Electrophysiological Insights into the Adaptability of Bletilla striata to Bicarbonate Stress in Karst Habitats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Bicarbonate Treatment and Concentration Maintenance
2.3. Chlorophyll Content and Biomass
2.4. Photosynthesis
2.5. Intrinsic Electrophysiological Parameters of B. striata Leaves
2.6. Intracellular Water Use Dynamics
2.7. Characterization of Nutrient Translocation Capacity
2.8. Metabolic Activity
2.9. Inherent Conduction Capacity and Inherent Conduction Resistance
2.10. Statistical Analysis
3. Results
3.1. Influence of HCO3− on Growth Characteristics and Chlorophyll Content of B. striata
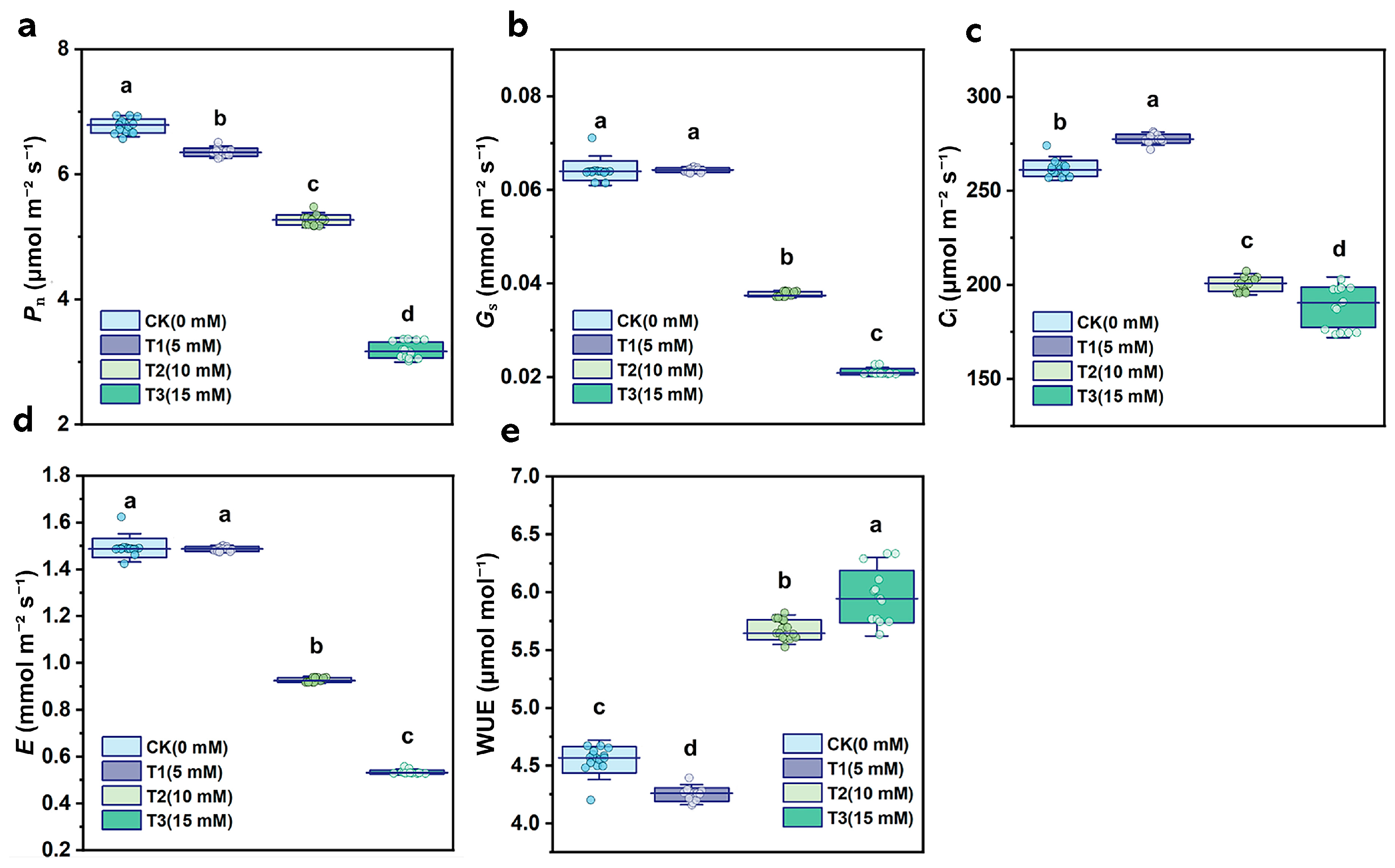
3.2. Influence of HCO3− on Photosynthetic Characteristics of B. striata
3.3. Influence of HCO3− on Electrophysiological Parameters of B. striata
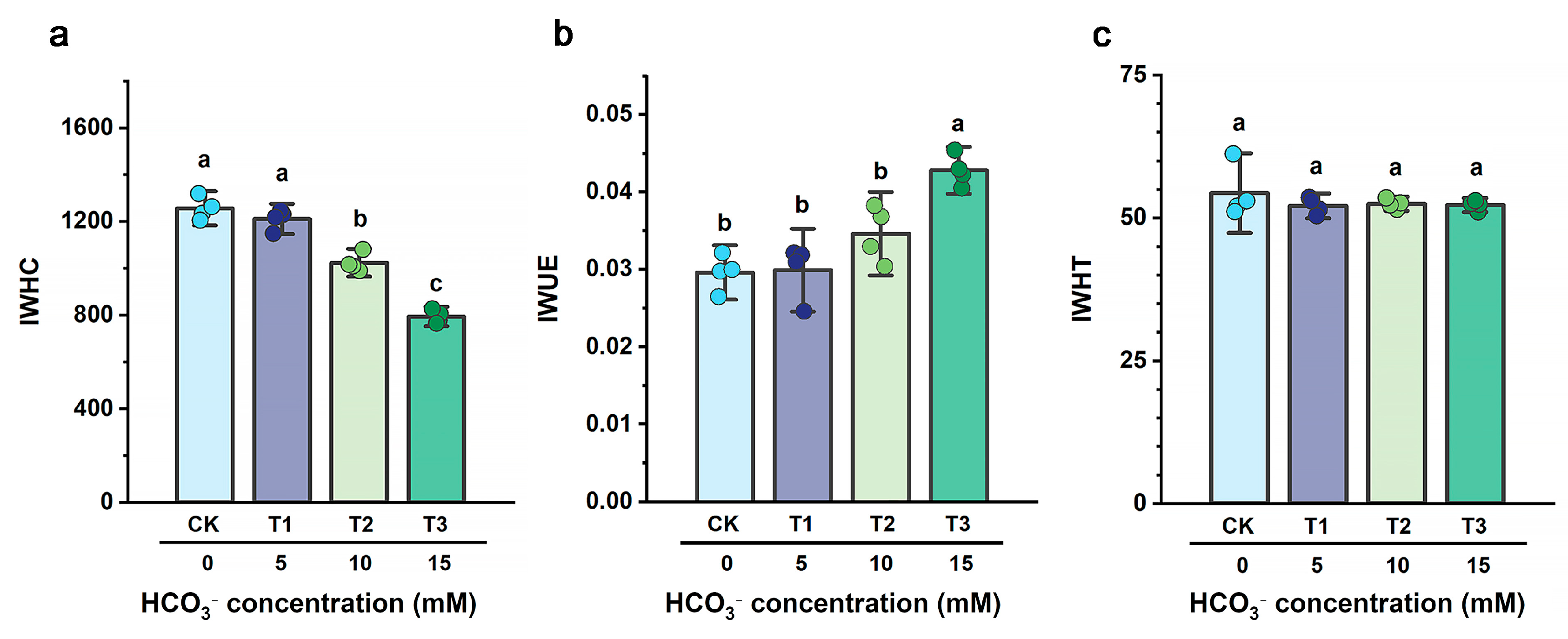
3.4. Influence of HCO3− on Intracellular Water Metabolism Capacity of B. striata
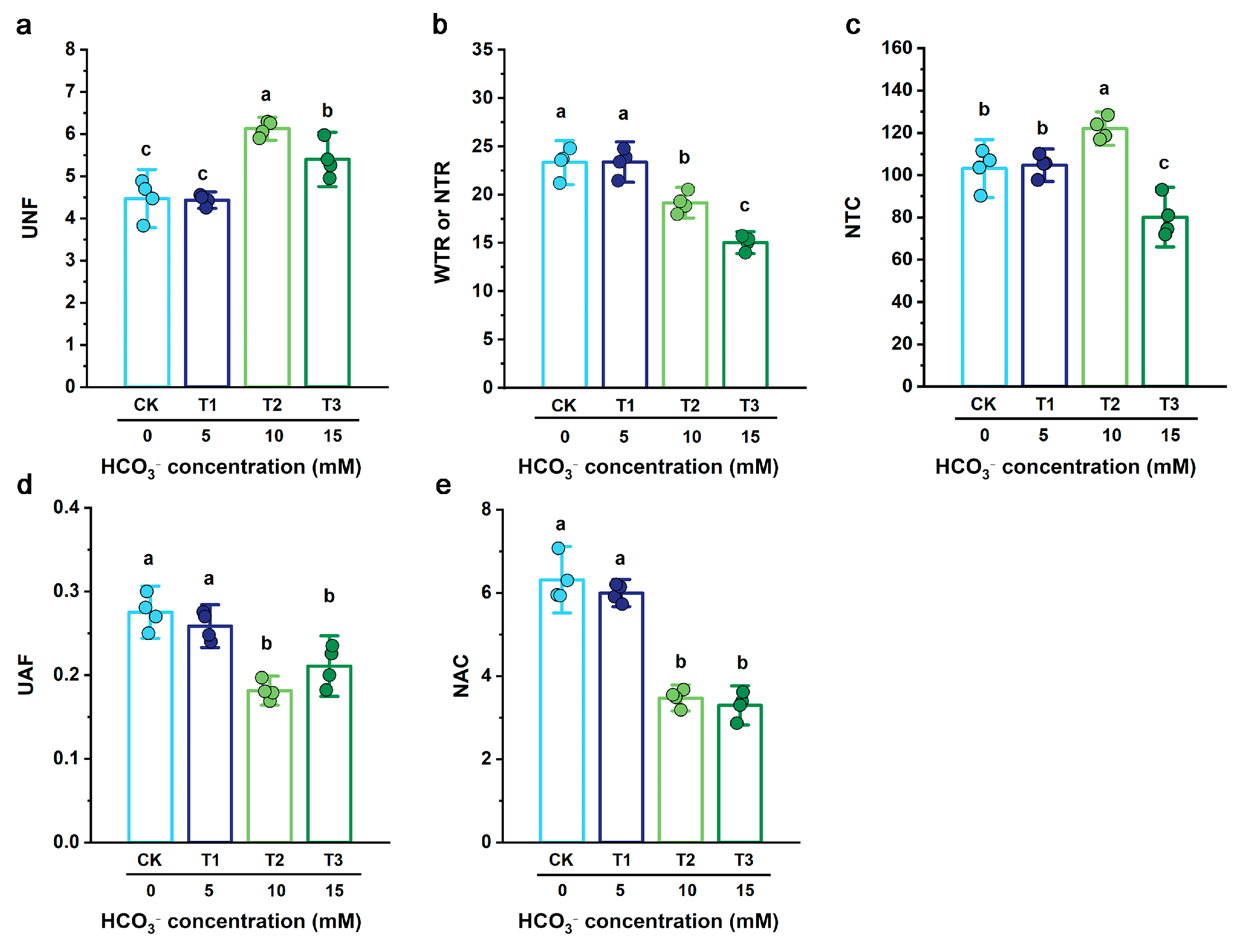
3.5. Influence of HCO3− on Nutrient Transport Dynamics of B. striata
3.6. Influence of HCO3− on Metabolic Indices of B. striata
3.7. Influence of HCO3− on Inherent Conduction Capacity and Inherent Conduction Resistance of B. striata
4. Discussion
4.1. The Effects of Bicarbonate Ions on the Growth and Photosynthesis of B. striata
4.2. Mechanism of HCO3− on Intracellular Water, Nutrient Translocation, and Metabolic Processes in B. striata
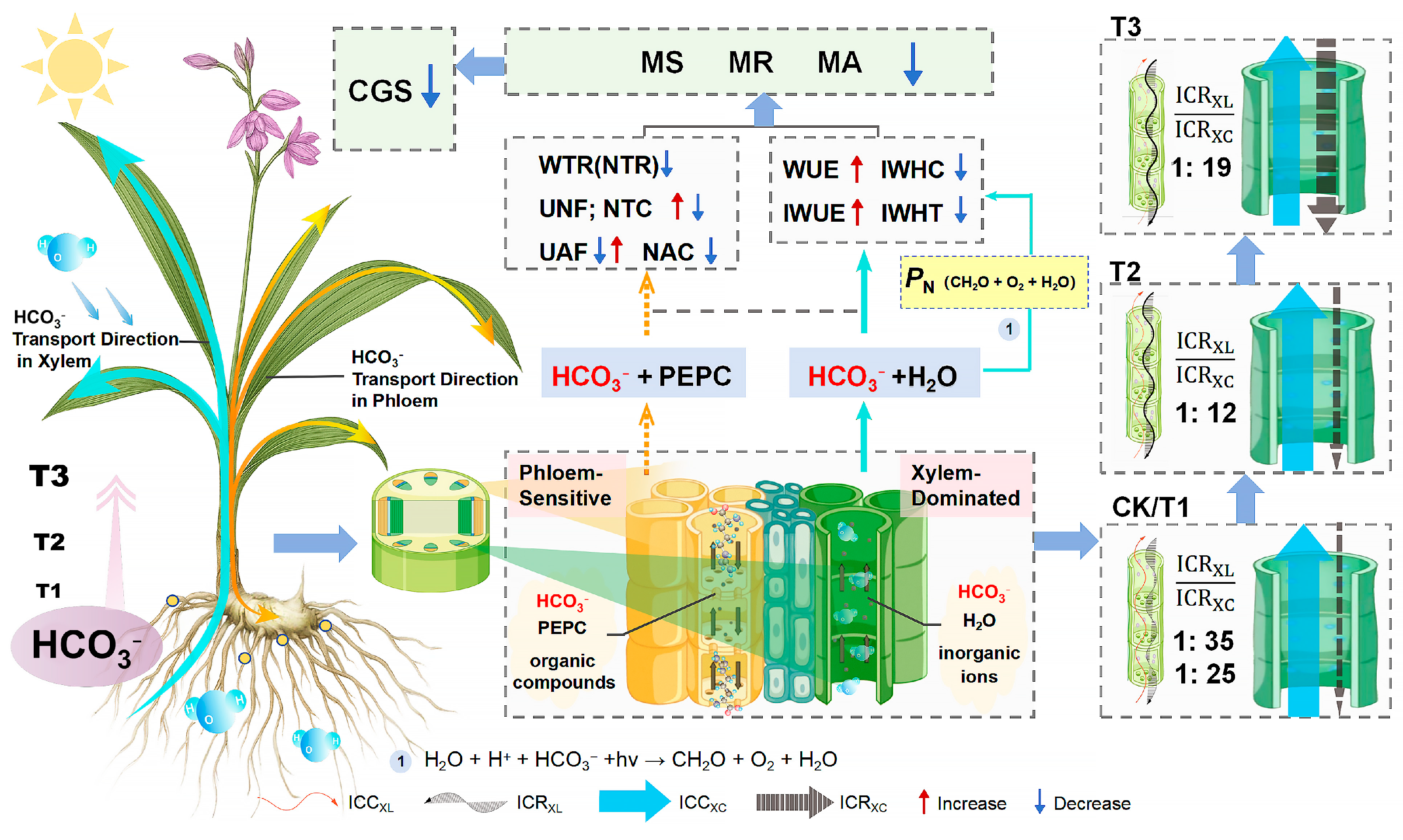
4.2.1. Electrophysiological Responses of B. striata to HCO3− Stress
4.2.2. Coupled Mechanisms of Water, Nutrient, and Metabolic Regulation in B. striata Under HCO3− Stress
4.3. Xylem-Dominated Transport of Bicarbonate in B. striata, Phloem Exhibits Greater Sensitivity but an Increased Contribution to HCO3− Translocation
4.4. Karst Adaptability and Stress-Enhanced Quality Formation in Saddle-Type B. striata
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Capacitance |
| R | Resistance |
| Z | Impedance |
| XC | Capacitive reactance |
| XL | Inductive reactance |
| IR | Intrinsic resistance |
| IZ | Intrinsic impedance |
| IXC | Intrinsic capacitive reactance |
| IXL | Intrinsic inductive reactance |
| ICP | Intrinsic capacitance |
| IWHC | Intracellular water holding capacity |
| IWHT | Intracellular water holding time |
| IWUE | Intracellular water use efficiency |
| WTR | Water transfer rate |
| UNF | Nutrient flux per unit area |
| UAF | Active transport flow of nutrient |
| NTC | Nutrient translocation capacity |
| NAC | Nutrient active translocation capacity |
| NTR | Nutrient translocation rate |
| MF | Metabolic flow |
| MS | Metabolic strength |
| MR | Metabolic rate |
| MA | Metabolic activity |
| GCS | Growth comprehensive score |
| ICCR | Inherent conduction capacity based on R |
| ICCZ | Inherent conduction capacity based on Z |
| ICCXC | Inherent conduction capacity based on Xc |
| ICCXL | Inherent conduction capacity based on XL |
| ICRR | Inherent conduction resistance based on R |
| ICRZ | Inherent conduction resistance based on Z |
| ICRXC | Inherent conduction resistance based on XC |
| ICRXL | Inherent conduction resistance based on XL |
| rICCXC | Relative inherent conduction capacity based on XC (normalized to CK = 1) |
| rICCXL | Relative inherent conduction capacity based on XL (normalized to CK = 1) |
| rICRXC | Relative inherent conduction resistance based on XC (normalized to CK = 1) |
| rICRXL | Relative inherent conduction resistance based on XL (normalized to CK = 1) |
| H+ | Hydrogen ion |
| ATP | Adenosine triphosphate |
| Pn | Net photosynthetic rate |
| GS | Stomatal conductance |
| Ci | Intercellular CO2 concentration |
| E | Transpiration rate |
| WUE | water use efficiency |
Appendix A
| Macroelement | Quantity of Matter (mM) |
|---|---|
| KNO3 | 6 |
| NH4Cl | 0.75 |
| NH4H2PO4 | 0.25 |
| Ca(NO3)2•4H2O | 4 |
| MgSO4•7H2O | 2 |
| A trace element | |
| KCl | 2 |
| H3BO3 | 50 |
| CuSO4•5H2O | 0.2 |
| ZnSO4•7H2O | 4 |
| MnSO4•4H2O | 4 |
| (NH4)6Mo7O24•4H2O | 0.2 |
| Carnallite | |
| Fe(Na)EDTA | 2 |
References
- Liu, Z.H.; Dreybrodt, W.; Wang, H.J. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- He, H.B.; Liu, Z.H.; Li, D.L.; Liu, X.; Han, Y.Q.; Sun, H.L.; Zhao, M.; Shao, M.Y.; Shi, L.X.; Hao, P.Y.; et al. Effects of carbon limitation and carbon fertilization on karst lake-reservoir productivity. Water Res. 2024, 261, 122036. [Google Scholar] [CrossRef]
- Li, D.J.; Zhang, Q.S.; Xiao, K.C.; Wang, Z.C.; Wang, K.L. Divergent responses of biological nitrogen fixation in soil, litter and moss to temperature and moisture in a karst forest, southwest China. Soil Biol. Biochem. 2018, 118, 1–7. [Google Scholar] [CrossRef]
- Qu, R.; Han, G.L. Effects of high Ca and Mg stress on plants water use efficiency in a karst ecosystem. PeerJ 2022, 10, e13925. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.H.; Wei, S.H.; Ji, L.; Yan, S.P. A potential CO2 carrier to improve the utilization of HCO3− by plant-soil ecosystem for carbon sink enhancement. J. Adv. Res. 2025, 73, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 2009; p. 210. [Google Scholar]
- He, X.R.; Wang, X.X.; Fang, J.C.; Zhao, Z.F.; Huang, L.H.; Guo, H.; Zheng, X.H. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017, 195, 20–38. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, C.H.; Wu, Y.; Wang, B.; Kui, M.H.; Xu, J.; Ma, H.S.; Li, J.P.; Zeng, J.S.; Gao, W.H.; et al. Protective effects of degraded Bletilla striata polysaccharides against UVB-induced oxidative stress in skin. Int. J. Biol. Macromol. 2024, 277, 134462. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, L.; Gu, F.; Huang, R.; Liu, L.; Nian, Y.; Zhang, Y.; Song, C. Exploration of the anti-inflammatory, analgesic, and wound healing activities of Bletilla striata polysaccharide. Int. J. Biol. Macromol. 2024, 261, 129874. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, Z.Z.; Wen, Y.J.; Zhou, J.; Zhou, W.H.; Mao, J.; Lv, K.; Cao, Y.P.; Jiang, L. The synergistic gelation of novel Bletilla striata polysaccharide with hyaluronic acid: Characterization, rheology. Food Chem. 2025, 467, 142359. [Google Scholar] [CrossRef]
- Wu, M.K. Research on Ecological Cultivation Techniques of Bletilla striata; Science Press: Beijing, China, 2018. [Google Scholar]
- Zhang, J.X.; Yang, L.L.; Liu, H.; Luo, M.; Song, Z.Q.; Wu, M.K. A new cultivar of Bletilla striata ‘Guiji No.1’. Acta Hortic. Sin. 2022, 49, 203–204. [Google Scholar] [CrossRef]
- Valipour, M.; Khoshgoftarmanesh, A.H.; Baninasab, B. Physiological responses of hawthorn (Crataegus persica Pojark.) and quince (Cydonia oblonga Mill.) rootstocks to bicarbonate-induced iron deficiency in nutrient solution. J. Plant Nutr. Soil Sci. 2018, 181, 905–913. [Google Scholar] [CrossRef]
- Sagervanshi, A.; Naeem, A.; Kaiser, H.; Pitann, B.; Mühling, K.H. Early growth reduction in Vicia faba L. under alkali salt stress is mainly caused by excess bicarbonate and related to citrate and malate over accumulation. Environ. Exp. Bot. 2021, 192, 104636. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Chen, Y.L.; Liu, S.Z.; Li, F.; Sun, M.D.; Liang, Z.X.; Sun, Z.; Yu, F.T.; Rengel, Z.; Li, H.G. Bicarbonate rather than high pH in growth medium induced Fe-deficiency chlorosis in dwarfing rootstock quince A (Cydonia oblonga Mill.) but did not impair Fe nutrition of vigorous rootstock Pyrus betulifolia. Front. Plant Sci. 2023, 14, 1237327. [Google Scholar] [CrossRef]
- Liu, X.J.; Hu, B.; Chu, C.C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Tang, C.; Li, H.; Xia, H.; Fan, S.; Kong, L. Bicarbonate-dependent detoxification by mitigating ammonium-induced hypoxic stress in Triticum aestivum root. Biology 2024, 13, 101. [Google Scholar] [CrossRef]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.S.; Xu, J.L. Saline-alkali tolerance in rice: Physiological response, molecular mechanism, and QTL identification and application to breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Cao, X.; Wu, C.; Wei, X.; Jiao, P.; Liu, S.; Ma, Y.; Guan, S. Unraveling saline-alkali stress tolerance: Contrasting morpho-physiological, biochemical, and ionic responses in maize (Zea mays L.) genotypes. Plant Physiol. Biochem. 2025, 229, 110349. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef]
- Dąbrowska-Bronk, J.; Komar, D.N.; Rusaczonek, A.; Kozłowska-Makulska, A.; Szechyńska-Hebda, M.; Karpiński, S. β-Carbonic anhydrases and carbonic ions uptake positively influence Arabidopsis photosynthesis, oxidative stress tolerance and growth in light dependent manner. J. Plant Physiol. 2016, 203, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Gamarra Reinoso, L.; Majláth, I.; Dernovics, M.; Fábián, A.; Jose, J.; Jampoh, E.A.; Hamow, K.Á.; Soós, V.; Sági, L.; Éva, C. Root-based inorganic carbon uptake increases the growth of Arabidopsis thaliana and changes transporter expression and nitrogen and sulfur metabolism. Front. Plant Sci. 2024, 15, 1448432. [Google Scholar] [CrossRef] [PubMed]
- Dolui, D.; Saha, I.; Sarkar, B.; Ghosh, A.; Adak, M.K. Bicarbonate toxicity and elevated pH in plants: Metabolism, regulation and tolerance. In Plant Stress Physiology; Springer: Singapore, 2021; pp. 87–115. [Google Scholar] [CrossRef]
- Feng, X.L.; Liu, R.; Li, C.J.; Zhang, H.; Slot, M. Contrasting responses of two C4 desert shrubs to drought but consistent decoupling of photosynthesis and stomatal conductance at high temperature. Environ. Exp. Bot. 2023, 209, 105295. [Google Scholar] [CrossRef]
- Misra, B.B.; Yin, Z.; Geng, S.; de Armas, E.; Chen, S. Metabolomic responses of Arabidopsis suspension cells to bicarbonate under light and dark conditions. Sci. Rep. 2016, 6, 35778. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Rao, S. Root-Derived Bicarbonate Assimilation in Plants; Springer Nature: Singapore, 2023. [Google Scholar] [CrossRef]
- Wegner, L.H.; Zimmermann, U. Bicarbonate-induced alkalinization of the xylem sap in intact maize seedlings as measured in situ with a novel xylem pH probe. Plant Physiol. 2004, 136, 3469–3477. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, M.; Knoblauch, J.; Mullendore, D.L.; Savage, J.A.; Babst, B.A.; Beecher, S.D.; Dodgen, A.C.; Jensen, K.H.; Holbrook, N.M. Testing the Münch hypothesis of long distance phloem transport in plants. eLife 2016, 5, e15341. [Google Scholar] [CrossRef] [PubMed]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Hedrich, R.; Salvador-Recatalà, V.; Dreyer, I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, D. Inaugural Editorial for the Journal of Plant Electrobiology. J. Plant Electrobiol. 2025, 1, 1–6. [Google Scholar] [CrossRef]
- Coatsworth, P.; Cotur, Y.; Naik, A.; Asfour, T.; Collins, A.S.; Olenik, S.; Zhou, Z.; Gonzalez-Macia, L.; Chao, D.Y.; Bozkurt, T.; et al. Time-resolved chemical monitoring of whole plant roots with printed electrochemical sensors and machine learning. Sci. Adv. 2024, 10, eadj6315. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Li, H.; Fang, L.; Xing, D.K. Plant’s electrophysiological information manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Y.Y.; Xing, D.K. The differential response of intracellular water metabolism derived from intrinsic electrophysiological information in Morus alba L. and Broussonetia papyrifera (L.) Vent. subjected to water shortage. Horticulturae 2022, 8, 182. [Google Scholar] [CrossRef]
- Xia, A.T.; Wu, Y.Y.; Zhai, K.; Xiang, D.S.; Li, L.; Qin, Z.H.; Twagirayezu, G. Plant electrophysiological parameters represent leaf intracellular water–nutrient metabolism and immunoregulations in Brassica rapa during Plasmodiophora infection. Plants 2025, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Wang, Z.L.; Lu, X.N.; Dai, L.Y.; Tang, C.L.; Hang, Y.; Tian, B. Physiological and electrophysiological characteristics of continuously cropped Pinellia ternata and a novel electrical information-based monitoring approach for its cropping obstacles. Front. Plant Sci. 2025, 16, 1614478. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.Y.; Xing, D.K.; Zhang, Q.; Quispe Puma, M.R.; Chen, Q. Dynamic traits of intracellular water and salt based on electrophysiological measurements during adaptations of three mangrove species under salinity stresses. Horticulturae 2025, 11, 309. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, C.; Tian, B.; Han, L.; Liu, D.D.; Li, G.; Gui, S.H.; Smagghe, G.; Chen, X.S.; Wu, X.M. Consortia of Bacillus sp. LY05 and Bacillus cereus LGY06 immobilized on coconut shell charcoal remediates pendimethalin and cadmium contaminated sites in situ and alleviates peanut’s continuous cropping obstacles. J. Hazard. Mater. 2025, 492, 138168. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, H.B.; Li, Y.Y.; Zhang, S.Q. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Li, M.H.; Wu, M.J.; Xing, D.K.; Liu, Y.J.; Yao, X.P.; Yu, R.; Xu, X.J.; Mao, J.P. A Method and Device for Determining Plant Leaf Physiological Electrical Parameters, Leaf Water Retention Capacity and Transport Capacity. China Patent CN109655496B, 10 September 2021. [Google Scholar]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Mengel, K. Iron availability in plant tissues—Iron chlorosis on calcareous soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, H.; Tang, Z.H.; Zu, Y.G.; Liu, Y. High NaHCO3 stress causes direct injury to Nicotiana tabacum roots. J. Plant Interact. 2013, 9, 56–61. [Google Scholar] [CrossRef]
- Nie, W.J.; He, Q.H.; Ma, J.Z.; Guo, H.G.; Shi, Q.H. Exogenous 2,4-Epibrassinolide alleviates alkaline stress in cucumber by modulating photosynthetic performance. Plants 2025, 14, 54. [Google Scholar] [CrossRef]
- Guerrieri, R.; Belmecheri, S.; Ollinger, S.V.; Asbjornsen, H.; Jennings, K.; Xiao, J.; Stocker, B.D.; Martin, M.; Hollinger, D.Y.; Bracho-Garrillo, R.; et al. Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 16909–16914. [Google Scholar] [CrossRef]
- Broeckx, L.S.; Fichot, R.; Verlinden, M.S.; Ceulemans, R. Seasonal variations in photosynthesis, intrinsic water-use efficiency and stable isotope composition of poplar leaves in a short-rotation plantation. Tree Physiol. 2014, 34, 701–715. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.Q. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef]
- Jia, B.; Cui, H.L.; Zhang, D.J.; Hu, B.S.; Li, Y.; Shen, Y.; Cai, X.X.; Sun, X.L.; Sun, M.Z. The conserved evolution of plant H+-ATPase family and the involvement of soybean H+-ATPases in sodium bicarbonate stress responses. Plant Physiol. Biochem. 2023, 204, 108133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Lv, J.M.; Su, Y.; Wu, Y.Y. Appropriate sodium bicarbonate concentration enhances the intracellular water metabolism, nutrient transport and photosynthesis capacities of Coix lacryma-jobi L. Agronomy 2023, 13, 1790. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lucena, C.; Romera, F.J.; Rojas, C.L.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of Strategy I plants. Funct. Plant Biol. 2007, 34, 1002–1009. [Google Scholar] [CrossRef]
- Guo, S.H.; Niu, Y.J.; Zhai, H.; Han, N.; Du, Y.P. Effects of alkaline stress on organic acid metabolism in roots of grape hybrid rootstocks. Sci. Hortic. 2018, 227, 255–260. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.F.; Zhang, X.Y.; Li, S.G. The strategies of water–carbon regulation of plants in a subtropical primary forest on karst soils in China. Biogeosciences 2018, 15, 4193–4203. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Gortan, E.; Nardini, A.; Salleo, S.; Jansen, S. Pit membrane chemistry influences the magnitude of ion-mediated enhancement of xylem hydraulic conductance in four Lauraceae species. Tree Physiol. 2011, 31, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Kumar, R.; Boedi Iswanto, A.B.; Kim, J.Y. Callose balancing at plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef]
- Giaquinta, R. Phloem loading of sucrose: pH dependence and selectivity. Plant Physiol. 1977, 59, 750–755. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Wang, D.; Dong, Y.; Li, J.; Lu, F.; Tao, W.; Guo, Y.; Xiang, W.; Wen, M.; et al. Trade-off strategies between drought resistance and growth rate of dominant tree species in karst forests within heterogeneous habitats. Sci. Rep. 2025, 15, 26381. [Google Scholar] [CrossRef]


| Treatment | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| Root (FW, g/Plant) | 3.59 ± 0.40 a | 3.44 ± 0.46 a | 2.69 ± 0.22 b | 2.39 ± 0.32 b |
| Stem (FW, g/Plant) | 6.12 ± 0.44 a | 5.91 ± 0.85 ab | 5.41 ± 0.66 bc | 4.83 ± 0.72 c |
| Leaves (FW, g/Plant) | 2.64 ± 0.47 a | 2.59 ± 0.30 a | 2.17 ± 0.51 b | 1.71 ± 0.33 c |
| Total Biomass (FW, g/Plant) | 12.35 ± 0.41 a | 11.94 ± 0.41 a | 10.27 ± 0.25 b | 8.94 ± 0.71 c |
| Treatment | IC (pF) | IR (MΩ) | IZ (MΩ) | IXC (MΩ) | IXL (MΩ) |
|---|---|---|---|---|---|
| CK (0 mM) | 44.38 ± 3.16 a | 4.18 ± 0.43 c | 1.24 ± 0.05 c | 1.20 ± 0.08 c | 4.35 ± 0.48 b |
| T1 (5 mM) | 42.03 ± 2.72 a | 4.45 ± 0.38 c | 1.24 ± 0.12 c | 1.27 ± 0.08 c | 4.98 ± 0.14 b |
| T2 (10 mM) | 32.90 ± 2.30 b | 8.53 ± 0.57 b | 1.60 ± 0.11 b | 1.62 ± 0.11 b | 9.20 ± 0.87 a |
| T3 (15 mM) | 22.77 ± 1.05 c | 10.35 ± 0.81 a | 2.29 ± 0.09 a | 2.33 ± 0.11 a | 10.99 ± 1.20 a |
| Treatment | MS | MR | MA | GCS |
|---|---|---|---|---|
| CK (0 mM) | 8.22 ± 0.18 a | 144.59 ± 20.54 a | 9.02 ± 0.35 a | 89.09 ± 4.26 a |
| T1 (5 mM) | 7.97 ± 0.24 a | 138.55 ± 12.81 a | 8.58 ± 0.47 a | 87.2 ± 4.36 a |
| T2 (10 mM) | 6.21 ± 0.30 b | 67.61 ± 7.69 b | 5.68 ± 0.39 b | 78.04 ± 5.20 b |
| T3 (15 mM) | 5.11 ± 0.14 c | 50.65 ± 5.29 c | 4.51 ± 0.18 c | 62.89 ± 3.18 c |
| Treatment | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| ICCXc/ICCXL | 453.66% | 432.26% | 401.16% | 422.18% |
| ICRXc/ICRXL | 35.01% | 25.32% | 12.30% | 19.01% |
| rICCXc | 1 | 94.33% | 73.34% | 62.91% |
| rICCXL | 1 | 99.00% | 82.93% | 67.61% |
| rICRXc | 1 | 104.69% | 132.29% | 235.85% |
| rICRXL | 1 | 144.78% | 376.49% | 434.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wu, Y.; Meng, H.; Xiao, J.; Wu, M.; Wang, Z. Electrophysiological Insights into the Adaptability of Bletilla striata to Bicarbonate Stress in Karst Habitats. Agronomy 2025, 15, 2628. https://doi.org/10.3390/agronomy15112628
Zhang J, Wu Y, Meng H, Xiao J, Wu M, Wang Z. Electrophysiological Insights into the Adaptability of Bletilla striata to Bicarbonate Stress in Karst Habitats. Agronomy. 2025; 15(11):2628. https://doi.org/10.3390/agronomy15112628
Chicago/Turabian StyleZhang, Juke, Yanyou Wu, Hanqing Meng, Juyue Xiao, Mingkai Wu, and Ziyang Wang. 2025. "Electrophysiological Insights into the Adaptability of Bletilla striata to Bicarbonate Stress in Karst Habitats" Agronomy 15, no. 11: 2628. https://doi.org/10.3390/agronomy15112628
APA StyleZhang, J., Wu, Y., Meng, H., Xiao, J., Wu, M., & Wang, Z. (2025). Electrophysiological Insights into the Adaptability of Bletilla striata to Bicarbonate Stress in Karst Habitats. Agronomy, 15(11), 2628. https://doi.org/10.3390/agronomy15112628







