Effect of Soybean Meal on Nutritional Content, Fermentation Profile, and Bacterial Community Structure of Napier Grass Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Fermentation Characteristics and Chemical Compositions
2.3. Microbial Community Analysis
2.3.1. Bacterial DNA Extraction and PCR Amplification
2.3.2. High-Throughput Sequencing of Metagenomic DNA
2.4. Statistical Analysis of Data
3. Results
3.1. The Chemical Composition and Microbial Population of Fresh Material
3.2. Effect of Soybean Meal on Silage Fermentation, Microbial Population, and Chemical Composition of Napier Grass
3.3. Nutritional Composition of Napier Grass Silage
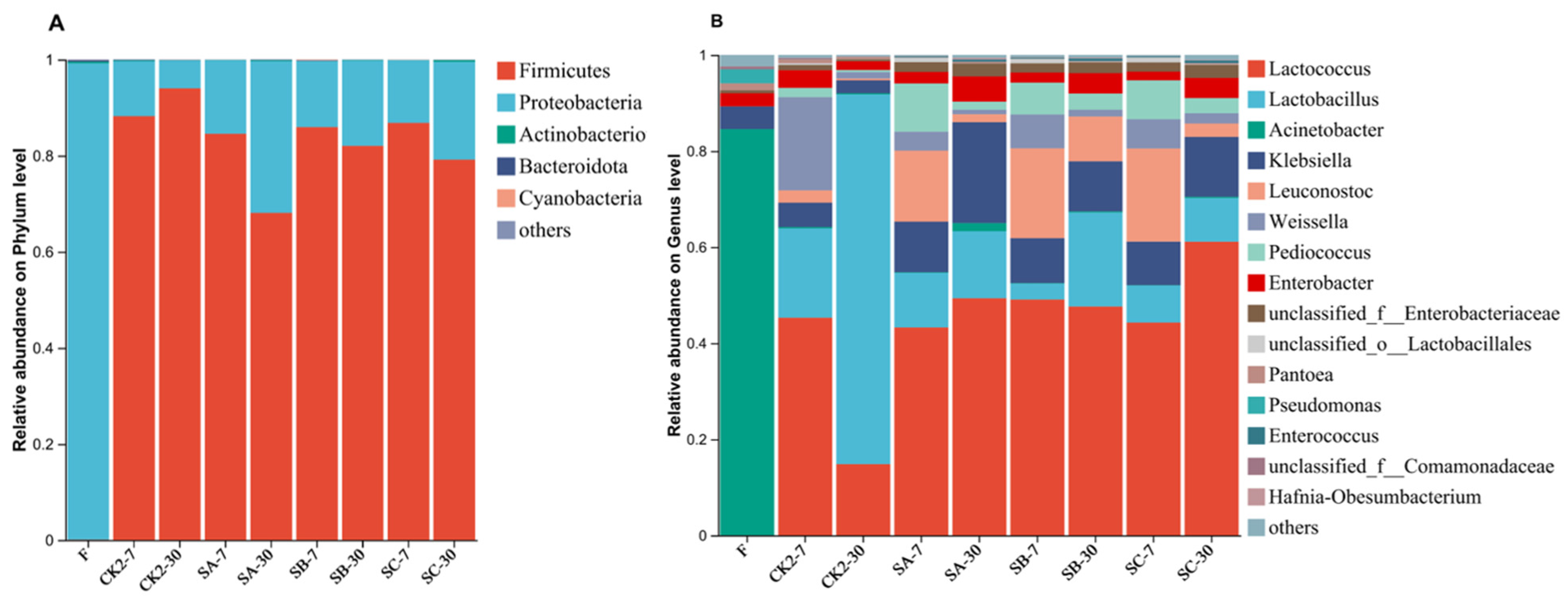
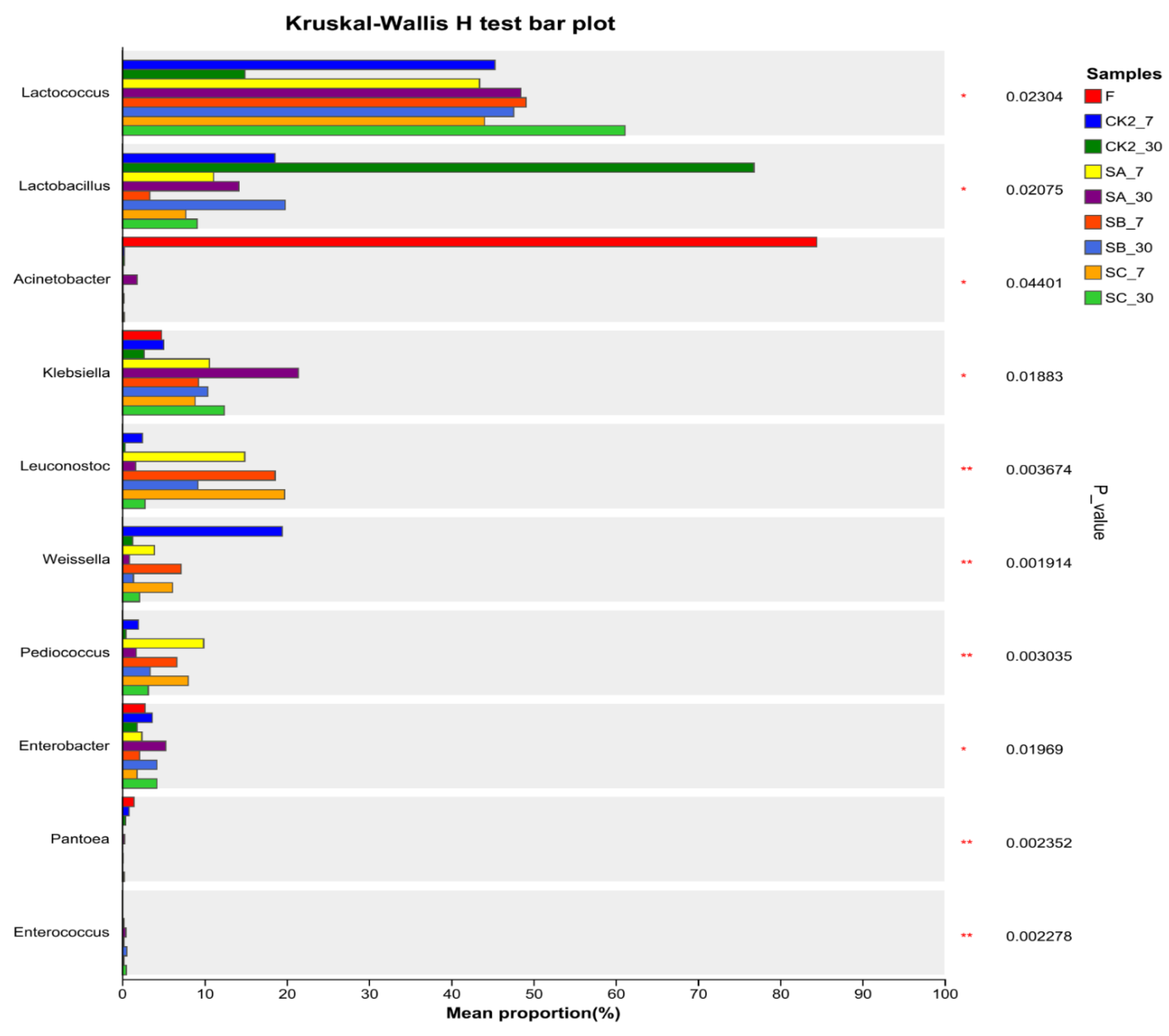
3.4. Effect of Soybean Meal on Bacterial Community of Napier Grass Silage
Bacterial Community Diversity and Abundance
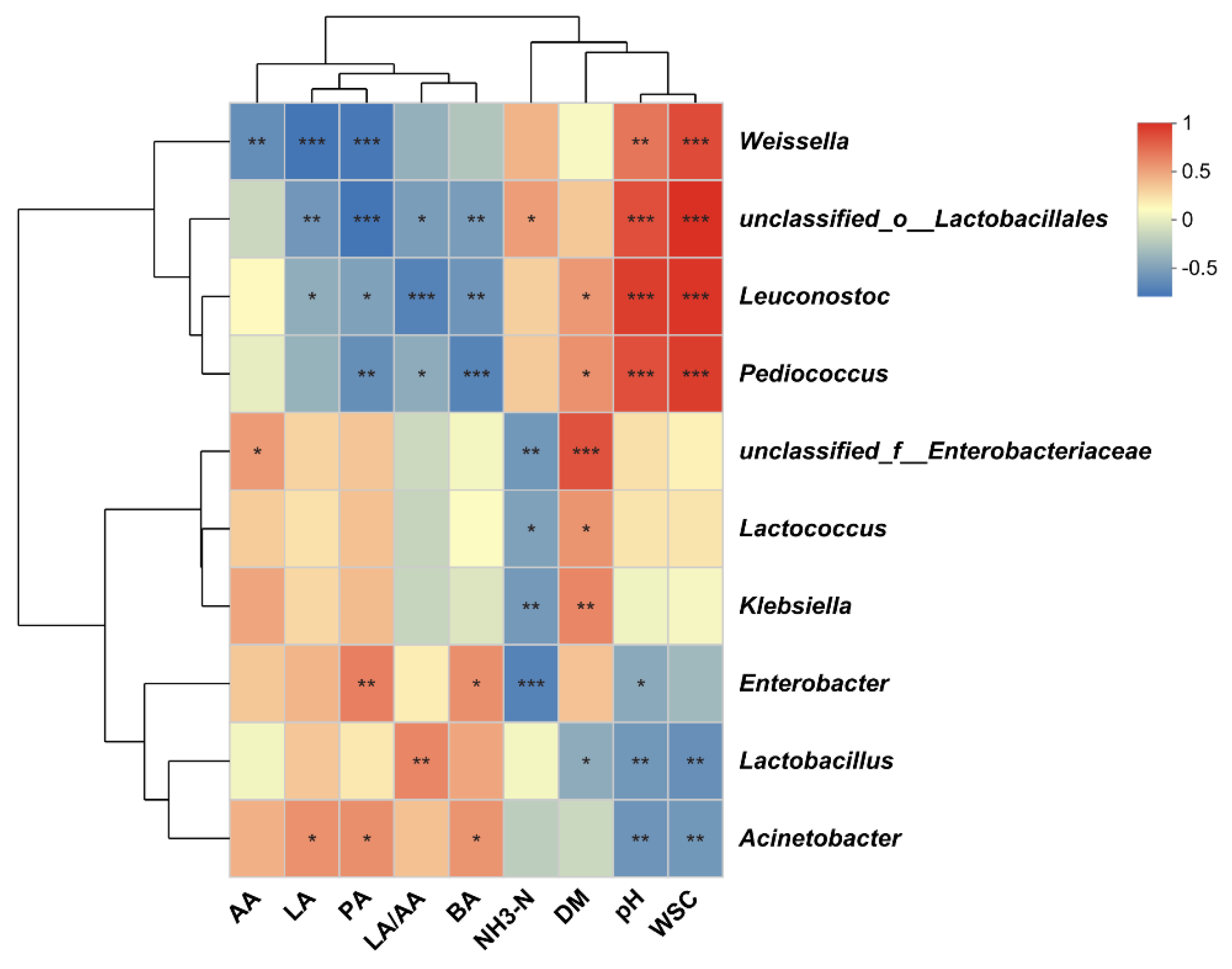
3.5. Correlation Analysis Between Bacterial Community Genus and Fermentation Indices
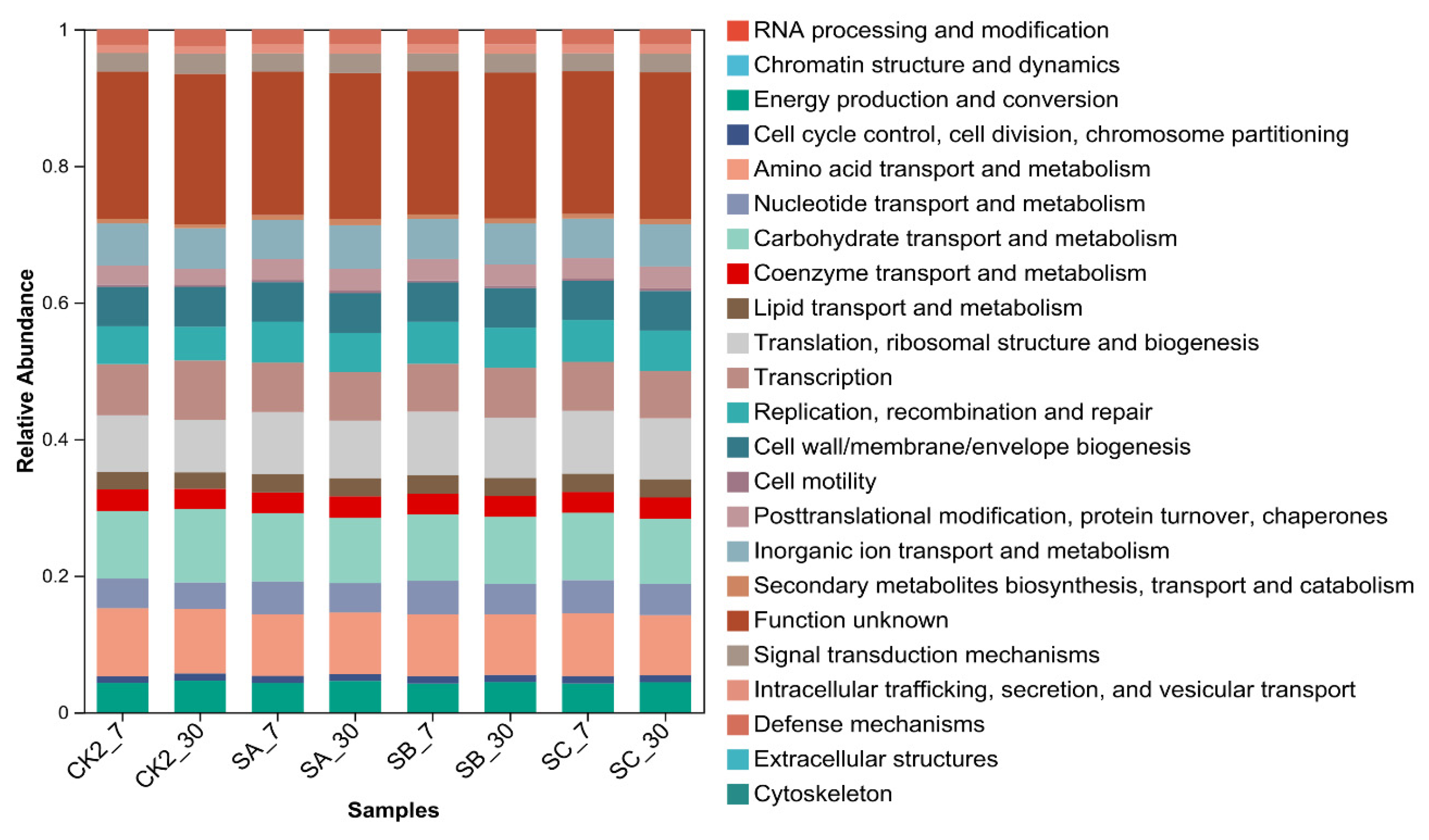
3.6. Bacterial Function Prediction
4. Discussion
4.1. Effect of Soybean Meal on the Fermentation Quality and Nutritional Composition of Napier Grass Ensiled for 90 Days
4.2. Effect of Soybean Meal on Bacterial Community Relative Abundance in Napier Grass Silage
4.3. Linkages Between Fermentation Characteristics, Bacterial Community and Predicted Bacterial Function
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kazemi, M.; Ghasemi, B.K.; Valizadeh, R. In Vitro and in Vivo Investigation of Persian Manna Plant Silage as an Alternative Forage for Fattening Lambs. Small Rumin. Res. 2023, 226, 107027. [Google Scholar] [CrossRef]
- Kazemi, M.; Valizadeh, R. Can Alhaji Maurorum as a Halophyte Plant Be Ensiled with Molasses and Saccharomyces Cerevisiae Well? AMB Express 2023, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Shi, Y.; Jiang, L.; Wang, X.; Zhu, G.; Zhou, G. Double-Cropping Systems Based on Maize, Sorghum, and Alfalfa: Impact on Nutritive Value and Silage Fermentation Quality. Agronomy 2025, 15, 630. [Google Scholar] [CrossRef]
- Kazemi, M.; Valizadeh, R.; Ibrahimi, K.A.E. Yogurt and Molasses Can Alter Microbial-Digestive and Nutritional Characteristics of Pomegranate Leaves Silage. AMB Express 2022, 12, 111. [Google Scholar] [CrossRef]
- Yan, L.; Hua, J.; Lu, W.; Zhao, H.; Chao, C.; Yan, L.; Lv, X. Effects of Silage from Different Maize Varieties on Growth Traits, Meat Quality, Slaughter Performance, Apparent Digestibility of Nutrients, and Rumen Microbiota in Hu Sheep. Front. Anim. Sci. 2025, 6, 1640756. [Google Scholar] [CrossRef]
- Bureenok, S.; Yuangklang, C.; Vasupen, K.; Schonewille, J.T.; Kawamoto, Y. The Effects of Additives in Napier Grass Silages on Chemical Composition, Feed Intake, Nutrient Digestibility and Rumen Fermentation. Asian-Australas. J. Anim. Sci. 2012, 25, 1248–1254. [Google Scholar] [CrossRef]
- Islam, M.R.; Garcia, S.C.; Sarker, N.R.; Roy, B.K.; Sultana, N.; Clark, C.E.F. The Role of Napier Grass (Pennisetum purpureum Schumach) for Improving Ruminant Production Efficiency and Human Nutrition in the Tropics. In Climate Change and Livestock Production: Recent Advances and Future Perspectives; Sejian, V., Chauhan, S.S., Devaraj, C., Malik, P.K., Bhatta, R., Eds.; Springer: Singapore, 2021; pp. 151–160. ISBN 978-981-16-9835-4. [Google Scholar]
- Lim, H.P.; Wan, A.W.A.; Low, J.H. Properties Characterization of Napier Grass (Pennisetum purpureum) as the Non-Wood Substitution for Natural Fiber Papermaking. J. Adv. Mech. Eng. Appl. 2020, 1, 27–35. [Google Scholar] [CrossRef]
- Guan, H.; Shuai, Y.; Yan, Y.; Ran, Q.; Wang, X.; Li, D.; Cai, Y.; Zhang, X. Microbial Community and Fermentation Dynamics of Corn Silage Prepared with Heat-Resistant Lactic Acid Bacteria in a Hot Environment. Microorganisms 2020, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.; Ohba, N.; Shimojo, M.; Furuse, M.; Masuda, Y. Effects of Adding Urea and Molasses on Napiergrass Silage Quality. Asian-Australas. J. Anim. Sci. 2000, 13, 1542–1547. [Google Scholar] [CrossRef]
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. In Soybean and Nutrition; El-Shemy, H., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-536-5. [Google Scholar]
- Zhang, Y.; Chen, S.; Zong, X.; Wang, C.; Shi, C.; Wang, F.; Wang, Y.; Lu, Z. Peptides Derived from Fermented Soybean Meal Suppresses Intestinal Inflammation and Enhances Epithelial Barrier Function in Piglets. Food Agric. Immunol. 2020, 31, 120–135. [Google Scholar] [CrossRef]
- Li, Y.; Gan, S.; Luo, L.; Yang, W.; Mo, L.; Shang, C. Optimization of Molasses and Soybean Meal Content to Enhance Tetramethylpyrazine Yield by Bacillus Sp. TTMP20. Molecules 2023, 28, 6515. [Google Scholar] [CrossRef]
- Abreu, J.G.D.; Kazama, D.C.D.S.; Peixoto, W.M.; Matter, E.; Cabral, L.D.S.; Balbinot, E.; Royer, P.O.; Ferreira, E.A. Use of NIRS Technology for Predicting the Nutritional Value of Silage Made from Tropical Grasses Enriched with Corn Ethanol Co-Products and Intercropped with Corn. Semin. Ciênc. Agrár. 2024, 46, 71–92. [Google Scholar] [CrossRef]
- Silva, J.K.D.; Oliveira, J.S.D.; Medeiros, A.N.D.; Santos, E.M.; Magalhães, T.D.S.; Ramos, A.O.; Bezerra, H.F.C. Elephant Grass Ensiled with Wheat Bran Compared with Corn Silage in Diets for Lactating Goats. Rev. Bras. Zootec. 2014, 43, 618–626. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, G.; Li, Z.; Liu, L.; Zhang, S.; Li, D. Effects of Different Crude Protein and Dietary Fiber Levels on the Comparative Energy and Nutrient Utilization in Sows and Growing Pigs. Animals 2020, 10, 495. [Google Scholar] [CrossRef]
- Yunus, M.; Ohba, N.; Tobisa, M.; Shimojo, M.; Masuda, Y. Effects of Preheated Additives on the Fermentation Quality of Napiergrass Silage. Asian-Australas. J. Anim. Sci. 2001, 14, 1564–1567. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Method 2001.11 Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds: Block Digestion Method Using Copper Catalyst and Steam Distillation into Boric Acid. In Official Methods of Analysis of AOAC International; Latimer, G.W., Ed.; Oxford University Press: New York, NY, USA, 2023; ISBN 978-0-19-761013-8. [Google Scholar]
- Arthur Thomas, T. An Automated Procedure for the Determination of Soluble Carbohydrates in Herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, M.; Feng, X.; Wang, P.; Cao, X.; Zhang, W. Established Methods and Comparison of 10 Organic Acids Based on Reversed Phase Chromatography and Hydrophilic Interaction Chromatography. CyTA-J. Food 2022, 20, 206–217. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio Cloud 2024: Update Single-cell and Multiomics Workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, N.; Heron, S. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991; ISBN 978-0-948617-22-5. [Google Scholar]
- Roy, A.; Roy, B.K.; Clark, C.E.F.; Bashar, M.K.; Sarker, N.R.; Sultana, N.; Billah, M.M.; Al-Mamun, M.; Islam, M.R. Management Strategies for Napier Grass (Pennisetum purpureum Schumach Cv Pakchong): Impact on Dry Matter Yield, Nutritive Characteristics and Cattle Growth. Animals 2025, 15, 1235. [Google Scholar] [CrossRef]
- Kung, L. Understanding the Biology of Silage Preservation to Maximize Quality and Protect the Environment. In Proceedings of the Alfalfa & Forage Symposium and Corn/Cereal Silage Conference, Visalia, CA, USA, 1–2 December 2010. [Google Scholar]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Tian, J.; Wang, X.; Zhang, J. Dried Soybean Curd Residue: A Promising Absorbent for Cleaner Production of High-Quality Silage. J. Clean. Prod. 2021, 324, 129300. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Liu, Z.; Zhang, G.; Zhang, Y. Fermentation Quality and Microbial Community of Corn Stover or Rice Straw Silage Mixed with Soybean Curd Residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Dong, L.K.; Choon, C.K. Role of LAB in silage fermentation: Effect on nutritional quality and organic acid production—An Overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J. Silage Additives. In Cultural History and Modern Production Technology of Silage; Cai, Y., Ataku, K., Eds.; Springer Nature: Singapore, 2025; pp. 117–144. ISBN 978-981-96-5786-5. [Google Scholar]
- Yin, X.; Fan, Y.; Tian, R.; Tang, R.; Tian, J.; Zhang, J. Improving the Quality and Reducing Harmful Microbes of Total Mixed Ration Silage with Dried Soybean Curd Residue. Chem. Biol. Technol. Agric. 2023, 10, 86. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Fu, L.; Zhou, Y.; Chen, J.; Wang, G.; Ran, Q.; Hu, L.; Hu, R.; Zhou, J.; et al. Dynamic Changes in Microorganisms and Metabolites During Silage Fermentation of Whole Winter Wheat. Vet. Sci. 2025, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural Populations of Lactic Acid Bacteria Associated with Silage Fermentation as Determined by Phenotype, 16S Ribosomal RNA and recA Gene Analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus Spp. from an Inoculant and of Weissella and Leuconostoc Spp. from Forage Crops on Silage Fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Ávila, C.L.S.; Pinto, J.C.; Carvalho, B.F.; Dias, D.R.; Schwan, R.F. Fermentative Profile and Bacterial Diversity of Corn Silages Inoculated with New Tropical Lactic Acid Bacteria. J. Appl. Microbiol. 2016, 120, 266–279. [Google Scholar] [CrossRef]
- Lin, H.; Lin, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Exploring the Bacterial Community and Fermentation Characteristics during Silage Fermentation of Abandoned Fresh Tea Leaves. Chemosphere 2021, 283, 131234. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; You, S.; Jiang, X.; Li, Y.; Wang, R.; Ge, G.; Jia, Y. Evaluating the Fermentation Characteristics, Bacterial Community, and Predicted Functional Profiles of Native Grass Ensiled with Different Additives. Front. Microbiol. 2022, 13, 1025536. [Google Scholar] [CrossRef]
- Jatkauskas, J.; Vrotniakienė, V.; Stoškus, R. Variations in Fermentation, Bacterial Population and Aerobic Stability in Maize Silage. Zemdirb.-Agric. 2018, 105, 377–382. [Google Scholar] [CrossRef]
- Graf, K.; Ulrich, A.; Idler, C.; Klocke, M. Bacterial Community Dynamics during Ensiling of Perennial Ryegrass at Two Compaction Levels Monitored by Terminal Restriction Fragment Length Polymorphism. J. Appl. Microbiol. 2016, 120, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ni, K.; Zhang, Y.; Lin, Y.; Yang, F. Fermentation Characteristics, Chemical Composition and Microbial Community of Tropical Forage Silage under Different Temperatures. Asian-Australas. J. Anim. Sci. 2019, 32, 665–674. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.; Wang, S.; Li, J.; Shao, T. Separating the Effects of Chemical and Microbial Factors on Fermentation Quality and Bacterial Community of Napier Grass Silage by Using Gamma-Ray Irradiation and Epiphytic Microbiota Transplantation. Anim. Feed Sci. Technol. 2021, 280, 115082. [Google Scholar] [CrossRef]
- McDonald, L.C.; Fleming, H.P.; Hassan, H.M. Acid Tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl. Environ. Microbiol. 1990, 56, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Lu, D.D.; Chen, J.Y.; Yu, B.; Liang, J.B.; Mi, J.D.; Candyrine, S.C.L.; Liao, X.D. Effects of Fermented Soybean Meal on Carbon and Nitrogen Metabolisms in Large Intestine of Piglets. Animal 2018, 12, 2056–2064. [Google Scholar] [CrossRef]

| Items 1 | Mean + SD 2 |
|---|---|
| pH | 5.75 ± 0.01 |
| BC (mEq/kg DM) | 41.12 |
| DM (g/kg DM) | 287.30 ± 0.03 |
| WSC (g/kg DM) | 53.30 ± 0.41 |
| CP (g/kg DM) | 40.82 ± 0.49 |
| NDF (g/kg DM) | 634.67 ± 0.27 |
| ADF (g/kg DM) | 364.89 ± 0.11 |
| ADL (g/kg DM) | 47.29 ± 0.70 |
| Lactic acid bacteria (log10 CFU/g FM) | 8.46 ± 0.20 |
| Aerobic bacteria (log10 CFU/g FM) | 9.14 ± 0.51 |
| Yeasts (log10 CFU/g FM) | 9.32 ± 0.99 |
| Items 1 | T 2 | Ensiling Days 3 | SEM 4 | p-Value 5 | M C p 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 15 | 30 | 60 | 90 | T | D | T × D | L | Q | |||
| LA (g/kg DM) | CK | 12.59 Bc | 20.66 Ab | 19.04 Bb | 25.49 a | 21.28 Bb | 1.112 | 0.012 | <0.001 | <0.001 | <0.001 | <0.001 |
| SA | 11.56 Bc | 18.43 Ab | 21.78 Ab | 28.63 a | 26.35 Aa | 1.598 | <0.001 | 0.002 | ||||
| SB | 12.76 Bc | 14.21 Bc | 22.14 Ab | 27.11 a | 24.61 Aab | 1.499 | <0.001 | 0.005 | ||||
| SC | 15.97 Ac | 13.89 Bd | 23.07 Ab | 26.24 a | 24.49 Aab | 1.272 | <0.001 | 0.009 | ||||
| SEM 4 | 0.538 | 0.861 | 0.488 | 0.590 | 0.576 | |||||||
| p-value | 0.003 | <0.001 | <0.001 | 0.894 | 0.014 | |||||||
| AA (g/kg DM) | CK | 1.99 Bb | 3.15 b | 3.81 ab | 5.94 a | 4.36 Bab | 0.388 | 0.006 | <0.001 | 0.300 | <0.001 | 0.049 |
| SA | 4.03 AB | 4.66 | 5.52 | 5.91 | 6.00 A | 0.256 | 0.004 | 0.373 | ||||
| SB | 3.91 ABb | 4.04 b | 5.14 b | 6.61 a | 5.17 ABb | 0.273 | <0.001 | 0.033 | ||||
| SC | 4.44 Aab | 4.20 b | 5.48 ab | 5.89 ab | 6.28 Aa | 0.261 | 0.003 | 0.809 | ||||
| SEM 4 | 0.372 | 0.212 | 0.283 | 0.155 | 0.248 | |||||||
| p-value | 0.031 | 0.145 | 0.069 | 0.707 | 0.006 | |||||||
| LA/AA (g/kg DM) | CK | 3.05 b | 6.56 Aa | 5.05 ab | 4.30 ab | 4.89 Aab | 0.400 | 0.034 | 0.002 | 0.026 | 0.566 | 0.097 |
| SA | 2.87 b | 4.14 Bab | 4.02 ab | 4.86 a | 4.44 ABab | 0.228 | 0.014 | 0.151 | ||||
| SB | 3.27 b | 3.53 Bab | 4.45 ab | 4.10 ab | 4.76 ABa | 0.182 | 0.005 | 0.683 | ||||
| SC | 3.71 | 3.33 B | 4.32 | 4.48 | 3.91 B | 0.159 | 0.156 | 0.337 | ||||
| SEM 4 | 0.347 | 0.406 | 0.230 | 0.111 | 0.133 | |||||||
| p-value | 0.541 | <0.001 | 0.444 | 0.809 | 0.015 | |||||||
| PA (g/kg DM) | CK | 0.00 | 0.63 B | 0.03 | 0.39 B | 0.65 | 0.105 | <0.001 | <0.001 | <0.001 | 0.140 | 0.765 |
| SA | NDc | 2.99 Aa | 0.08 bc | 0.29 Bbc | 0.51 b | 0.292 | <0.001 | <0.001 | ||||
| SB | NDc | 3.50 Aa | 0.14 bc | 0.80 Bb | 0.38 bc | 0.340 | 0.004 | <0.001 | ||||
| SC | NDc | 3.70 Aa | 0.46 bc | 1.63 Ab | 0.41 c | 0.351 | 0.013 | <0.001 | ||||
| SEM 4 | 0.001 | 0.366 | 0.065 | 0.168 | 0.075 | |||||||
| p-value | 0.217 | <0.001 | 0.067 | 0.001 | 0.286 | |||||||
| BA (g/kg DM) | CK | 1.83 A | 2.07 A | 1.45 | 2.28 | 2.05 A | 0.166 | <0.001 | <0.001 | 0.002 | 0.614 | 0.746 |
| SA | 0.02 Bd | 0.71 Bc | 1.50 ab | 1.78 a | 1.37 ABb | 0.166 | <0.001 | <0.001 | ||||
| SB | 0.02 Bc | 0.70 Bb | 1.67 a | 1.63 a | 1.13 Bb | 0.163 | <0.001 | <0.001 | ||||
| SC | 0.02 Bd | 0.67 Bc | 1.59 b | 1.75 a | 1.65 ABab | 0.175 | <0.001 | <0.001 | ||||
| SEM 4 | 0.261 | 0.175 | 0.051 | 0.128 | 0.128 | |||||||
| p-value | 0.005 | <0.001 | 0.289 | 0.171 | 0.016 | |||||||
| Items 1 | T 2 | Ensiling Days 3 | SEM 4 | p-Value 5 | M C p 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 15 | 30 | 60 | 90 | T | D | T × D | L | Q | |||
| pH | CK | 4.04 Da | 3.66 Bb | 3.98 a | 4.06 Ba | 3.97 Ba | 0.043 | <0.001 | <0.001 | <0.001 | 0.203 | 0.164 |
| SA | 4.44 Ca | 4.07 Ab | 3.82 c | 4.14 Bb | 4.40 Aa | 0.061 | 0.896 | <0.001 | ||||
| SB | 4.55 Ba | 4.24 Aab | 4.06 b | 4.25 ABab | 4.41 Aab | 0.052 | 0.309 | 0.001 | ||||
| SC | 4.63 Aa | 4.08 Ab | 4.07 b | 4.37 Aa | 4.42 Aa | 0.059 | 0.544 | <0.001 | ||||
| SEM 4 | 0.066 | 0.067 | 0.053 | 0.039 | 0.057 | |||||||
| p-value | <0.001 | < 0.001 | 0.296 | <0.001 | < 0.001 | |||||||
| DM (g/kg FW) | CK | 331.44 B | 312.12 C | 306.07 C | 282.44 B | 296.64 C | 7.110 | <0.001 | 0.020 | 0.033 | 0.073 | 0.418 |
| SA | 376.79 AB | 366.44 B | 351.69 B | 358.00 A | 381.63 B | 4.658 | 0.970 | 0.042 | ||||
| SB | 390.60 A | 408.59 A | 402.83 A | 379.38 A | 409.02 AB | 3.965 | 0.745 | 0.839 | ||||
| SC | 384.74 ABb | 418.61 Aab | 442.52 Aa | 395.55 Ab | 426.84 Aab | 5.680 | 0.066 | 0.055 | ||||
| SEM 4 | 5.447 | 12.309 | 14.370 | 13.966 | 13.893 | |||||||
| p-value | 0.044 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| WSC (g/kg DM) | CK | 24.57 Ba | 16.86 Db | 16.94 Cb | 9.47 Cd | 10.39 Cc | 1.409 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| SA | 25.75 Ba | 25.55 Ca | 17.42 Cb | 12.02 Ad | 14.53 Bc | 1.460 | <0.001 | <0.001 | ||||
| SB | 27.65 Aa | 26.42 Bb | 21.74 Bc | 10.74 Be | 15.85 Ad | 1.654 | <0.001 | <0.001 | ||||
| SC | 28.48 Ab | 32.82 Aa | 23.29 Ac | 12.14 Ae | 15.53 Ad | 1.996 | <0.001 | <0.001 | ||||
| SEM 4 | 0.461 | 1.640 | 0.788 | 0.315 | 0.630 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| ETH (g/kg DM) | CK | 3.75 c | 5.90 Aabc | 5.11 Abc | 8.01 Aa | 7.24 Aab | 0.433 | <0.001 | <0.001 | 0.074 | <0.001 | 0.288 |
| SA | 2.47 d | 3.20 Bcd | 3.61 Bbc | 4.84 Ba | 4.47 Bab | 0.235 | <0.001 | 0.118 | ||||
| SB | 2.42 b | 2.71 Bb | 3.41 Bab | 4.64 Ba | 4.02 Ba | 0.233 | <0.001 | 0.227 | ||||
| SC | 2.80 c | 3.08 Bbc | 3.32 Bbc | 5.37 Ba | 4.37 Bab | 0.266 | <0.001 | 0.480 | ||||
| SEM 4 | 0.199 | 0.386 | 0.236 | 0.405 | 0.426 | |||||||
| p-value | 0.067 | <0.001 | 0.002 | <0.001 | 0.004 | |||||||
| NH3-N (g/kg TN) | CK | 51.49 Ad | 47.75 Ae | 66.41 Aa | 63.17 Ab | 57.30 Ac | 1.808 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| SA | 14.01 Bd | 17.37 Bc | 13.44 Cd | 25.14 Ba | 20.72 Bb | 1.138 | <0.001 | 0.957 | ||||
| SB | 13.96 Bb | 11.04 Cc | 16.42 Ba | 12.17 Cc | 11.86 Dc | 0.509 | 0.013 | 0.004 | ||||
| SC | 8.64 Cd | 10.29 Cc | 12.31 Cb | 7.51 De | 13.19 Ca | 0.555 | <0.001 | 0.063 | ||||
| SEM 4 | 4.954 | 4.430 | 6.560 | 6.315 | 5.346 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Items 1 | Treatment 2 | Ensiling Days 3 | SEM 4 | p-Value 5 | M C p 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 15 | 30 | 60 | 90 | T | D | T × D | L | Q | |||
| LAB (Log10 CFU/g FW) | CK | 9.65 ABa | 9.38 a | 9.57 a | 8.62 b | 7.01 c | 0.261 | 0.095 | <0.001 | 0.182 | <0.001 | <0.001 |
| SA | 9.47 Bab | 9.96 a | 9.02 ab | 8.31 b | 6.67 c | 0.315 | <0.001 | 0.005 | ||||
| SB | 9.89 Aa | 9.68 a | 9.19 b | 8.36 c | 7.45 d | 0.234 | <0.001 | <0.001 | ||||
| SC | 9.92 Aa | 9.72 ab | 9.37 b | 8.49 c | 7.18 d | 0.263 | <0.001 | <0.001 | ||||
| SEM 4 | 0.062 | 0.085 | 0.090 | 0.062 | 0.148 | |||||||
| p-value | 0.011 | 0.304 | 0.569 | 0.527 | 0.370 | |||||||
| AR (Log10 CFU/g FW) | CK | 9.46 a | 8.42 Ba | 6.18 c | 6.54 Ac | 4.58 d | 0.459 | 0.063 | <0.001 | 0.658 | <0.001 | 0.525 |
| SA | 9.58 a | 9.65 Aa | 7.16 b | 5.60 Bc | 4.57 d | 0.536 | <0.001 | 0.151 | ||||
| SB | 9.81 a | 9.35 ABa | 7.04 b | 5.26 Bc | 5.32 c | 0.503 | <0.001 | 0.046 | ||||
| SC | 9.56 a | 9.51 ABa | 7.13 b | 5.33 Bc | 4.88 c | 0.521 | <0.001 | 0.807 | ||||
| SEM 4 | 0.090 | 0.178 | 0.155 | 0.167 | 0.148 | |||||||
| p-value | 0.580 | 0.038 | 0.037 | 0.002 | 0.240 | |||||||
| Yeast (Log10 CFU/g FW) | CK | 8.97 Ba | 8.72 a | 6.82 b | 6.13 bc | 5.09 Bc | 0.400 | 0.011 | <0.001 | <0.001 | <0.001 | 0.724 |
| SA | 9.20 ABa | 9.58 a | 7.38 b | 6.06 c | 5.05 Bd | 0.454 | <0.001 | 0.001 | ||||
| SB | 9.55 Aa | 9.55 a | 7.36 b | 5.87 c | 5.22 ABd | 0.468 | <0.001 | 0.152 | ||||
| SC | 9.54 Aa | 9.70 a | 7.15 b | 5.66 c | 5.57 Ac | 0.467 | <0.001 | 0.226 | ||||
| SEM 4 | 0.078 | 0.162 | 0.083 | 0.091 | 0.070 | |||||||
| p-value | <0.001 | 0.055 | 0.135 | 0.080 | 0.005 | |||||||
| Items 1 | Experimental Silage 2 | SEM 3 | p-Value 4 | p-Value of Contrast 5 | ||||
|---|---|---|---|---|---|---|---|---|
| CK | SA | SB | SC | ANOVA | L | Q | ||
| a CP (g/kg TN) | 40.59 D | 144.74 C | 166.57 B | 201.50 A | 17.311 | <0.001 | <0.001 | <0.001 |
| b NDF (g/kg DM) | 610.61 A | 562.11 C | 574.53 B | 575.20 B | 5.229 | <0.001 | 0.049 | <0.001 |
| c ADF (g/kg DM) | 391.34 A | 369.69 B | 357.71 C | 360.23 C | 3.837 | <0.001 | <0.001 | <0.001 |
| d ADL (g/kg DM) | 43.40 A | 38.27 B | 35.72 B | 35.94 B | 0.949 | <0.001 | <0.001 | 0.009 |
| e HC (g/kg DM) | 219.27 A | 192.41 B | 216.82 A | 214.97 A | 3.135 | <0.001 | 0.713 | <0.001 |
| f CE (g/kg DM) | 347.94 A | 331.42 B | 321.99 C | 324.28 C | 2.952 | <0.001 | <0.001 | <0.001 |
| Ensiling Days 1 | Treatments 2 | Reads | OTUs | Shannon | Chaio1 | Simpson | Coverage |
|---|---|---|---|---|---|---|---|
| 0 | FM | 65,353 | 144 | 0.979 | 177.35 | 0.66 | 0.999 |
| 7 | CK | 54,204 | 106 | 1.732 | 127.04 | 0.29 | 0.9994 |
| 7 | SA | 62,279 | 98 | 2.258 | 119.92 | 0.15 | 0.9993 |
| 7 | SB | 56,435 | 105 | 2.15 | 133.72 | 0.17 | 0.9993 |
| 7 | SC | 63,933 | 98 | 2.19 | 119.79 | 0.17 | 0.9993 |
| 30 | CK | 52,312 | 93 | 1.19 | 119.74 | 0.50 | 0.9993 |
| 30 | SA | 48,542 | 124 | 1.95 | 148.14 | 0.22 | 0.9992 |
| 30 | SB | 50,453 | 116 | 2.01 | 163.78 | 0.21 | 0.999 |
| 30 | SC | 46,958 | 140 | 1.98 | 178.5 | 0.25 | 0.9989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, A.I.H.; Zhao, J.; Dong, Z.; Li, J.; Yuan, X.; Shao, T. Effect of Soybean Meal on Nutritional Content, Fermentation Profile, and Bacterial Community Structure of Napier Grass Silage. Agronomy 2025, 15, 2634. https://doi.org/10.3390/agronomy15112634
Mansoor AIH, Zhao J, Dong Z, Li J, Yuan X, Shao T. Effect of Soybean Meal on Nutritional Content, Fermentation Profile, and Bacterial Community Structure of Napier Grass Silage. Agronomy. 2025; 15(11):2634. https://doi.org/10.3390/agronomy15112634
Chicago/Turabian StyleMansoor, Abdelrahim I. H., Jie Zhao, Zhihao Dong, Junfeng Li, Xianjun Yuan, and Tao Shao. 2025. "Effect of Soybean Meal on Nutritional Content, Fermentation Profile, and Bacterial Community Structure of Napier Grass Silage" Agronomy 15, no. 11: 2634. https://doi.org/10.3390/agronomy15112634
APA StyleMansoor, A. I. H., Zhao, J., Dong, Z., Li, J., Yuan, X., & Shao, T. (2025). Effect of Soybean Meal on Nutritional Content, Fermentation Profile, and Bacterial Community Structure of Napier Grass Silage. Agronomy, 15(11), 2634. https://doi.org/10.3390/agronomy15112634








