Application of Nanostructured Semiconductor Oxides TiO2-Based as Additives in the Germination Process of Alfalfa
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis of TiO2 and Ag-TiO2 by Sol–Gel Method and Physicochemical Material’s Characterization
2.2. Germination Experiments
Germination Percentage
2.3. Metabolites Quantification
2.3.1. Quantification of Total Flavonoids
2.3.2. Quantification of Total Phenol
2.4. Antioxidant Capacity
2.4.1. Antioxidant Capacity Determination by DPPH (2,2-Diphenyl-1-picrylhydrazyl)
2.4.2. Determination of Antioxidant Capacity by ABTS (2,2-Azino-bis-3-ethylthiazoline benzenesulfonic acid-6)
2.5. Chlorophyll Index Measurements
2.6. Data Analysis
3. Results
3.1. Physicochemical Characterization of TiO2 and Ag-TiO2 NPs
3.2. Physical Assessment on Sprouts
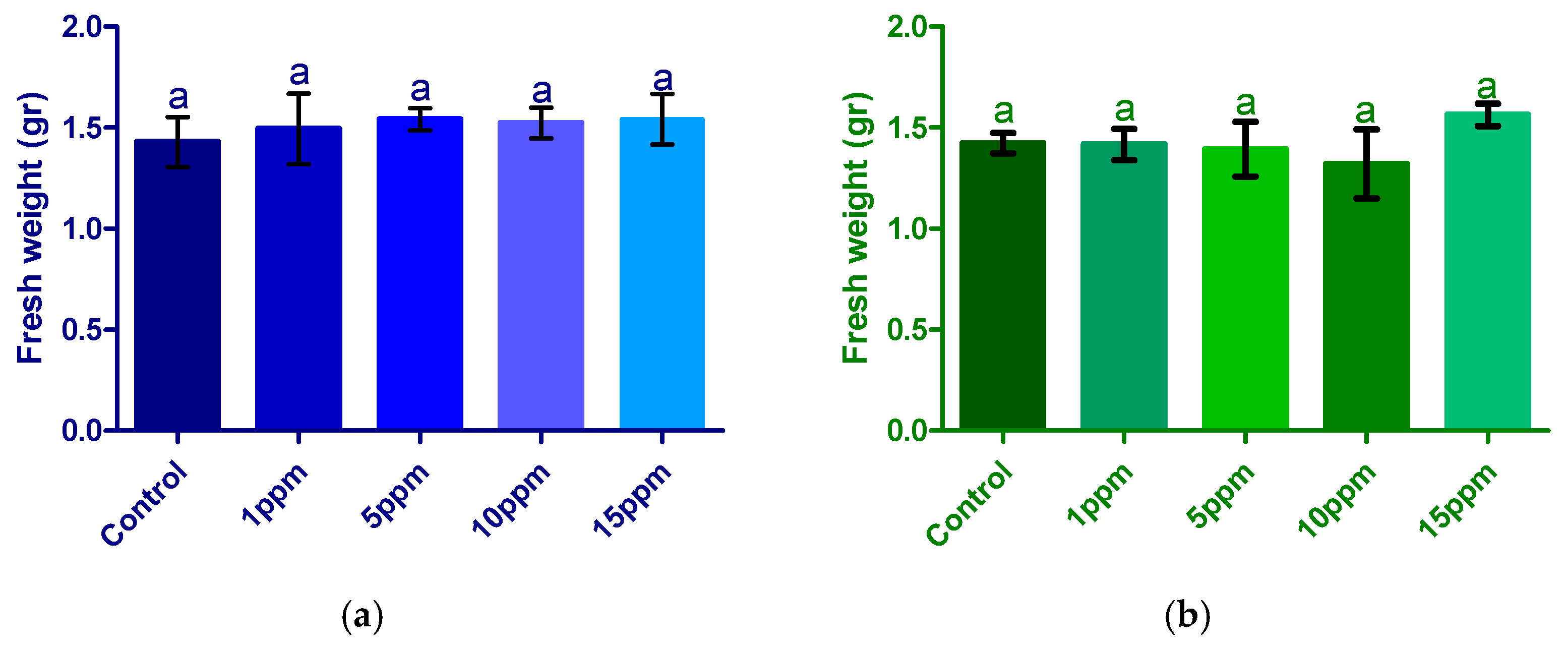
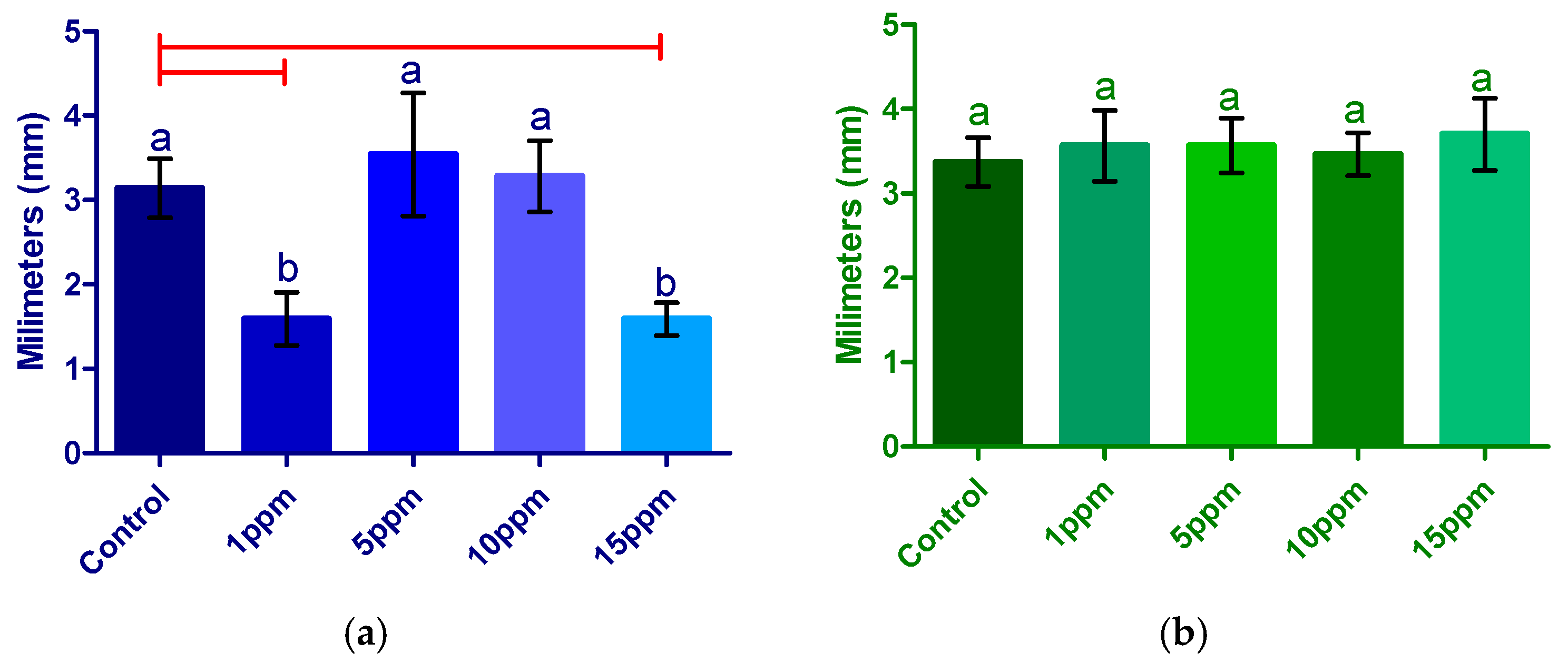
3.2.1. Fresh Weight
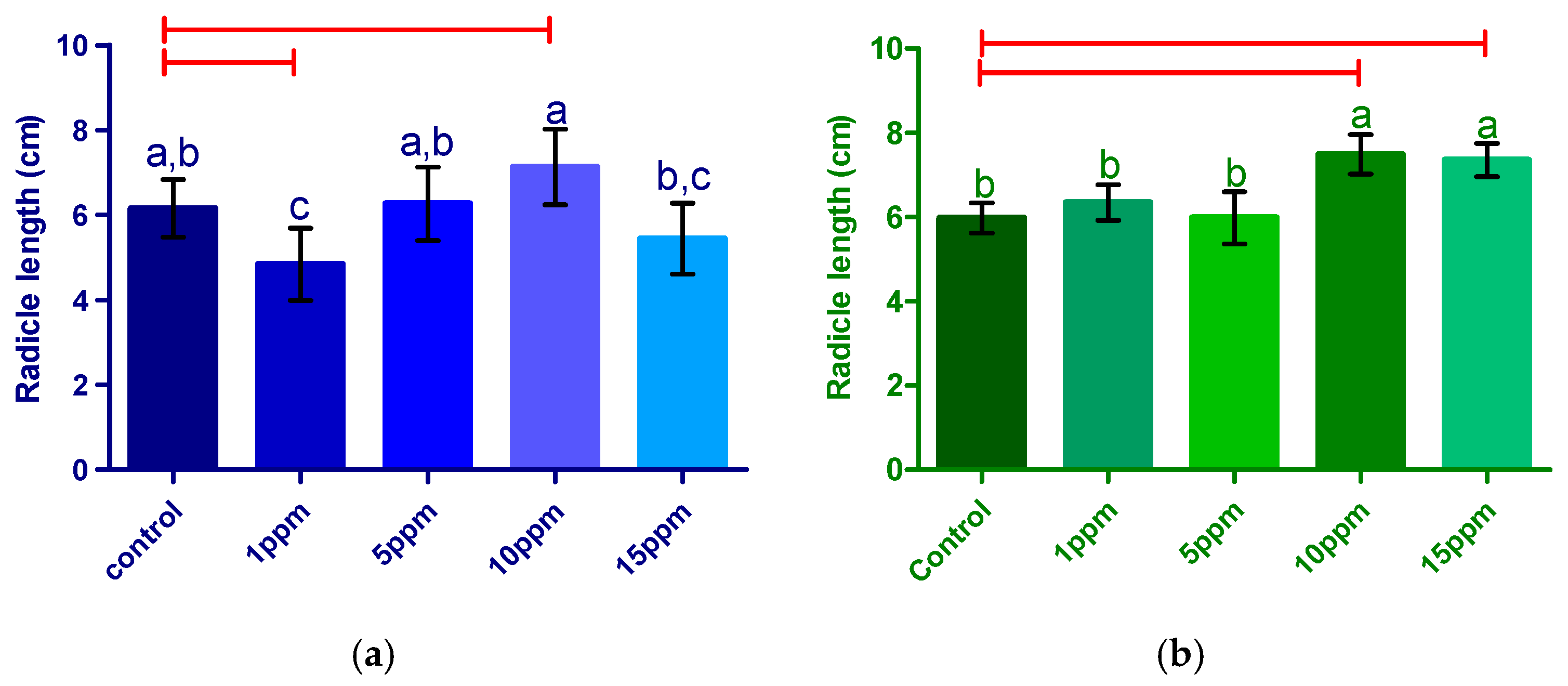
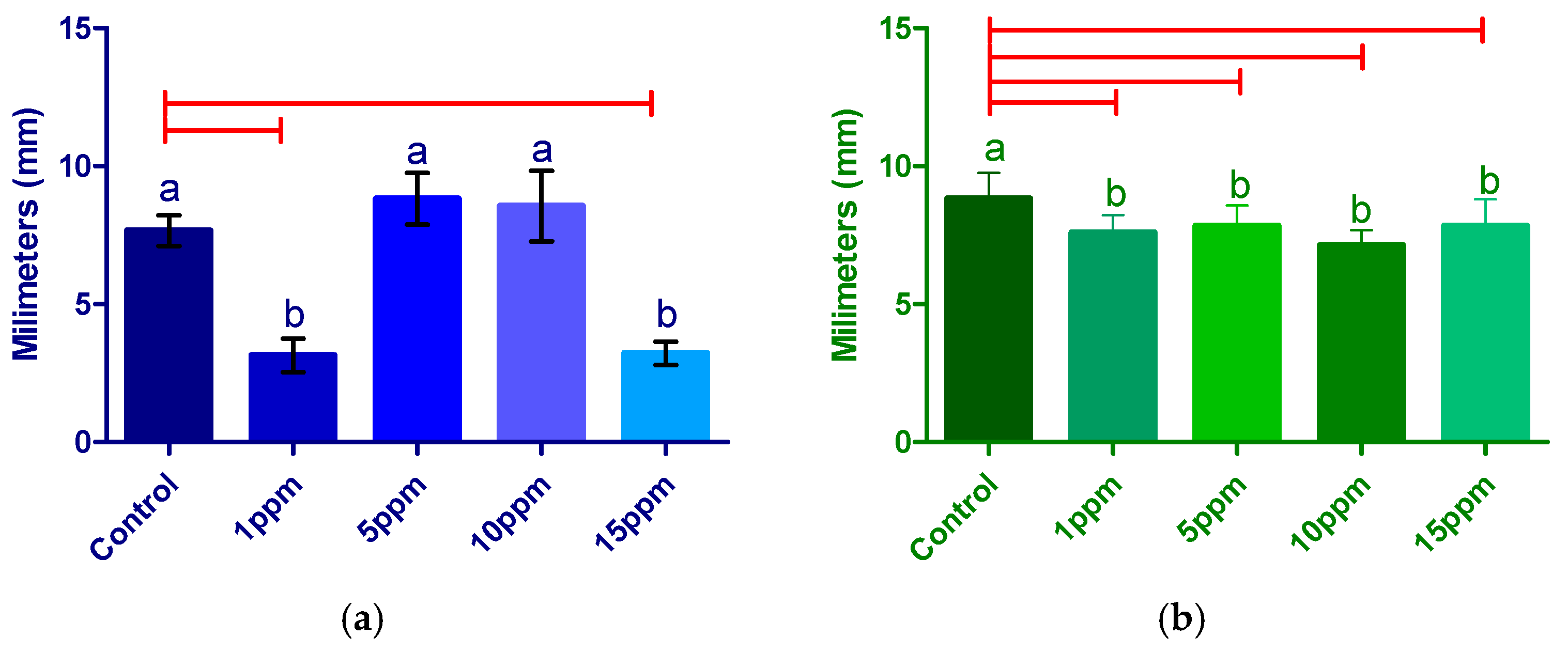
3.2.2. Radicle Length
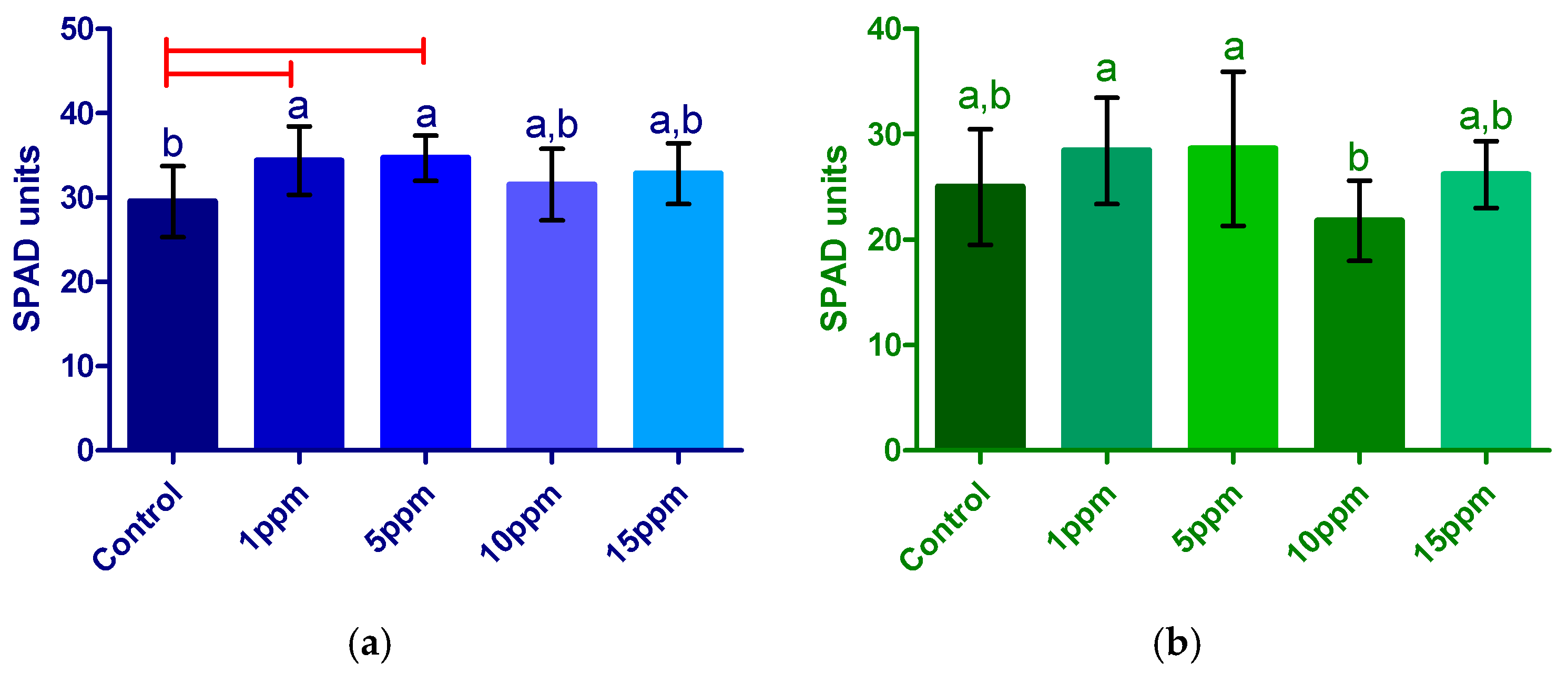
3.2.3. Chlorophyll Index
3.2.4. Morphological Changes on Leaves
3.3. Metabolic Assessment (Antioxidant Capacity and Total Phenols and Flavonoids)
3.3.1. Determination of Total Phenols
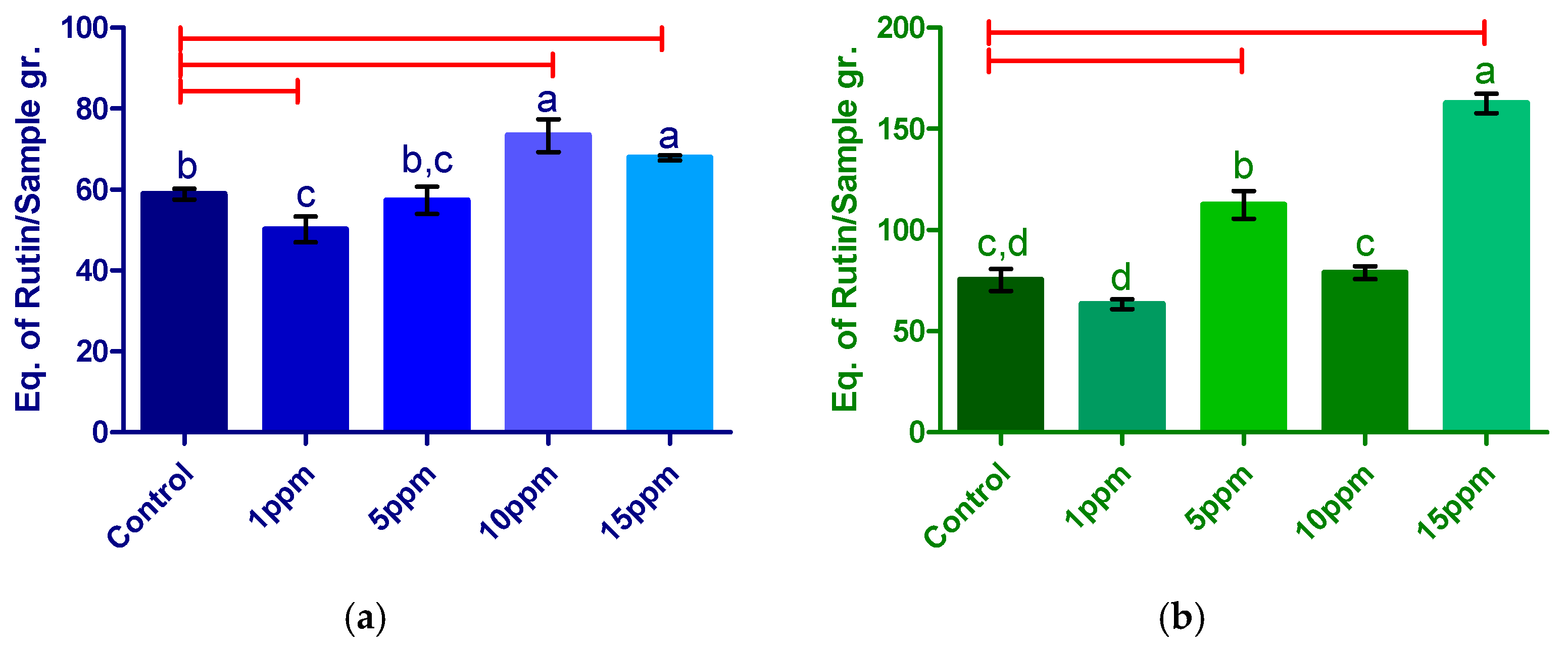
3.3.2. Determination of Total Flavonoids
3.3.3. Determination of Antioxidant Capacity in Sprouts with TiO2 and Ag-TiO2 Treatment
| DPPH | ||
|---|---|---|
| Treatment | Concentration (ppm) | % of IHB 1 |
| Control | 0 | 48.62 ± 2.82 A |
| TiO2 | 1 | 28.30 ± 3.52 C* |
| 5 | 40.85 ± 2.82 B* | |
| 10 | 17.72 ± 1.43 D* | |
| 15 | 13.96 ± 1.20 D* | |
| Control | 0 | 57.40 ± 1.93 AB |
| Ag-TiO2 | 1 | 54.00 ± 1.72 AB |
| 5 | 49.97 ± 3.53 B | |
| 10 | 49.17 ± 5.66 B | |
| 15 | 60.45 ± 3.49 A | |
| ABTS | ||
| Control | 0 | 70.36 ± 1.42 A |
| TiO2 | 1 | 67.49 ± 1.04 AB |
| 5 | 68.52 ± 2.76 AB | |
| 10 | 62.78 ± 2.97 BC* | |
| 15 | 55.65 ± 4.13 C* | |
| Control | 0 | 56.09 ± 3.48 B |
| Ag-TiO2 | 1 | 64.12 ± 2.99 A* |
| 5 | 65.35 ± 2.47 A* | |
| 10 | 46.78 ± 1.66 C* | |
| 15 | 48.41 ± 0.62 C* | |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Y.; Klumpp, E.; Bol, R.; Amelung, W. Uptake of metallic nanoparticles containing essential (Cu, Zn and Fe) and non-essential (Ag, Ce and Ti) elements by crops: A meta-analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1512–1533. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Joshi, A.S.; Perumalsamy, H.; Mijakovic, I.; Singh, P. Advancing sustainable agriculture: A critical review of smart and eco-friendly nanomaterial applications. J. Nanobiotechnol. 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Sharma, P.; Sultan, H.; Alam, P.; Sehar, S.; Rajput, V.D.; Hayat, S. Nano-priming: Improving plant nutrition to support the establishment of sustainable agriculture under heavy metal stress. Plant Nano Biol. 2024, 10, 100096. [Google Scholar] [CrossRef]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle applications in agriculture: Overview and response of plant-associated microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
- Ansari, M.M.; Shin, M.; Kim, M.; Ghosh, M.; Kim, S.-H.; Son, Y.-O. Nano-enabled strategies in sustainable agriculture for enhanced crop productivity: A comprehensive review. J. Environ. Manag. 2024, 372, 123420. [Google Scholar] [CrossRef]
- Chandoliya, R.; Sharma, S.; Sharma, V.; Joshi, R.; Sivanesan, I. Titanium Dioxide Nanoparticle: A Comprehensive Review on Synthesis, Applications and Toxicity. Plants 2024, 13, 2964. [Google Scholar] [CrossRef]
- Bakshi, M.; Kumar, S. Investigating Impacts of Nanosized Titanium Dioxide (Nano-TiO2) on Maize (Zea mays L.) and Their Ensuing Implications on Soil Physiochemistry: A Soil Exposure Study. Water Air Soil Pollut. 2023, 234, 559. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis and Nanomaterials. From Biostimulation to Toxicity. In Plant Biostimulation with Nanomaterials; Juárez-Maldonado, A., Benavides-Mendoza, A., Ojeda-Barrios, D.L., Tortella Fuentes, G., Seabra, A.B., Eds.; Springer Nature: Singapore, 2025; pp. 1–19. [Google Scholar] [CrossRef]
- Sharma, M.M.M.; Kapoor, D.; Loyal, A.; Kumar, R.; Sharma, P.; Husen, A. Plant Response to Silver Nanoparticles in Terms of Growth, Development, Production, and Protection: An Overview. In Plant Response to Silver Nanoparticles: Plant Growth, Development, Production, and Protection; Husen, A., Ed.; Springer Nature: Singapore, 2025; pp. 1–22. [Google Scholar] [CrossRef]
- Alfosea-Simón, F.J.; Burgos, L.; Alburquerque, N. Silver Nanoparticles Help Plants Grow, Alleviate Stresses, and Fight Against Pathogens. Plants 2025, 14, 428. [Google Scholar] [CrossRef]
- Muzammil, S.; Ashraf, A.; Siddique, M.H.; Aslam, B.; Rasul, I.; Abbas, R.; Afzal, M.; Faisal, M.; Hayat, S. A review on toxicity of nanomaterials in agriculture: Current scenario and future prospects. Sci. Prog. 2023, 106, 00368504231221672. [Google Scholar] [CrossRef]
- Narang, J.; Kapoor, B.; Sharma, S.; Gill, N.S. Mechanistic insights: How nanoparticles modulate plant hormones and defense response signaling under stress. Plant Nano Biol. 2025, 13, 100174. [Google Scholar] [CrossRef]
- Mathur, P.; Chakraborty, R.; Aftab, T.; Roy, S. Engineered nanoparticles in plant growth: Phytotoxicity concerns and the strategies for their attenuation. Plant Physiol. Biochem. 2023, 199, 107721. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F.R. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef]
- Kokina, I.; Plaksenkova, I.; Jermaļonoka, M.; Petrova, A. Impact of iron oxide nanoparticles on yellow medick (Medicago falcata L.) plants. J. Plant Interact. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- De Souza-Torres, A.; Govea-Alcaide, E.; Gomez-Padilla, E.; Masunaga, S.H.; Effenberger, F.B.; Rossi, L.M.; Lopez-Sanchez, R.; Jardim, R.F. Fe3O4 nanoparticles and Rhizobium inoculation enhance nodulation, nitrogen fixation and growth of common bean plants grown in soil. Rhizosphere 2021, 17, 100275. [Google Scholar] [CrossRef]
- Aruna, A.; Namasivayam, S.K.R.; Chandu, P.S. Evaluation of phytotoxicity of metallic nanoparticles against Amaranthus retroflexus. Asian J. Microbiol. Biotechnol. Environ. Sci. 2014, 16, 41–46. [Google Scholar]
- Karimi, J.; Mohsenzadeh, S. Effects of silicon oxide nanoparticles on growth and physiology of wheat seedlings. Russ. J. Plant Physiol. 2016, 63, 119–123. [Google Scholar] [CrossRef]
- Imtiaz, M.; Kamran, M.; Arshad, A.; Sardar, S.; Shah, T. Effect of Exogenous Application of Titanium Dioxide Nanoparticles on Growth, Yield, and Yield Components of Wheat (Triticum aestivum L.) under Drought Stress Conditions. Planta Anim. 2025, 4, 217–234. [Google Scholar] [CrossRef]
- Trela-Makowej, A.; Orzechowska, A.; Szymańska, R. Less is more: The hormetic effect of titanium dioxide nanoparticles on plants. Sci. Total Environ. 2024, 910, 168669. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The Size-Dependent effects of silver nanoparticles on germination, early seedling development and polar metabolite profile of wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Altabbaa, S.; Mann, N.A.; Chauhan, N.; Utkarsh, K.; Thakur, N.; Mahmoud, G.A.-E. Era connecting nanotechnology with agricultural sustainability: Issues and challenges. Nanotechnol. Environ. Eng. 2023, 8, 481–498. [Google Scholar] [CrossRef]
- Esquivel, K.; Nava, R.; Zamudio-Méndez, A.R.; Vega González, M.; Jaime-Acuña, Ó.; Escobar-Alarcón, L.; Peralta-Hernández, J.M.; Pawelec, B.; García Fierro, J.L. Microwave-assisted synthesis of (S)Fe/TiO2 systems: Effects of synthesis conditions and dopant concentration on photoactivity. Appl. Catal. B Environ. 2013, 140, 213–224. [Google Scholar] [CrossRef]
- Basahi, M. Seed germination with titanium dioxide nanoparticles enhances water supply, reserve mobilization, oxidative stress and antioxidant enzyme activities in pea. Saudi J. Biol. Sci. 2021, 28, 6500–6507. [Google Scholar] [CrossRef] [PubMed]
- Doğaroğlu, Z. Effects of TiO2 and ZnO nanoparticles on germination and antioxidant system of wheat (Triticum aestivum L.). Appl. Ecol. Environ. Res. 2017, 15, 1499–1510. [Google Scholar] [CrossRef]
- Parveen, A.; Rao, S. Effect of Nanosilver on Seed Germination and Seedling Growth in Pennisetum glaucum. J. Clust. Sci. 2015, 3, 693–701. [Google Scholar] [CrossRef]
- Parola-Contreras, I.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Reynoso-Camacho, R.; Pérez-Ramírez, I.F.; Ocampo-Velázquez, R.V.; Rojas-Molina, A.; Luna-Vazquez, F.; Tovar-Pérez, E.G.; Parola-Contreras, I.; et al. Phenolic compounds and antioxidant activity of methanolic extracts from leaves and flowers of chilcuague (Heliopsis longipes, Asteraceae). Bot. Sci. 2021, 99, 149–160. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Jimenez-Garcia, S.N.; Guevara-González, R.G.; Feregrino-Perez, A.A.; Contreras-Medina, L.M.; Torres-Pacheco, I. Elicitor Mixtures Significantly Increase Bioactive Compounds, Antioxidant Activity, and Quality Parameters in Sweet Bell Pepper. J. Chem. 2015, 2015, e269296. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Meneses-Reyes, A.E.; Jimenez-Garcia, S.N.; Mercado Luna, A.; García Trejo, J.F.; Contreras-Medina, L.M.; Feregrino-Perez, A.A. Polyphenol Content and Antioxidant Activity of Stevia and Peppermint as a Result of Organic and Conventional Fertilization. J. Food Qual. 2021, 2021, e6620446. [Google Scholar] [CrossRef]
- Doğaroğlu, Z.G.; Ece, F.; Çiftci, B.N.; Yıldırımcan, S.; Erat, S. Evaluation of stress factor on wheat (Triticum aestivum): The effect of ZnO and Ni-doped ZnO nanoparticles. Toxicol. Environ. Chem. 2021, 103, 382–398. [Google Scholar] [CrossRef]
- Vishwakarma, M.; Kulhare, P.S.; Tagore, G.S. Estimation of Chlorophyll Using SPAD meter. Int. J. Environ. Clim. Change 2023, 13, 1901–1912. [Google Scholar] [CrossRef]
- Datye, A.; DeLaRiva, A. Scanning Electron Microscopy (SEM). In Springer Handbook of Advanced Catalyst Characterization; Wachs, I.E., Bañares, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 359–380. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Backscattered Electrons. In Scanning Electron Microscopy and X-Ray Microanalysis; Springer: New York, NY, USA, 2018; pp. 15–28. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Structural and optical characterization of Ag-doped TiO2 nanoparticles prepared by a sol–gel method. Res. Chem. Intermed. 2012, 38, 1443–1453. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sattayaporn, S.; Saito, N.; Sujaridworakun, P. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation. Appl. Surf. Sci. 2022, 573, 151617. [Google Scholar] [CrossRef]
- Damkale, S.R.; Arbuj, S.S.; Umarji, G.G.; Rane, S.B.; Kale, B.B. Highly crystalline anatase TiO2 nanocuboids as an efficient photocatalyst for hydrogen generation. RSC Adv. 2021, 11, 7587–7599. [Google Scholar] [CrossRef]
- Porkodi, K.; Arokiamary, S.D. Synthesis and spectroscopic characterization of nanostructured anatase titania: A photocatalyst. Mater. Charact. 2007, 58, 495–503. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Sukarman; Kristiawan, B.; Budiana, E.P.; Khoirudin; Abdulah, A. Multi-technique characterization of TiO2 nanoparticles: Crystallite size, microstrain, and phase analysis for nanomaterial applications—A review. Hybrid Adv. 2025, 11, 100523. [Google Scholar] [CrossRef]
- Alam, M.A.; Ahmed, S.; Bishwas, R.K.; Mostofa, S.; Jahan, S.A. X-ray crystallographic diffraction study by whole powder pattern fitting (WPPF) method: Refinement of crystalline nanostructure polymorphs TiO2. S. Afr. J. Chem. Eng. 2025, 51, 68–77. [Google Scholar] [CrossRef]
- Padilla, F.M.; Teresa Peña-Fleitas, M.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agron. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Tripathi, S.; Tiwari, K.; Mahra, S.; Victoria, J.; Rana, S.; Tripathi, D.K.; Sharma, S. Nanoparticles and root traits: Mineral nutrition, stress tolerance and interaction with rhizosphere microbiota. Planta 2024, 260, 34. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Park, T.J.; Kallerhoff, J.; Arshad, M. Dose-dependent physiological responses of Triticum aestivum L. to soil applied TiO2 nanoparticles: Alterations in chlorophyll content, H2O2 production, and genotoxicity. Agric. Ecosyst. Environ. 2018, 255, 95–101. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of Poplars and Arabidopsis Exposed to Silver Nanoparticles and Ag+ at Sublethal Concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Park, S. Nanomaterials in agriculture: A pathway to enhanced plant growth and abiotic stress resistance. Plants 2025, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Petersen, E.J.; Challis, K.E.; Rabb, S.A.; Holbrook, R.D.; Ranville, J.F.; Nelson, B.C.; Xing, B. Multiple Method Analysis of TiO2 Nanoparticle Uptake in Rice (Oryza sativa L.) Plants. Environ. Sci. Technol. 2017, 51, 10615–10623. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Khan, I.; Hasan, M.; Kausar, R.; Shehzad, J.; Mustafa, G. Plant Molecular Responses to Nanoparticle Stress. In Plant and Nanoparticles; Chen, J.-T., Ed.; Springer Nature: Singapore, 2022; pp. 239–264. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.-T.; Rui, Y.-K.; Ren, J.-Y.; Hou, T.-Q.; Wu, S.-J.; Rui, M.-M.; Jiang, F.-P.; Liu, L.-M. Effect of Different Nanoparticles on Seed Germination and Seedling Growth in Rice. In Proceedings of the 2nd Annual International Conference on Advanced Material Engineering (AME 2016), Dubai, United Arab Emirates, 23–24 January 2016; Atlantis Press: Dordrecht, The Netherlands, 2016; pp. 166–173. [Google Scholar] [CrossRef]
- Haghighi, M.; Teixeira Da Silva, J.A. The effect of N-TiO2 on tomato, onion, and radish seed germination. J. Crop Sci. Biotechnol. 2014, 17, 221–227. [Google Scholar] [CrossRef]
- Kushwah, K.S.; Patel, S. Effect of Titanium Dioxide Nanoparticles (TiO2 NPs) on Faba bean (Vicia faba L.) and Induced Asynaptic Mutation: A Meiotic Study. J. Plant Growth Regul. 2020, 39, 1107–1118. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; De Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Li, S.; Yin, L.; Huang, J.; Chen, C. Toxicity of silver nanoparticles to Arabidopsis: Inhibition of root gravitropism by interfering with auxin pathway. Environ. Toxicol. Chem. 2017, 36, 2773–2780. [Google Scholar] [CrossRef]
- Vatankhah, A.; Aliniaeifard, S.; Moosavi-Nezhad, M.; Abdi, S.; Mokhtarpour, Z.; Reezi, S.; Tsaniklidis, G.; Fanourakis, D. Plants exposed to titanium dioxide nanoparticles acquired contrasting photosynthetic and morphological strategies depending on the growing light intensity: A case study in radish. Sci. Rep. 2023, 13, 5873. [Google Scholar] [CrossRef]
- Gordillo-Delgado, F.; Zuluaga-Acosta, J.; Restrepo-Guerrero, G. Effect of the suspension of Ag-incorporated TiO2 nanoparticles (Ag-TiO2 NPs) on certain growth, physiology and phytotoxicity parameters in spinach seedlings. PLoS ONE 2020, 15, e0244511. [Google Scholar] [CrossRef]
- Liu, W.; Zeb, A.; Lian, J.; Wu, J.; Xiong, H.; Tang, J.; Zheng, S. Interactions of metal-based nanoparticles (MBNPs) and metal-oxide nanoparticles (MONPs) with crop plants: A critical review of research progress and prospects. Environ. Rev. 2020, 28, 294–310. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Reyes-Díaz, M.; Nunes-Nesi, A.; Recio, G.; Carmona, E.; Corgne, A.; Rengel, Z.; Inostroza-Blancheteau, C. Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci. Hortic. 2020, 269, 109418. [Google Scholar] [CrossRef]
- Missaoui, T.; Smiri, M.; Chemingui, H.; Hafiane, A. Effect of Nanosized TiO2 on Redox Properties in Fenugreek (Trigonella foenum graecum L.) during Germination. Environ. Process. 2021, 8, 843–867. [Google Scholar] [CrossRef]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Jiang, H.S.; Yin, L.Y.; Ren, N.N.; Zhao, S.T.; Li, Z.; Zhi, Y.; Shao, H.; Li, W.; Gontero, B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017, 11, 157–167. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Deryabin, A.N. Regulation of Pro-/Antioxidant Balance in Higher Plants by Nanoparticles of Metals and Metal Oxides. Russ. J. Plant Physiol. 2023, 70, 14. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism (s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J. Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef] [PubMed]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag Nanoparticles on Seed Germination and Seedling Growth of Green Beans in Normal and Chill Temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res. 2025, 71, 107–125. [Google Scholar] [CrossRef]
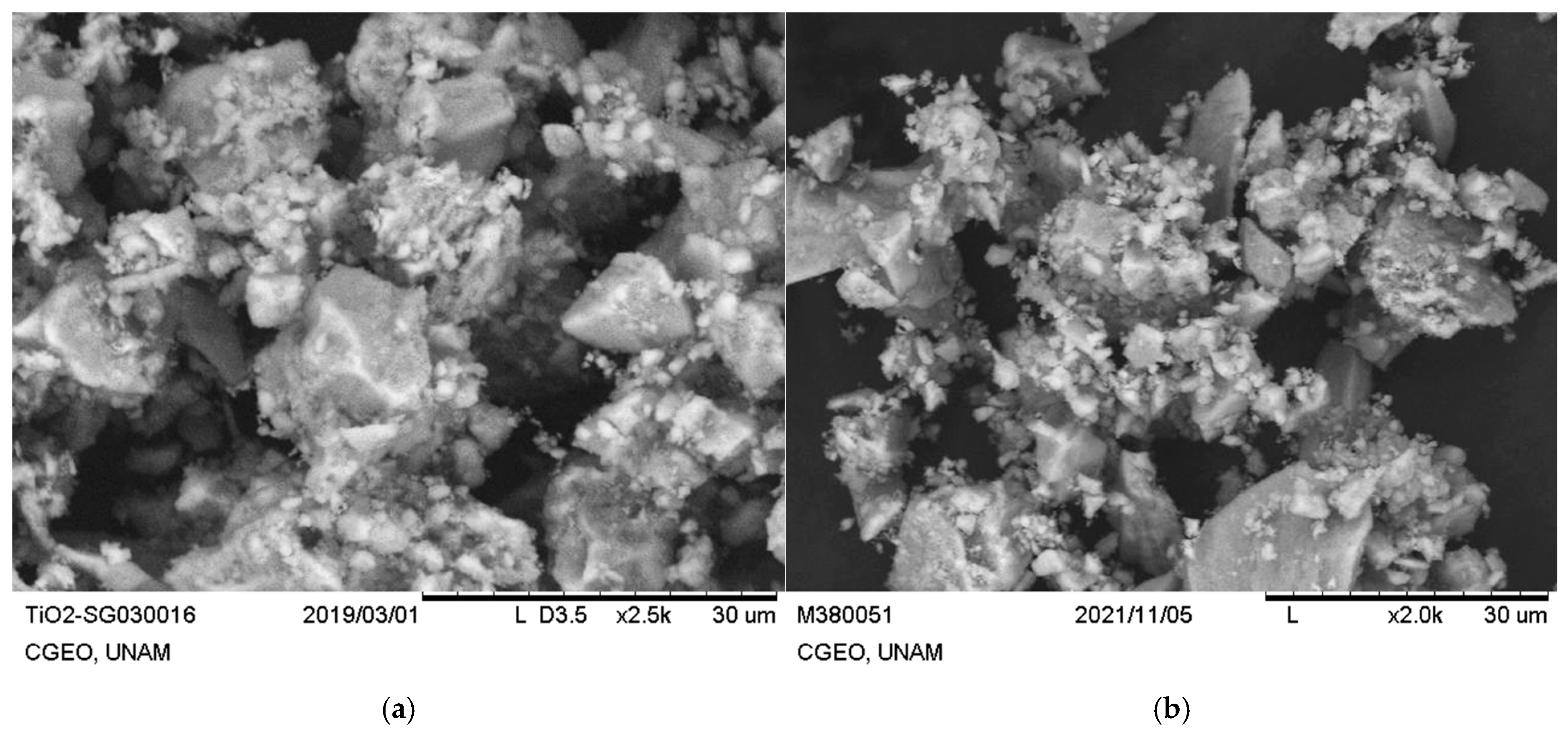



| Material | Scherrer (nm) | W-H (nm) |
|---|---|---|
| TiO2 | 8.65 | 19.80 |
| Ag-TiO2 | 9.62 | 14.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Barras, A.; Rodríguez-Jurado, S.; Aguirre-Becerra, H.; Pérez-García, C.E.; Esquivel Escalante, K.; Feregrino-Pérez, A.A. Application of Nanostructured Semiconductor Oxides TiO2-Based as Additives in the Germination Process of Alfalfa. Agronomy 2025, 15, 2580. https://doi.org/10.3390/agronomy15112580
Martínez-Barras A, Rodríguez-Jurado S, Aguirre-Becerra H, Pérez-García CE, Esquivel Escalante K, Feregrino-Pérez AA. Application of Nanostructured Semiconductor Oxides TiO2-Based as Additives in the Germination Process of Alfalfa. Agronomy. 2025; 15(11):2580. https://doi.org/10.3390/agronomy15112580
Chicago/Turabian StyleMartínez-Barras, Alexis, Susana Rodríguez-Jurado, Humberto Aguirre-Becerra, Claudia E. Pérez-García, Karen Esquivel Escalante, and Ana A. Feregrino-Pérez. 2025. "Application of Nanostructured Semiconductor Oxides TiO2-Based as Additives in the Germination Process of Alfalfa" Agronomy 15, no. 11: 2580. https://doi.org/10.3390/agronomy15112580
APA StyleMartínez-Barras, A., Rodríguez-Jurado, S., Aguirre-Becerra, H., Pérez-García, C. E., Esquivel Escalante, K., & Feregrino-Pérez, A. A. (2025). Application of Nanostructured Semiconductor Oxides TiO2-Based as Additives in the Germination Process of Alfalfa. Agronomy, 15(11), 2580. https://doi.org/10.3390/agronomy15112580







