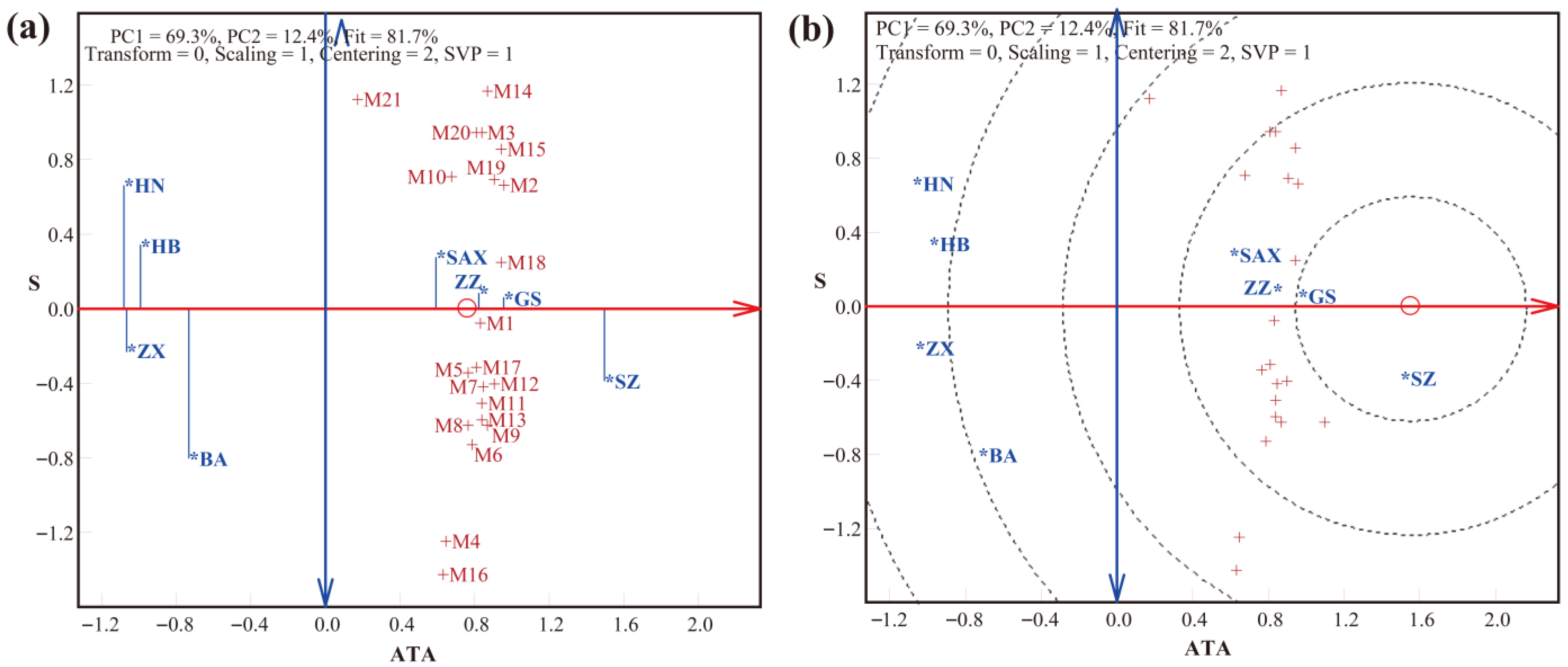
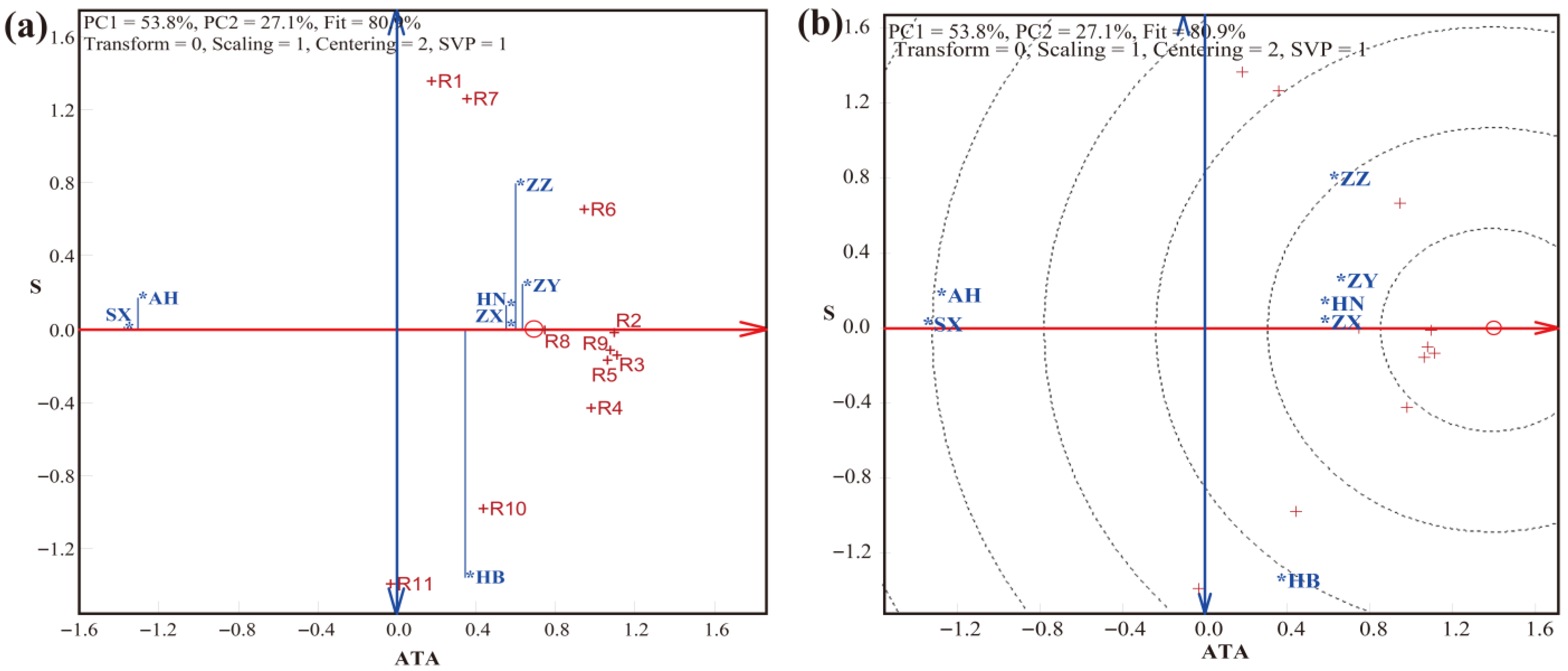
3.2. LLG Biplot Analysis of Trueness, Precision, and Accuracy in Detection by the SNP Method
3.3. Analysis of Detection Accuracy and Uncertainty of the SNP Detection Method Based on Single-Genotype Analysis
3.4. Analysis of Detection Accuracy and Uncertainty of the SNP Detection Method Based on Single-Sample Analysis
3.5. Analysis of Detection Precision and Uncertainty of the SNP Detection Method Based on Multi-Genotype Combined Analysis of Variance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Yuan, D.; Lu, H.; Jian, Y.; Li, X.; Huang, A.; Luo, Z.; Lu, Q.; Tan, Y.; Zhang, Y.; et al. The results of rice germplasm EDV test by genomic analysis and related discussions. Sci. Sin. Vitae 2020, 50, 633–649. [Google Scholar] [CrossRef]
- Wei, Z.; Li, H.; Li, J.; Yasir, A.G.; Ma, Y.; Qiu, L. Accurate identification of varieties by nucleotide polymorphisms and establishment of scannable variety IDs for soybean germplasm. Acta Agron. Sin. 2018, 44, 315–323. [Google Scholar] [CrossRef]
- Jamali, S.H.; Cockram, J.; Hickey, L.T. Insights into deployment of DNA markers in plant variety protection and registration. Theor. Appl. Genet. 2019, 132, 1911–1929. [Google Scholar] [CrossRef]
- UPOV (International Union for the Protection of New Varieties of Plants). Guidelines for DNA-Profiling: Molecular Marker Selection and Database Construction; UPOV/INF/17/2; UPOV (International Union for the Protection of New Varieties of Plants): Geneva, Switzerland, 2010. [Google Scholar]
- ISTA (International Seed Testing Association). Method Validation Reports on Rules Proposals for the International Rules for Seed Testing 2017 Edition; ISTA OM16-06; ISTA (International Seed Testing Association): Geneva, Switzerland, 2017. [Google Scholar]
- Van Inghelandt, D.; Melchinger, A.E.; Lebreton, C.; Stich, B. Population structure and genetic diversity in a commercial maize breeding program assessed with SSR and SNP markers. Theor. Appl. Genet. 2010, 120, 1289–1299. [Google Scholar] [CrossRef]
- Röder, M.S.; Wendehake, K.; Korzun, V.; Bredemeijer, G.; Laborie, D.; Bertrand, L.; Isaac, P.; Rendell, S.; Jackson, J.; Cooke, R.J.; et al. Construction and analysis of a microsatellite-based database of european wheat varieties. Theor. Appl. Genet. 2002, 106, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bredemeijer, G.; Cooke, R.J.; Ganal, M.W.; Peeters, R.; Isaac, P.; Noordijk, Y.; Rendell, S.; Jackson, J.; Röder, M.S.; Wendehake, K.; et al. Construction and testing of a microsatellite database containing more than 500 tomato varieties. Theor. Appl. Genet. 2002, 105, 1019–1026. [Google Scholar] [CrossRef]
- Reid, A.; Hof, L.; Felix, G.; Rucker, B.; Tams, S.; Milczynska, E.; Esselink, D.; Uenk, G.; Vosman, B.; Weitz, A. Construction of an integrated microsatellite and key morphological characteristic database of potato varieties on the EU common catalogue. Euphytica 2011, 182, 239–249. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhang, J.; Zhang, J.; Li, J. Enhancement of plant variety protection and regulation using molecular marker technology. Acta Agron. Sin. 2022, 48, 1853–1870. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Samartza, I.; et al. DNA barcoding and fertilization strategies in Sideritis syriaca subsp. syriaca, a local endemic plant of Crete with high medicinal value. Int. J. Mol. Sci. 2024, 25, 1891. [Google Scholar] [PubMed]
- NY/T 4022-2021; Maize (Zea mays L.) Variety Genuineness Identification: SNP Based Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2021. Available online: https://hbba.sacinfo.org.cn/attachment/onlineRead/0c9ed07cbe3b38f0129c0e420923580f5b49002d92aa1699d6164f667de39f73 (accessed on 10 January 2025).
- ISTA (International Seed Testing Association). Method Validation Reports on Rules Proposals for the International Rules for Seed Testing 2023 Edition. Part 3: Validation of a New DNA-Based Method for Testing Pisum Varieties; ISTA OGM22-06-Part 3; ISTA (International Seed Testing Association): Geneva, Switzerland, 2023. [Google Scholar]
- Xu, N.; Jin, S.; Jin, F.; Liu, L.; Xu, J.; Liu, F.; Ren, X.; Sun, Q.; Xu, X.; Pang, B. Genetic similarity and its detection accuracy analysis of wheat varieties based on SNP markers. Acta Agron. Sin. 2024, 50, 887–896. [Google Scholar] [CrossRef]
- Ro, N.; Haile, M.; Yoon, H.; Yu, D.; Ko, H.; Cho, G.; Woo, H.; Sung, P. Development of informative SNP markers for Capsicumspecies identification using phenotypic and genomic data. Sci. Hortic. 2025, 351, 114417. [Google Scholar] [CrossRef]
- ISO 5725-1; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 1: General principles and Definitions; ISO (International Standards Organization): Geneva, Switzerland, 1994.
- Ellison, S.L.R.; Williams, A. Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Laboratory of the Government Chemist: London, UK, 2012; pp. 7–9. [Google Scholar]
- IOC (International Standards Organization); IEC (International Electrotechnical Commission). Guide to the Expression of Uncertainty in Measurement. Part 1: Introduction; ISO/IEC Guide 98-Part 1; ISO (International Standards Organization): Geneva, Switzerland; IEC (International Electrotechnical Commission): Geneva, Switzerland, 2024. [Google Scholar]
- ISO 13528; Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison. ISO (International Standards Organization): Geneva, Switzerland, 2022.
- ISO 5725-4; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 4: Basic Methods for the Determination of the Trueness of a Standard Measurement Method. ISO (International Standards Organization): Geneva, Switzerland, 1994.
- NY/T 2745-2021; Rice (Oryza sativa L.) Variety Genuineness Identification: SNP Based Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2021. Available online: https://hbba.sacinfo.org.cn/portal/online/f34b856d930383c47fe37d85228854a1b82be2b2bb8a3926ffeb9da773aa83db (accessed on 10 January 2025).
- NY/T 4021-2021; Wheat (Triticum aestivum L.) Variety Genuineness Identification: SNP Based Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2021. Available online: https://hbba.sacinfo.org.cn/portal/online/cb9572bf672f36f7f6f1dd479e46398a36702b3d7d29599ae9247a07ff46cb86 (accessed on 10 January 2025).
- Tang, Q.; Zhang, C. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Yan, W. A systematic narration of some key concepts and procedures in plant breeding. Front. Plant Sci. 2021, 12, 724517. [Google Scholar] [CrossRef]
- Yan, W. Singular-value partitioning in biplot analysis of multienvironment trial data. Agron. J. 2002, 94, 990–996. [Google Scholar] [CrossRef]
- Achard, F.; Butruille, M.; Madjarac, S.; Nelson, P.T.; Duesing, J.; Laffont, J.; Nelson, B.; Xiong, J.; Mikel, M.A.; Smith, J.S.C. Single nucleotide polymorphisms facilitate distinctness-uniformity-stability testing of soybean cultivars for plant variety protection. Crop Sci. 2020, 60, 2280–2303. [Google Scholar] [CrossRef]
- Székács, A.; Weiss, G.; Quist, D.; Hilbeck, A. Inter-laboratory comparison of cry1ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food Agric. Immunol. 2012, 23, 99–121. [Google Scholar] [CrossRef]
- Singh, N.; Choudhury, D.R.; Singh, A.K.; Kumar, S.; Srinivasan, K.; Tyagi, R.K.; Singh, N.K.; Singh, R. Comparison of SSR and SNP markers in estimation of genetic diversity and population structure of Indian rice varieties. PLoS ONE 2013, 8, e84136. [Google Scholar] [CrossRef]
- Xu, K.; Wu, J.; Li, F.; Wu, X. Comparison between SSR and SNP systems of genetic diversity analysis in Brassica napus L. Oil Crop Sci. 2018, 3, 86–91. [Google Scholar]
- Esteves, F.; Gaspar, J.; De Sousa, B.; Antunes, F.; Mansinho, K.; Matos, O. Clinical relevance of multiple single-nucleotide polymorphisms in Pneumocystis jiroveciiPneumonia: Development of a multiplex PCR-single-base-extension methodology. J. Clin. Microbiol. 2011, 49, 1810–1815. [Google Scholar] [CrossRef]
- ISO 5725-2; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard measurement Method. ISO (International Standards Organization): Geneva, Switzerland, 1994.
- Jbeily, A.C.; Haubelt, G.; Myburgh, J.; Svacinka, R. Results of an international ring test for the determination of the rheological properties of wheat flour dough using the haubelt flourgraph e 7 (ICC standard no. 180). Qual. Assur. Saf. Crops Foods 2014, 6, 469–477. [Google Scholar] [CrossRef]
- Zhu, J. Discussion on repeatability and reproducibility of measuring method in ISO 5725. China Stand. 2013, 58, 80–87. [Google Scholar]
- Yan, W.; Frégeau-Reid, J. Genotype by yield*trait (GYT) biplot: A novel approach for genotype selection based on multiple traits. Sci. Rep. 2018, 8, 8242. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
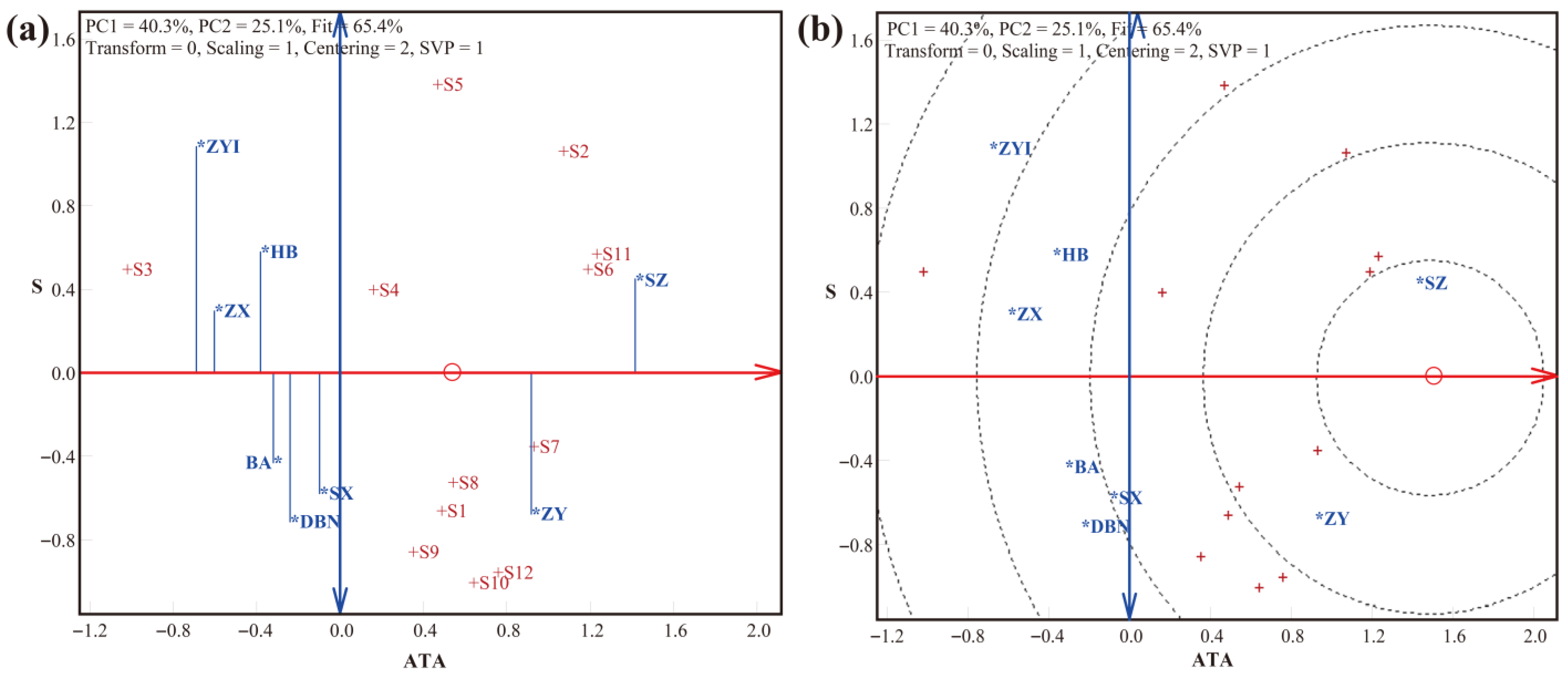
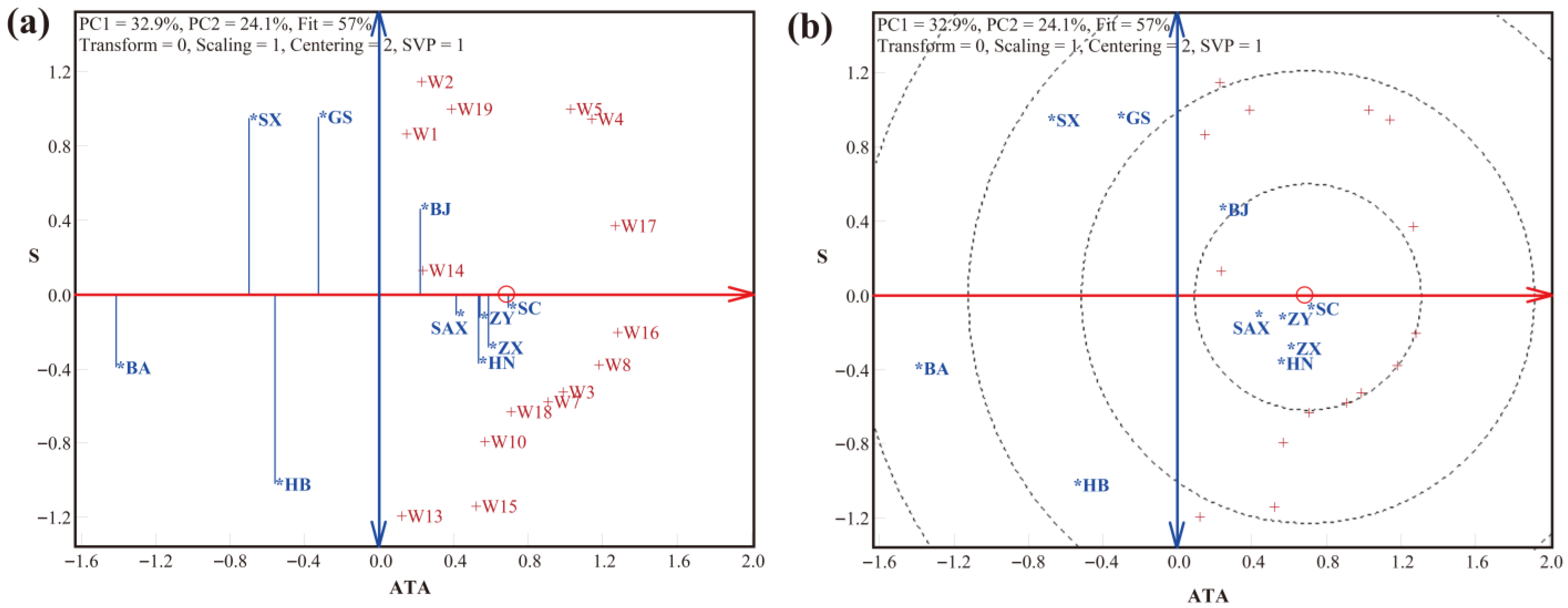
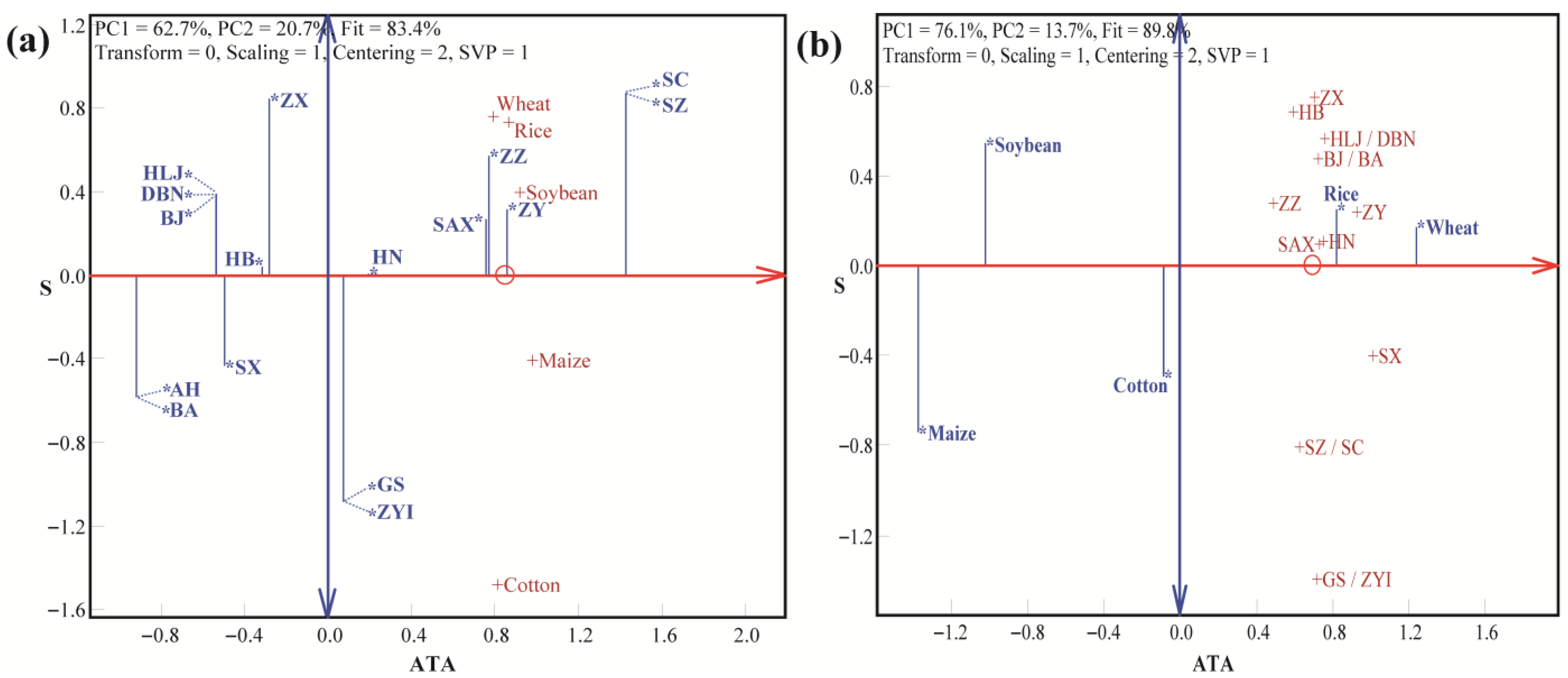
| Crop | SNP Number | Laboratory Number (p) | Variety Number (q) | Type | Weight (g) |
|---|---|---|---|---|---|
| Cotton | 58 | 9 | 19 | DNA | 1.0 × 10−5 |
| Maize | 96 | 8 | 21 | Seed | 18.0 |
| Rice | 96 | 7 | 11 | Seed | 2.0 |
| Soybean | 65 | 8 | 12 | Seed | 15.0 |
| Wheat | 96 | 10 | 15 | Seed powder | 0.5 |
| Laboratory Name Initials | Province | Detection Platform | Crop Detected | ||||
|---|---|---|---|---|---|---|---|
| Cotton | Maize | Rice | Soybean | Wheat | |||
| ZX | Beijing | LGC SNP Line | √ | √ | √ | √ | √ |
| BA | Beijing | IMAP | √ | √ | √ | √ | |
| HB | Hebei | LGC SNP Line | √ | √ | √ | √ | |
| ZY | Beijing | Array tape | √ | √ | √ | √ | |
| HN | Henan | LGC SNP Line | √ | √ | √ | √ | |
| SX | Shanxi | LGC SNP Line | √ | √ | √ | √ | |
| SAX | Shaanxi | LGC SNP Line | √ | √ | √ | ||
| BJ | Beijing | Quantitative PCR | √ | ||||
| SZ | Guangdong | LGC SNP Line | √ | √ | |||
| ZZ | Beijing | Quantitative PCR | √ | √ | √ | ||
| GS | Gansu | Quantitative PCR | √ | √ | |||
| HLJ | Heilongjiang | LGC SNP Line | √ | ||||
| SC | Sichuan | Array tape | √ | ||||
| ZYI | Gansu | LGC SNP Line | √ | √ | |||
| AH | Anhui | LGC SNP Line | √ | ||||
| DBN | Beijing | Array tape | √ | ||||
| Statistic | Single-Genotype Analysis Method for Genotype j Individually | Multi-Genotype Joint Analysis Method for All Genotypes Simultaneously |
|---|---|---|
| Repeatability standard deviation (σr) | ||
| Inter-laboratory standard deviation (σL) | ||
| Reproducibility standard deviation (σR) | ||
| Ratio of the reproducibility to the repeatability standard deviation (γ) | ||
| Coefficient of uncertainty (A) | ||
| Coefficient of extended uncertainty (EA) | ||
| Least significant difference among labs at the 0.05 probability level (LSD0.05,L) | ||
| Least significant difference among genotypes at the 0.05 probability level (LSD0.05,G) | / | |
| Test accuracy (TA) |
| Crop | Source | df | SS | SStrmt (%) | MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Cotton | Laboratory | 8 | 538.40 | 43.9 | 67.30 | 31.42 | 0.000 |
| Genotype | 18 | 129.37 | 10.5 | 7.19 | 3.36 | 0.000 | |
| Laboratory × Genotype | 144 | 559.20 | 45.6 | 3.88 | 1.81 | 0.000 | |
| Error | 342 | 732.66 | 2.14 | ||||
| Maize | Laboratory | 7 | 2587.13 | 54.4 | 369.59 | 244.89 | 0.000 |
| Genotype | 20 | 544.60 | 11.4 | 27.23 | 18.04 | 0.000 | |
| Laboratory × Genotype | 140 | 1626.82 | 34.2 | 11.62 | 7.70 | 0.000 | |
| Error | 336 | 507.09 | 1.51 | ||||
| Rice | Laboratory | 6 | 47.99 | 32.0 | 8.00 | 29.63 | 0.000 |
| Genotype | 10 | 34.74 | 23.1 | 3.47 | 12.87 | 0.000 | |
| Laboratory × Genotype | 60 | 67.43 | 44.9 | 1.12 | 4.16 | 0.000 | |
| Error | 154 | 41.57 | 0.27 | ||||
| Soybean | Laboratory | 7 | 117.51 | 5.9 | 16.79 | 12.68 | 0.000 |
| Genotype | 11 | 1390.37 | 70.0 | 126.40 | 95.46 | 0.000 | |
| Laboratory × Genotype | 77 | 477.98 | 24.1 | 6.21 | 4.69 | 0.000 | |
| Error | 192 | 254.23 | 1.32 | ||||
| Wheat | Laboratory | 9 | 44.35 | 21.5 | 4.93 | 15.92 | 0.000 |
| Genotype | 14 | 20.63 | 10.0 | 1.47 | 4.76 | 0.000 | |
| Laboratory × Genotype | 126 | 141.67 | 68.6 | 1.12 | 3.63 | 0.000 | |
| Error | 300 | 92.88 | 0.31 |
| Statistic | Cotton | Maize | Rice | Soybean | Wheat | Mean |
|---|---|---|---|---|---|---|
| σr | 1.44 ± 0.06 a | 1.19 ± 0.07 b | 0.49 ± 0.06 c | 1.03 ± 0.16 b | 0.54 ± 0.04 c | 0.94 |
| [1.32, 1.56] | [1.05, 1.33] | [0.37, 0.61] | [0.71, 1.35] | [0.46, 0.62] | [0.78, 1.09] | |
| σL | 1.07 ± 0.17 bc | 2.83 ± 0.23 a | 0.68 ± 0.06 cd | 1.27 ± 0.17 b | 0.56 ± 0.05 d | 1.28 |
| [0.73, 1.41] | [2.37, 3.29] | [0.56, 0.80] | [0.93, 1.61] | [0.46, 0.66] | [1.01, 1.55] | |
| σR | 1.89 ± 0.12 b | 3.09 ± 0.22 a | 0.85 ± 0.06 c | 1.68 ± 0.20 b | 0.80 ± 0.04 c | 1.66 |
| [1.65, 2.13] | [2.65, 3.53] | [0.73, 0.97] | [1.28, 2.08] | [0.72, 0.88] | [1.41, 1.92] | |
| γ | 1.33 ± 0.09 c | 2.70 ± 0.19 a | 1.91 ± 0.20 b | 1.78 ± 0.18 bc | 1.56 ± 0.12 bc | 1.86 |
| [1.15, 1.51] | [2.32, 3.08] | [1.51, 2.31] | [1.42, 2.14] | [1.32, 1.80] | [1.54, 2.17] | |
| A | 0.48 ± 0.02 c | 0.65 ± 0.01 a | 0.65 ± 0.02 a | 0.59 ± 0.02 b | 0.50 ± 0.01 c | 0.57 |
| [0.44, 0.52] | [0.63, 0.67] | [0.61, 0.69] | [0.55, 0.63] | [0.48, 0.52] | [0.54, 0.61] | |
| AσR | 0.94 ± 0.09 b | 2.02 ± 0.16 a | 0.55 ± 0.04 c | 0.99 ± 0.12 b | 0.41 ± 0.03 c | 0.98 |
| [0.76, 1.12] | [1.70, 2.34] | [0.47, 0.63] | [0.75, 1.23] | [0.35, 0.47] | [0.81, 1.16] | |
| 98.08 ± 0.12 b | 96.19 ± 0.23 d | 99.21 ± 0.12 a | 97.18 ± 0.66 c | 99.48 ± 0.06 a | 98.03 | |
| [97.84, 98.32] | [95.73, 96.65] | [98.97, 99.45] | [95.86, 98.50] | [99.36, 99.60] | [97.55, 98.50] | |
| TA | 97.31 ± 0.17 b | 95.08 ± 0.31 c | 98.80 ± 0.10 a | 96.65 ± 0.66 b | 99.04 ± 0.06 a | 97.38 |
| [97.00, 97.62] | [94.46, 95.70] | [98.60, 99.00] | [95.33, 97.97] | [98.92, 99.16] | [96.86, 97.89] | |
| LSD0.05,L | 2.47 ± 0.10 a | 2.05 ± 0.12 b | 0.85 ± 0.10 c | 1.78 ± 0.27 b | 0.92 ± 0.07 c | 1.61 |
| [2.27, 2.67] | [1.81, 2.29] | [0.65, 1.05] | [1.24, 2.32] | [0.78, 1.06] | [1.35, 1.88] |
| Statistic | Cotton | Maize | Rice | Soybean | Wheat | Mean |
|---|---|---|---|---|---|---|
| σr | 1.46 [1.383, 1.551] | 1.23 [1.146, 1.319] | 0.52 [0.475, 0.572] | 1.15 [1.065, 1.246] | 0.56 [0.515, 0.603] | 0.98 [0.917, 1.058] |
| σL | 1.07 (0%) | 2.42 (−14.5%) | 0.48 (−29.4%) | 0.66 (−48%) | 0.32 (−42.9%) | 0.99 (−22.7%) |
| σR | 1.81 (−4.2%) | 2.71 (−12.3%) | 0.71 (−16.5%) | 1.32 (−21.4%) | 0.64 (−20%) | 1.44 (−13.3%) |
| σG | 0.43 | 1.04 | 0.39 | 2.28 | 0.2 | 0.87 |
| σLG | 0.76 | 1.84 | 0.53 | 1.28 | 0.52 | 0.99 |
| γ | 1.24 (−6.8%) | 2.21 (−18.1%) | 1.37 (−28.3%) | 1.15 (−35.4%) | 1.15 (−26.3%) | 1.42 (−23.7%) |
| A | 0.49 (2.1%) | 0.64 (−1.5%) | 0.59 (−9.2%) | 0.49 (−16.9%) | 0.44 (−12%) | 0.53 (−7%) |
| AσR | 0.89 (−5.3%) [0.836, 0.949] | 1.75 (−13.4%) [1.637, 1.868] | 0.42 (−23.6%) [0.384, 0.465] | 0.65 (−34.3%) [0.592, 0.711] | 0.28 (−31.7%) [0.259, 0.306] | 0.8 (−18.4%) [0.742, 0.860] |
| 98.08 | 96.19 | 99.21 | 97.18 | 99.48 | 98.03 | |
| TA | 97.36 (0.1%) | 95.33 (0.3%) | 98.94 (0.1%) | 96.88 (0.2%) | 99.18 (0.1%) | 97.54 (0.2%) |
| LSD0.05,L | 0.78 (−68.4%) | 0.70 (−65.9%) | 0.32 (−62.4%) | 0.66 (−62.9%) | 0.28 (−69.6%) | 0.55 (−65.8%) |
| LSD0.05,G | 0.54 | 0.43 | 0.25 | 0.53 | 0.23 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, G.; Jin, S.; Liu, L.; Yi, H.; Jin, F.; Xu, Q.; Kuang, M.; Ren, X.; Sun, Q.; et al. Improved Estimation and Graphical Representation of the Reliability Measures of the SNP Marker Method for Crop Variety Identification. Agronomy 2025, 15, 2670. https://doi.org/10.3390/agronomy15122670
Xu J, Wang G, Jin S, Liu L, Yi H, Jin F, Xu Q, Kuang M, Ren X, Sun Q, et al. Improved Estimation and Graphical Representation of the Reliability Measures of the SNP Marker Method for Crop Variety Identification. Agronomy. 2025; 15(12):2670. https://doi.org/10.3390/agronomy15122670
Chicago/Turabian StyleXu, Jianwen, Guangying Wang, Shiqiao Jin, Lihua Liu, Hongmei Yi, Fang Jin, Qun Xu, Meng Kuang, Xuezhen Ren, Quan Sun, and et al. 2025. "Improved Estimation and Graphical Representation of the Reliability Measures of the SNP Marker Method for Crop Variety Identification" Agronomy 15, no. 12: 2670. https://doi.org/10.3390/agronomy15122670
APA StyleXu, J., Wang, G., Jin, S., Liu, L., Yi, H., Jin, F., Xu, Q., Kuang, M., Ren, X., Sun, Q., Li, J., Xu, X., Pang, B., & Xu, N. (2025). Improved Estimation and Graphical Representation of the Reliability Measures of the SNP Marker Method for Crop Variety Identification. Agronomy, 15(12), 2670. https://doi.org/10.3390/agronomy15122670






