Long-Term Nitrogen Addition Promotes Microbial Mineralization of Organic Phosphorus Supporting Phosphorus Uptake in Spring Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling and Analyses
Sampling Strategy Rationale
2.4. Soil DNA Extraction and High-Throughput Sequencing
2.5. Statistical Analyses
3. Results
3.1. Effects of Different N Addition Rates on Different Forms of P in Soil
3.2. Effects of Different N Addition Rates on Soil Enzyme Activity, Microbial Biomass, and Physicochemical Properties
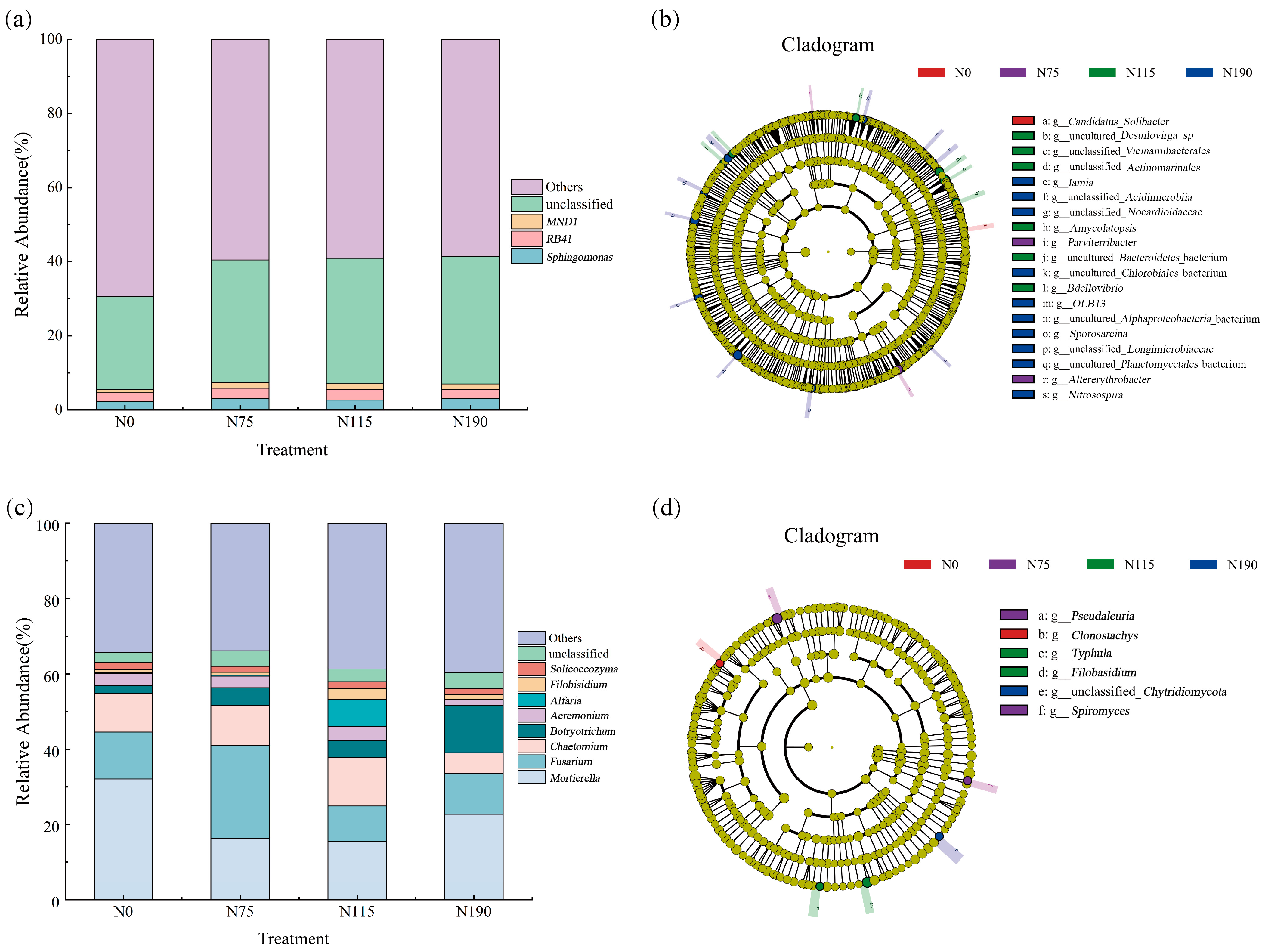
3.3. Effects of Different N Addition Rates on Microbial Community Structure
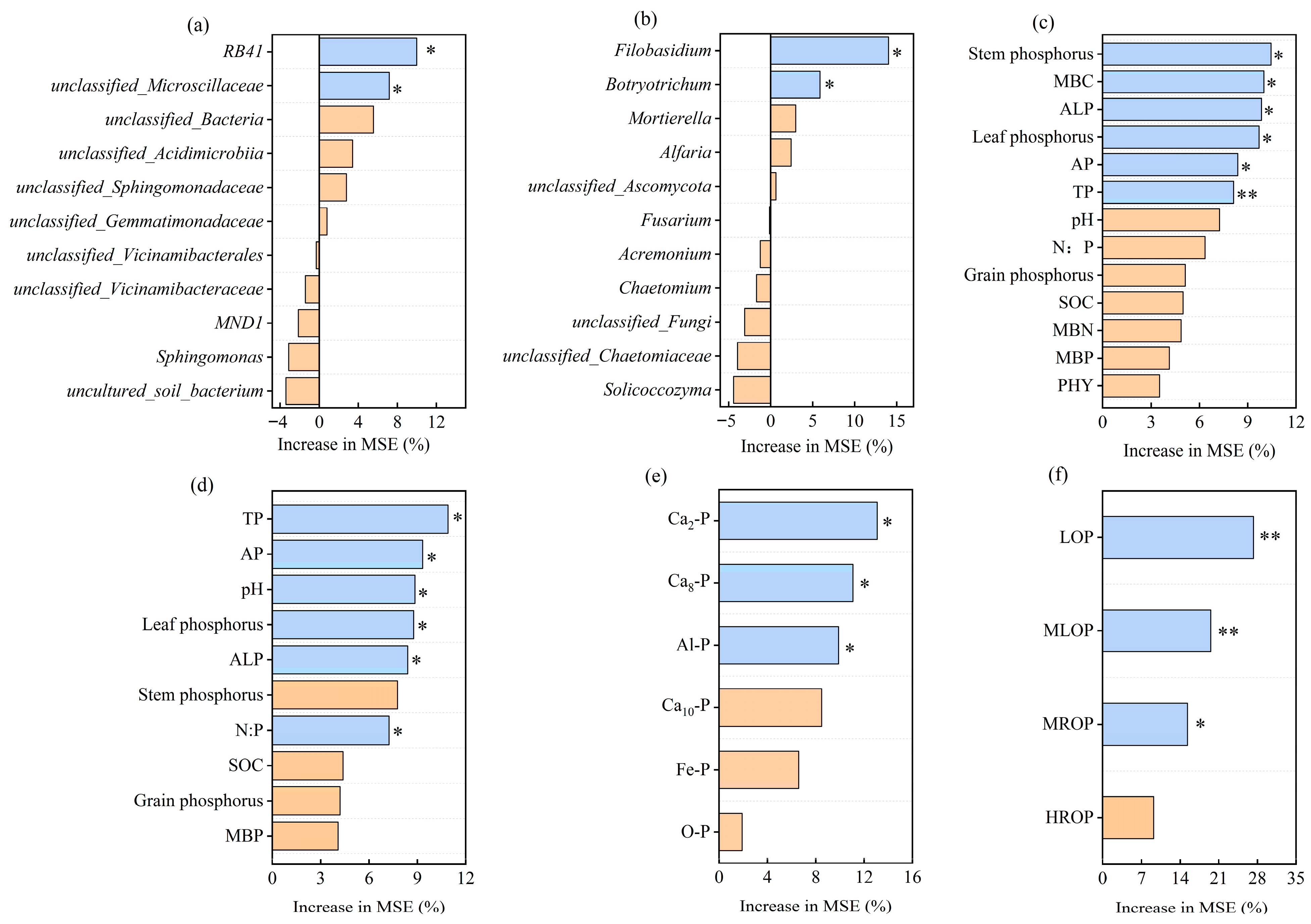
3.4. Correlations Between P Components and Soil Microbial Variables
4. Discussion
4.1. Effect of N Addition on Soil P Fractions
4.2. Effects of N Addition on Soil Microbial Variables
4.3. Mechanism of Microbial-Mediated Organic P Mineralization Under N Addition
5. Limitations and Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, R.E.; Craine, J.M.; Lany, N.K.; Jonard, M.; Ollinger, S.V.; Groffman, P.M.; Fulweiler, R.W.; Angerer, J.; Read, Q.D.; Reich, P.B.; et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 2022, 376, eabh3767. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Regus, J.U.; Wendlandt, C.E.; Bantay, R.M.; Gano-Cohen, K.A.; Gleason, N.J.; Hollowell, A.C.; O’Neill, M.R.; Shahin, K.K.; Sachs, J.L. Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 2017, 414, 159–170. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yan, Z.; Zhang, S.; Fan, B.; Cade-Menun, B.J.; Chen, Q. Nitrogen application favors soil organic phosphorus accumulation in calcareous vegetable fields. Biol. Fertil. Soils 2019, 55, 481–496. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef]
- Richards, S.; Marshall, R.; Lag-Brotons, A.J.; Semple, K.T.; Stutter, M. Phosphorus solubility changes following additions of bioenergy wastes to an agricultural soil: Implications for crop availability and environmental mobility. Geoderma 2021, 401, 115150. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest Soil. Glob. Change Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Change Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.X.; Zeng, X.M.; Lin, K.M.; Zhang, Q.F.; Cheng, L.; Zhou, J.C.; Lin, Q.Y.; Chen, Y.M.; Xu, J.G. Responses of soil phosphorus fractions and microorganisms to nitrogen application in a subtropical Phyllostachys pubescen forest. Ying Yong Sheng Tai Xue Bao 2020, 31, 753–760. [Google Scholar]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland Soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar]
- Jiao, Y.P.; Qi, P.; Wang, X.J.; Yao, Y.M.; Wu, J.; Cai, L.Q.; Zhang, R.Z. Effects of nitrogen and phosphorus fertilization on inorganic phosphorus forms of typical farmland soil in the dry farming area of the Loess Plateau. J. Plant Nutr. Fertil. 2020, 26, 1459–1472. [Google Scholar]
- Mirabello, M.J.; Yavitt, J.B.; Garcia, M.; Harms, K.E.; Turner, B.L.; Wright, S.J. Soil phosphorus responses to chronic nutrient fertilisation and seasonal drought in a humid lowland forest, Panama. Soil Res. 2013, 51, 215–221. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, W.; Delgado-Baquerizo, M.; Song, W.Z.; Ma, J.Y.; Wang, K.Y.; Ling, X.L. Cascading effects of N fertilization activate biologically driven mechanisms promoting P availability in a semi-arid grassland ecosystem. Funct. Ecol. 2021, 35, 1001–1011. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, H.; Niu, B.; Yin, L.N.; Wang, S.W.; Deng, X.P. Response of nitrogen fertilizer productivity of spring maize on the Loess Plateau to different tillage measures: A meta-analysis. Chin. J. Eco-Agric. 2024, 32, 755–765. [Google Scholar]
- Yamamoto, S.; Endo, T. Soils on the Loess Plateau. In Restoration and Development of the Degraded Loess Plateau, China; Tsunekawa, A., Liu, G., Yamanaka, N., Du, S., Eds.; Springer: Japan, Tokyo, 2014; pp. 35–47. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Yang, J.C.; Wang, Z.G.; Zhou, J.; Jiang, H.M.; Zhang, J.F.; Pan, P.; Han, Z.; Lu, C.; Li, L.L.; Ge, C.L. Inorganic phosphorus fractionation and its translocation dynamics in a low-P Soil. J. Environ. Radioact. 2012, 112, 64–69. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of Organic Phosphorus Substrates in Soils as Evaluated by NaHCO3 Extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.F.; McNeil, B.E.; Lovett, G.M.; Canham, C.D.; Driscoll, C.T.; Rustad, L.E.; Denny, E.; Hallett, R.A.; Arthur, M.A.; Boggs, J.L.; et al. Do Nutrient Limitation Patterns Shift from Nitrogen Toward Phosphorus with Increasing Nitrogen Deposition Across the Northeastern United States? Ecosystems 2012, 15, 940–957. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, F.; Yang, L.; Zhong, X.; Wang, M.; Zhou, J.; Chen, Y.; Yang, Y. Decreased soil organic P fraction associated with ectomycorrhizal fungal activity to meet increased P demand under N application in a subtropical forest ecosystem. Biol. Fertil. Soils 2018, 54, 149–161. [Google Scholar] [CrossRef]
- Nasto, M.K.; Alvarez-Clare, S.; Lekberg, Y.; Sullivan, B.W.; Townsend, A.R.; Cleveland, C.C. Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol. Lett. 2014, 17, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Blake, L.; Johnston, A.E.; Poulton, P.R.; Goulding, K.W.T. Changes in soil phosphorus fractions following positive and negative phosphorus balances for long periods. Plant Soil 2003, 254, 245–261. [Google Scholar] [CrossRef]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes Governing Phosphorus Availability in Temperate Soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphorus Forms and Concentrations in Leachate under Four Grassland Soil Types. Soil Sci. Soc. Am. J. 2000, 64, 1090–1099. [Google Scholar] [CrossRef]
- Hou, E.Q.; Tang, S.B.; Chen, C.R.; Kuang, Y.W.; Lu, X.K.; Heenan, M.; Wen, D.Z. Solubility of phosphorus in subtropical forest soils as influenced by low-molecular organic acids and key soil properties. Geoderma 2018, 313, 172–180. [Google Scholar] [CrossRef]
- Barrow, N.J. A mechanistic model for describing the sorption and desorption of phosphate by Soil. Eur. J. Soil Sci. 2015, 66, 9–18. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.-P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef]
- Petrović, D.; Szeler, K.; Kamerlin, S.C.L. Challenges and advances in the computational modeling of biological phosphate hydrolysis. Chem. Commun. 2018, 54, 3077–3089. [Google Scholar] [CrossRef]
- Zhang, W.N.; Yang, Z.; Yan, Y.P.; Wang, X.M.; Yin, H.; Xu, R.K.; Tan, W.F.; Feng, X.H. Research Progress on Soil Organic Phosphorus Mineralization and Its Regulation. Acta Pedol. Sin. 2025, 62, 334–347. [Google Scholar]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.S.; Hagedorn, F.; Penuelas, J.; Sardans, J.; Tan, X.P.; Yan, Z.B.; He, C.Q.; Ni, X.F.; Feng, Y.H.; Zhu, J.L.; et al. Differential Responses of Soil Phosphorus Fractions to Nitrogen and Phosphorus Fertilization: A Global Meta-Analysis. Glob. Biogeochem. Cycles 2024, 38, e2023GB008064. [Google Scholar] [CrossRef]
- Yu, Q.S.; Ma, S.H.; Ni, X.F.; Ni, X.L.; Guo, Z.M.; Tan, X.P.; Zhong, M.Y.; Abu Hanif, M.; Zhu, J.L.; Ji, C.J.; et al. Long-term phosphorus addition inhibits phosphorus transformations involved in soil arbuscular mycorrhizal fungi and acid phosphatase in two tropical rainforests. Geoderma 2022, 425, 116076. [Google Scholar] [CrossRef]
- Belinque, H.; Pucheu, N.; Kerber, N.; Rubio, G. Utilization of organic phosphorus sources by oilseed rape, sunflower, and soybean. J. Plant Nutr. Soil Sci. 2015, 178, 339–344. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, J.; Yu, H.; Wang, C.; Yang, Y.; Wang, J.; He, Z.; Yan, Q. Antimony efflux underpins phosphorus cycling and resistance of phosphate-solubilizing bacteria in mining soils. ISME J. 2023, 17, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Liang, W.; Tu, Z.N.; Li, R.H.; Zhang, Z.Q.; Ali, A.; Xiao, R. Succession of bacterial community during composting: Dissimilarity between compost mixture and biochar additive. Biochar 2021, 3, 229–237. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.R.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Lu, C.; Hong, Y.; Liu, J.; Gao, Y.; Ma, Z.; Yang, B.; Ling, W.; Waigi, M.G. A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut. 2019, 251, 773–782. [Google Scholar] [CrossRef]
- Liao, X.; Fang, W.; Lin, L.; Lu, H.L.; St Leger, R.J. Metarhizium robertsii produces an extracellular invertase (MrINV) that plays a pivotal role in rhizospheric interactions and root colonization. PLoS ONE 2013, 8, e78118. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil Phosphate Desorption Induced by a Phosphate-Solubilizing Fungus. Commun. Soil Sci. Plant Anal. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wu, X.H.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Wu, Z.X.; Hao, Z.P.; Sun, Y.Q.; Guo, L.P.; Huang, L.Q.; Zeng, Y.; Wang, Y.; Yang, L.; Chen, B.D. Comparison on the structure and function of the rhizosphere microbial community between healthy and root-rot Panax notoginseng. Appl. Soil Ecol. 2016, 107, 99–107. [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef]
- Samuelson, L.; Mathew, R.; Stokes, T.; Feng, Y.; Aubrey, D.; Coleman, M. Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For. Ecol. Manag. 2009, 258, 2431–2438. [Google Scholar] [CrossRef]
- Tu, L.H.; Chen, G.; Peng, Y.; Hu, H.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Soil Biochemical Responses to Nitrogen Addition in a Bamboo Forest. PLoS ONE 2014, 9, e102315. [Google Scholar] [CrossRef]
- Shen, R.-C.; Xu, M.; Chi, Y.-G.; Yu, S.; Wan, S.-Q. Soil Microbial Responses to Experimental Warming and Nitrogen Addition in a Temperate Steppe of Northern China. Pedosphere 2014, 24, 427–436. [Google Scholar] [CrossRef]
- He, R.Y.; Luo, Z.Z.; Li, L.L.; Niu, Y.N.; Zhang, Y.Q.; Li, L.L.; Liu, J.H.; Chen, Z.M. Long-Term Phosphate Addition Changes Soil P Accumulation via phoD-Harbouring Bacterial Community in Loess Plateau. Eur. J. Soil Sci. 2025, 76, e70067. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.; de Lima, J.M.; Olivares, F.L.; Moreira, F.M. Initial pH of medium affects organic acids production but do not affect phosphate solubilization. Braz. J. Microbiol. 2015, 46, 367–375. [Google Scholar] [CrossRef]
- Turner, B.L.; Driessen, J.P.; Haygarth, P.M.; McKelvie, I.D. Potential contribution of lysed bacterial cells to phosphorus solubilisation in two rewetted Australian pasture soils. Soil Biol. Biochem. 2003, 35, 187–189. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhou, Y.; Gu, Z.H.; Zhu, H.H.; Fu, S.L.; Yao, Q. The combined effects of cover crops and symbiotic microbes on phosphatase gene and organic phosphorus hydrolysis in subtropical orchard soils. Soil Biol. Biochem. 2015, 82, 119–126. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]



| Targets | N Addition | Time | N Addition × Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sig | F | p | Sig | F | p | Sig | F | p | |
| Ca2-P | *** | 64.437 | <0.001 | *** | 38.244 | <0.001 | ns | 2.341 | >0.05 |
| Ca8-P | * | 6.708 | <0.05 | ** | 30.425 | <0.01 | ** | 5.375 | <0.01 |
| Al-P | * | 4.991 | <0.05 | ** | 18.916 | <0.01 | *** | 6.292 | <0.001 |
| Fe-P | * | 8.854 | <0.05 | * | 9.154 | <0.05 | ** | 5.341 | <0.01 |
| Ca10-P | * | 6.659 | <0.05 | *** | 122.559 | <0.001 | *** | 15.416 | <0.001 |
| O-P | * | 5.756 | <0.05 | ns | 4.473 | >0.05 | *** | 14.603 | <0.001 |
| LOP | ** | 26.736 | <0.01 | *** | 39.910 | <0.001 | ** | 5.532 | <0.01 |
| MLOP | ** | 12.163 | <0.01 | ** | 11.878 | <0.01 | *** | 10.764 | <0.001 |
| MROP | *** | 76.868 | <0.001 | *** | 221.853 | <0.001 | ns | 1.331 | >0.05 |
| HROP | *** | 49.179 | <0.001 | *** | 37.794 | <0.001 | * | 2.632 | <0.05 |
| TP | ** | 14.838 | <0.01 | *** | 91.185 | <0.001 | *** | 62.549 | <0.001 |
| AP | * | 5.318 | <0.05 | ns | 0.840 | >0.05 | *** | 30.586 | <0.001 |
| Total inorganic P | *** | 150.182 | <0.001 | *** | 232.688 | <0.001 | *** | 12.238 | <0.001 |
| Total organic P | *** | 112.130 | <0.001 | *** | 214.667 | <0.001 | *** | 10.219 | <0.001 |
| Total inorganic P/Total organic P | *** | 36.637 | <0.001 | *** | 68.854 | <0.001 | *** | 19.654 | <0.001 |
| Year | Treatment | Alkaline Phosphatase /(mg g−1 24 h−1) | Phytase /(U g−1) | Microbial Biomass Carbon /(mg kg−1) | Microbial Biomass Nitrogen /(mg kg−1) | Microbial Biomass Phosphorus /(mg kg−1) |
|---|---|---|---|---|---|---|
| 2019 | N0 | 1.08 ± 0.01 c | 1.01 ± 0.38 c | 231.10 ± 5.88 c | 31.61 ± 1.19 c | 7.55 ± 0.56 c |
| N75 | 1.11 ± 0.04 c | 1.18 ± 0.03 b | 242.36 ± 6.75 b | 35.61 ± 0.93 ab | 12.12 ± 0.90 a | |
| N115 | 1.42 ± 0.02 a | 1.39 ± 0.07 a | 255.82 ± 3.88 a | 37.45 ± 1.12 a | 13.43 ± 0.49 a | |
| N190 | 1.33 ± 0.03 b | 1.06 ± 0.03 c | 246.33 ± 3.57 ab | 33.87 ± 0.22 bc | 10.78 ± 0.78 b | |
| 2020 | N0 | 1.57 ± 0.038 c | 1.08 ± 0.03 c | 208.67 ± 10.50 c | 45.15 ± 3.61 c | 15.03 ± 1.02 c |
| N75 | 1.63 ± 0.03 c | 1.15 ± 0.02 b | 227.04 ± 2.01 b | 53.25 ± 2.65 b | 23.17 ± 1.27 a | |
| N115 | 2.12 ± 0.02 a | 1.42 ± 0.06 a | 243.67 ± 2.52 a | 59.67 ± 3.06 a | 24.21 ± 1.87 a | |
| N190 | 1.70 ± 0.23 b | 1.12 ± 0.01 bc | 235.08 ± 5.57 ab | 54.65 ± 2.01 ab | 19.58 ± 0.27 b | |
| 2023 | N0 | 0.95 ± 0.44 c | 1.09 ± 0.02 c | 197.20 ± 7.80 c | 48.82 ± 0.36 c | 11.63 ± 0.38 c |
| N75 | 1.07 ± 0.64 c | 1.27 ± 0.05 b | 227.97 ± 6.57 b | 51.83 ± 0.34 b | 14.75 ± 0.33 a | |
| N115 | 1.51 ± 0.10 a | 1.36 ± 0.03 a | 247.43 ± 2.64 a | 53.26 ± 0.37 a | 15.26 ± 0.29 a | |
| N190 | 1.30 ± 0.60 b | 1.15 ± 0.03 c | 238.60 ± 2.66 a | 51.35 ± 0.27 b | 12.86 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qi, P.; Yin, X.; Wang, X.; Gan, R.; Xue, J.; Han, Y.; Lu, M.; Liang, G.; Li, H. Long-Term Nitrogen Addition Promotes Microbial Mineralization of Organic Phosphorus Supporting Phosphorus Uptake in Spring Wheat. Agronomy 2025, 15, 2632. https://doi.org/10.3390/agronomy15112632
Li H, Qi P, Yin X, Wang X, Gan R, Xue J, Han Y, Lu M, Liang G, Li H. Long-Term Nitrogen Addition Promotes Microbial Mineralization of Organic Phosphorus Supporting Phosphorus Uptake in Spring Wheat. Agronomy. 2025; 15(11):2632. https://doi.org/10.3390/agronomy15112632
Chicago/Turabian StyleLi, Huaqiang, Peng Qi, Xiaodong Yin, Xiaojiao Wang, Run Gan, Jianglong Xue, Yangzi Han, Meixia Lu, Guopeng Liang, and Hailiang Li. 2025. "Long-Term Nitrogen Addition Promotes Microbial Mineralization of Organic Phosphorus Supporting Phosphorus Uptake in Spring Wheat" Agronomy 15, no. 11: 2632. https://doi.org/10.3390/agronomy15112632
APA StyleLi, H., Qi, P., Yin, X., Wang, X., Gan, R., Xue, J., Han, Y., Lu, M., Liang, G., & Li, H. (2025). Long-Term Nitrogen Addition Promotes Microbial Mineralization of Organic Phosphorus Supporting Phosphorus Uptake in Spring Wheat. Agronomy, 15(11), 2632. https://doi.org/10.3390/agronomy15112632







