Interactive Effects of Planting Density and Row Spacing on Maize Root Distribution and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Field Experimental Design
2.3. Sampling and Measurement
2.4. Statistical Analysis
3. Results
3.1. Yield and Shoot Dry Weight
3.2. Shoot Nutrient Content (N, P, K) of a Single Maize Plant
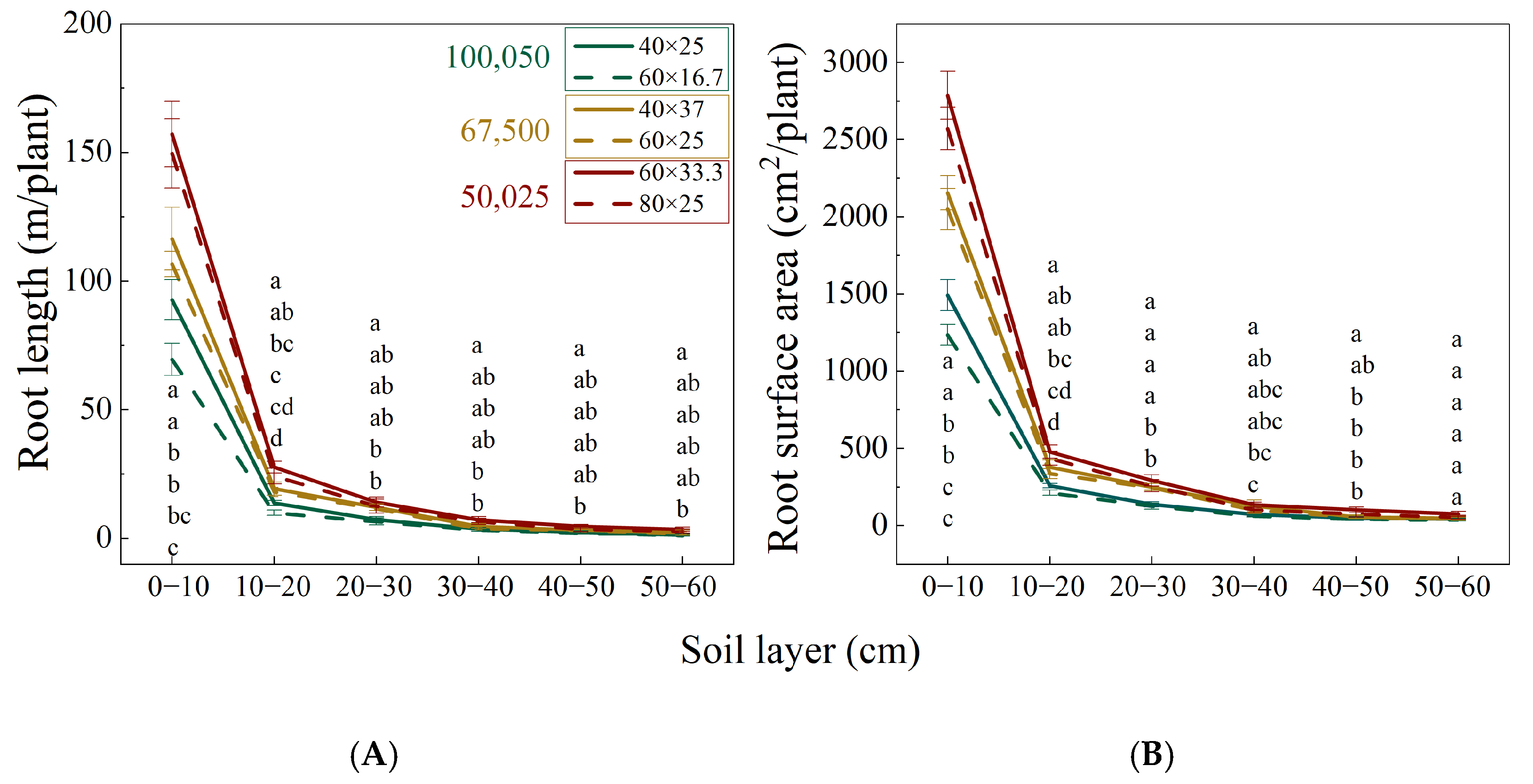
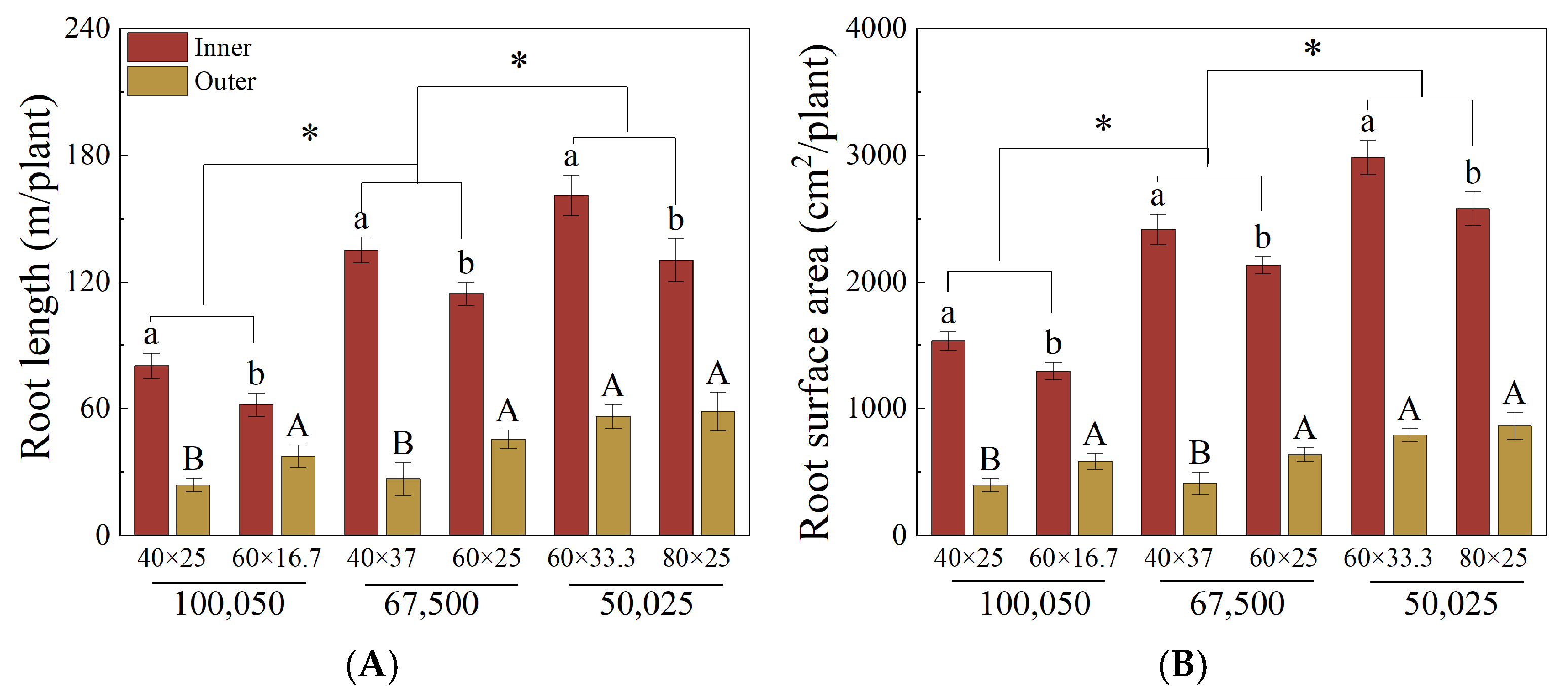
3.3. Vertical and Horizontal Root Distribution
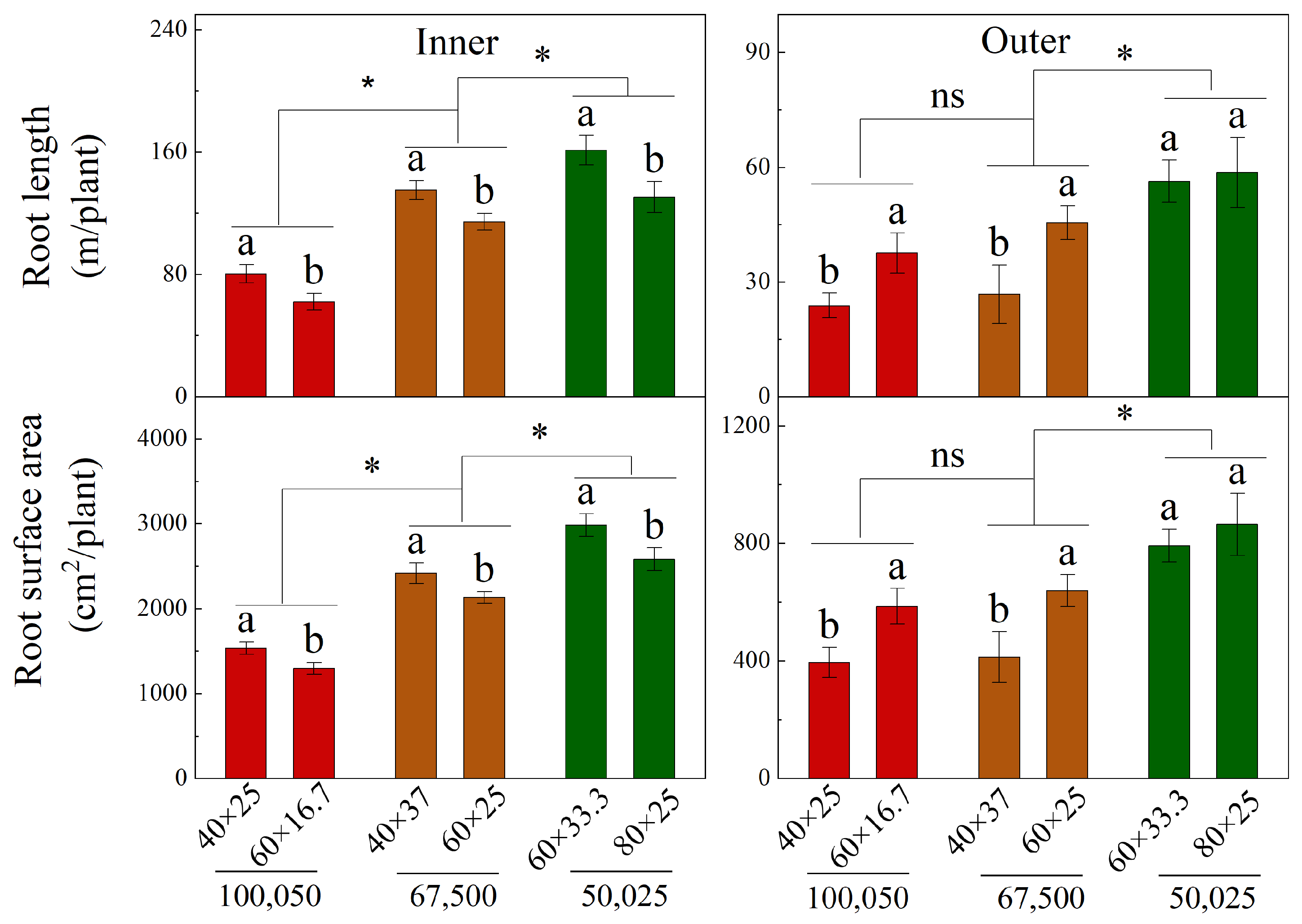
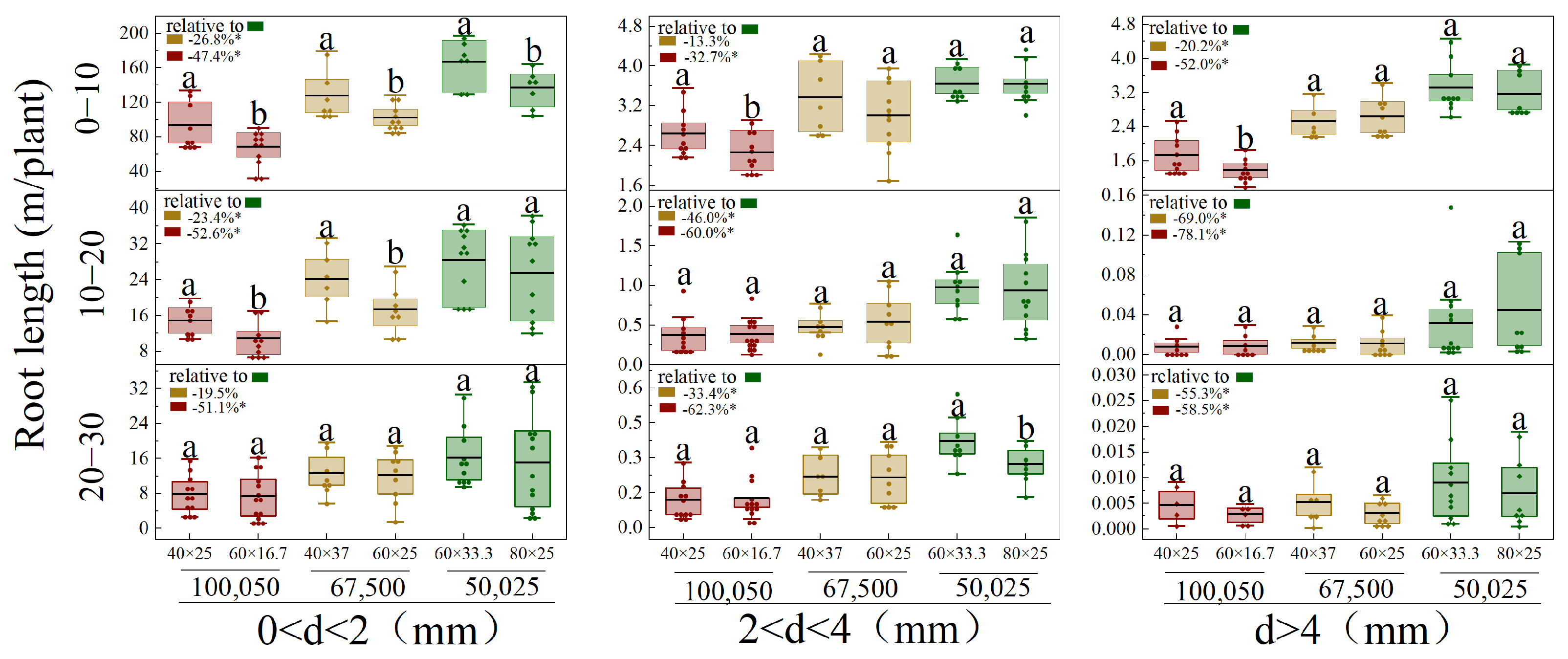
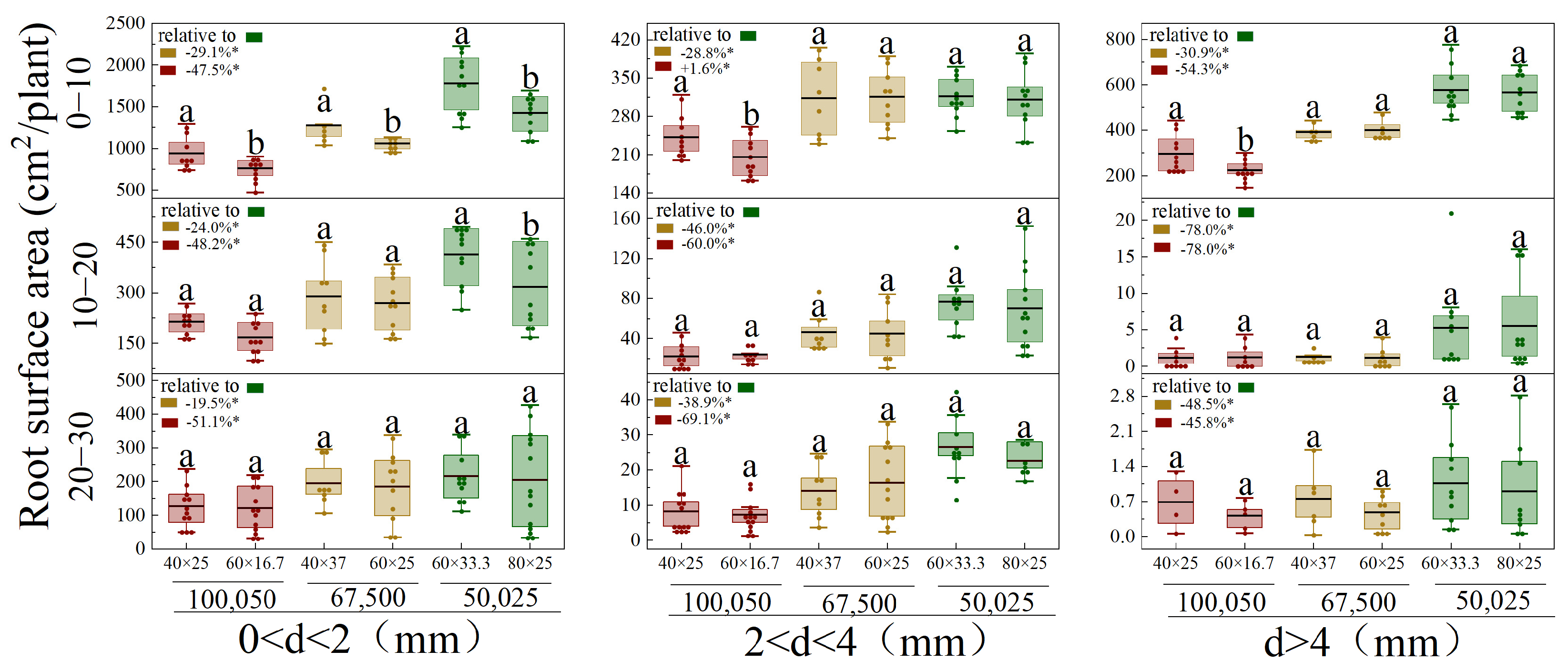
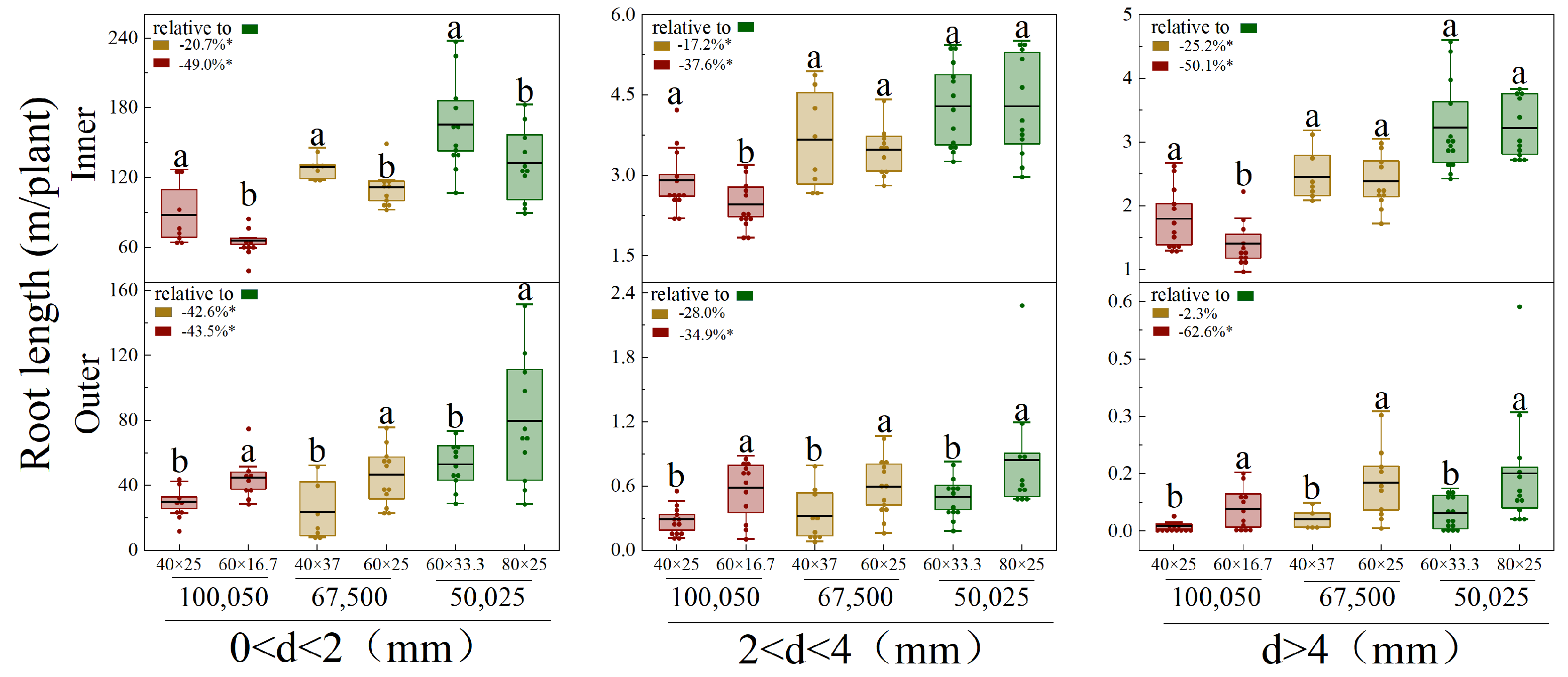
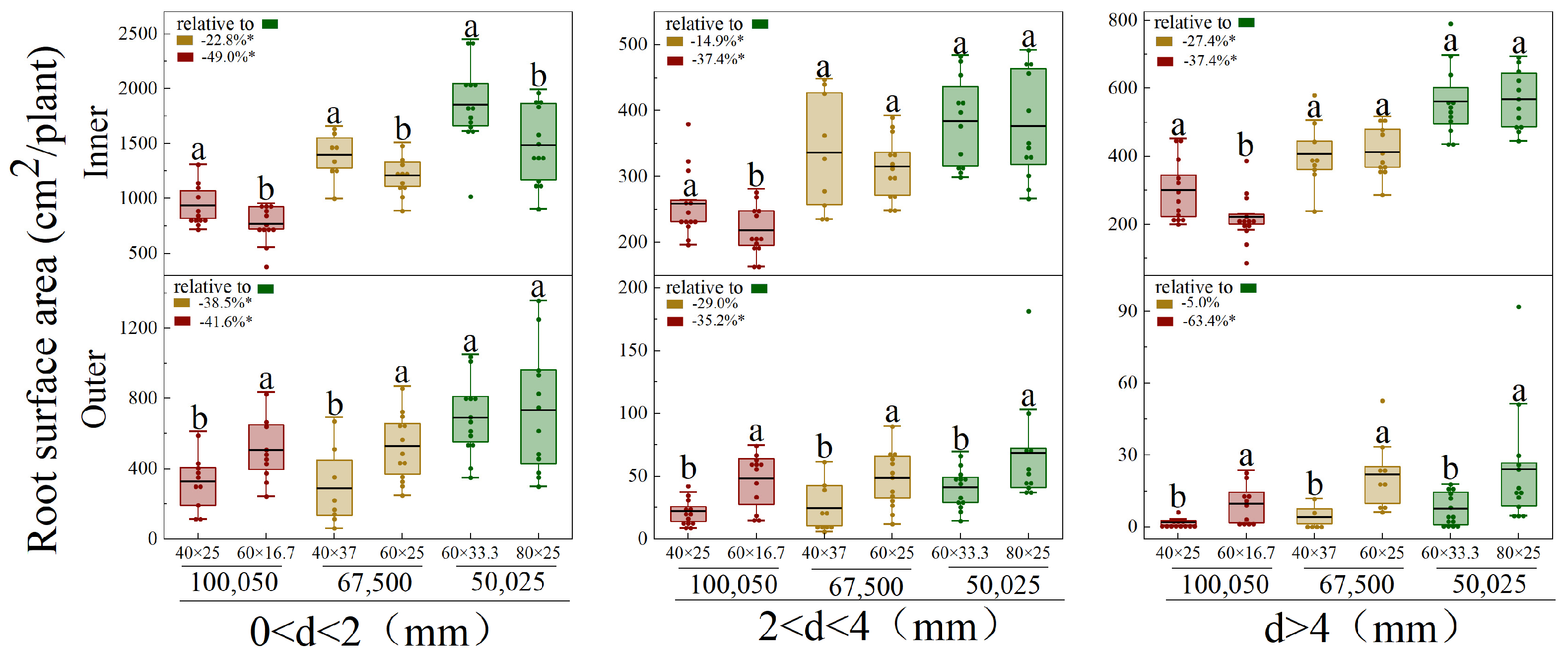
3.4. Vertical and Horizontal Root Distribution of Different Diameters
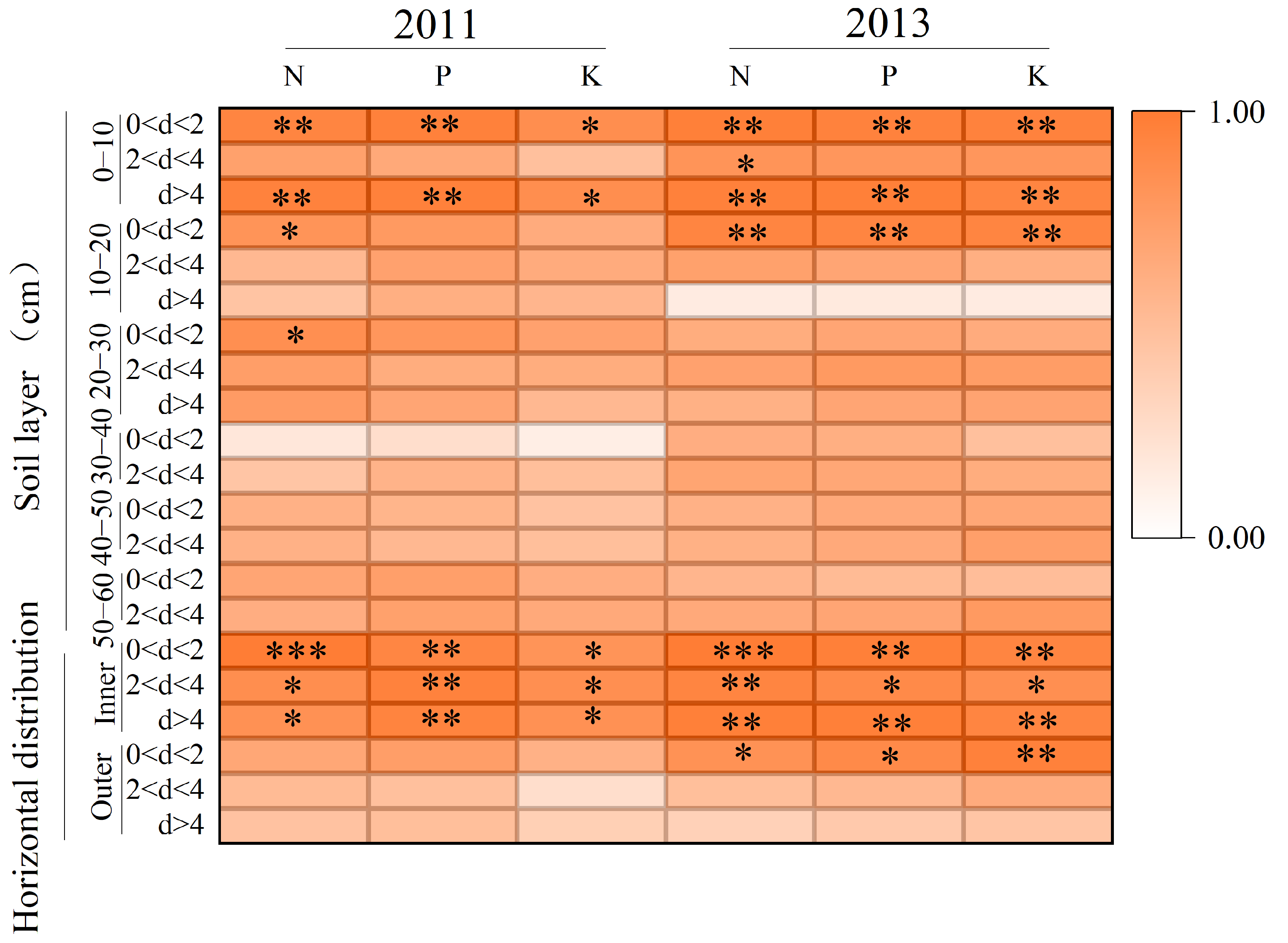
3.5. Root Length Correlation with Shoot Nutrient Content (N, P, K)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duvick, D. Genetic progress in yield of United States maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Assefa, Y.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Smith, S.; Ciampitti, I.A. Analysis of Long Term Study Indicates Both Agronomic Optimal Plant Density and Increase Maize Yield per Plant Contributed to Yield Gain. Sci. Rep. 2018, 8, 4937. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Vara Prasad, P.V.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Ciampitti, I.A. Yield Responses to Planting Density for US Modern Corn Hybrids: A Synthesis-Analysis. Crop Sci. 2016, 56, 2802–2817. [Google Scholar] [CrossRef]
- Bernhard, B.J.; Below, F.E. Plant population and row spacing effects on corn: Plant growth, phenology, and grain yield. Agron. J. 2020, 112, 2456–2465. [Google Scholar] [CrossRef]
- Borrás, L.; Maddonni, G.; Otegui, M. Leaf senescence in maize hybrids: Plant population, row spacing and kernel set effects. Field Crops Res. 2003, 82, 13–26. [Google Scholar] [CrossRef]
- Gao, J.; Shi, J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Grain yield and root characteristics of summer maize (Zea mays L.) under shade stress conditions. J. Agron. Crop Sci. 2017, 203, 562–573. [Google Scholar] [CrossRef]
- Gheysari, M.; Sadeghi, S.-H.; Loescher, H.W.; Amiri, S.; Zareian, M.J.; Majidi, M.M.; Asgarinia, P.; Payero, J.O. Comparison of deficit irrigation management strategies on root, plant growth and biomass productivity of silage maize. Agric. Water Manag. 2017, 182, 126–138. [Google Scholar] [CrossRef]
- Feng, G.; He, X.; Coulter, J.A.; Chen, Y.; Gao, Q.; Mi, G. Effect of limiting vertical root growth on maize yield and nitrate migration in clay and sandy soils in Northeast China. Soil Tillage Res. 2019, 195, 104407. [Google Scholar] [CrossRef]
- Song, R.; Wu, C.; Ma, L.; Guo, J.; Xing, F. Comparison of roots distribution in different maize plant type cultivars in the Songnen Plain. Chin. J. Appl. Ecol. 2003, 14, 1911–1913. [Google Scholar]
- Li, L.; Sun, J.; Zhang, F.; Guo, T.; Bao, X.; Smith, F.A.; Smith, S.E. Root distribution and interactions between intercropped species. Oecologia 2006, 147, 280–290. [Google Scholar] [CrossRef]
- Rubio, G.; Walk, T.; Ge, Z.; Yan, X.; Liao, H.; Lynch, J.P. Root gravitropism and below-ground competition among neighbouring plants: A modelling approach. Ann. Bot. 2001, 88, 929–940. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Shao, L.; Li, G.; Chen, X.; Liu, P.; Zhao, B.; Dong, S.; Zhang, J.; Zhao, B. Effect of root in different soil layers on plant growth and yield formation after anthesis in summer maize. Sci. Agric. Sin. 2013, 46, 4007–4017. [Google Scholar] [CrossRef]
- Niu, L.; Yan, Y.; Hou, P.; Bai, W.; Zhao, R.; Wang, Y.; Li, S.; Du, T.; Zhao, M.; Song, J.; et al. Influence of plastic film mulching and planting density on yield, leaf anatomy, and root characteristics of maize on the Loess Plateau. Crop J. 2020, 8, 548–564. [Google Scholar] [CrossRef]
- Sharratt, B.S.; McWilliams, D.A. Microclimatic and rooting characteristics of narrow-row versus conventional-row corn. Agron. J. 2005, 97, 1129–1135. [Google Scholar] [CrossRef]
- Bernhard, B.J.; Below, F.E. Plant population and row spacing effects on corn: Phenotypic traits of positive yield-responsive hybrids. Agron. J. 2020, 112, 1589–1600. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, K.; Wu, Q.; Dong, S.; Liu, P.; Zhang, J. Effects of narrow plant spacing on root distribution and physiological nitrogen use efficiency in summer maize. Crop J. 2013, 1, 77–83. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Wang, W.; Zhan, L.; Wang, J. Characteristics of fine-root biomass in poplar plantations with different planting densities and spacing configurations. J. Nanjing For. Univ. 2020, 44, 179. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L. Determination of total nitrogen in plant material 1. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Soon, Y.; Kalra, Y. A comparison of plant tissue digestion methods for nitrogen and phosphorus analyses. Can. J. Soil Sci. 1995, 75, 243–245. [Google Scholar] [CrossRef]
- Murányi, E. Effect of plant density and row spacing on maize (Zea mays L.) grain yield in different crop year. Columella-J. Agric. Environ. Sci. 2015, 2, 57–63. [Google Scholar]
- Testa, G.; Reyneri, A.; Blandino, M. Maize grain yield enhancement through high plant density cultivation with different inter-row and intra-row spacings. Eur. J. Agron. 2016, 72, 28–37. [Google Scholar] [CrossRef]
- Van Roekel, R.J.; Coulter, J.A. Agronomic responses of corn to planting date and plant density. Agron. J. 2011, 103, 1414–1422. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.; Qiu, X.; Liu, Z.; Sun, J.; Zhang, J.; Wang, H. Distribution of roots and root length density in a maize/soybean strip intercropping system. Agric. Water Manag. 2010, 98, 199–212. [Google Scholar] [CrossRef]
- Shao, H.; Shi, D.; Shi, W.; Ban, X.; Chen, Y.; Ren, W.; Chen, F.; Mi, G. Genotypic difference in the plasticity of root system architecture of field-grown maize in response to plant density. Plant Soil 2019, 439, 201–217. [Google Scholar] [CrossRef]
- Lambers, H.; Posthumus, F. The effect of light intensity and relative humidity on growth rate and root respiration of Plantago lanceolata and Zea mays. J. Exp. Bot. 1980, 31, 1621–1630. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Pellerin, S. Effect of mutual shading on the emergence of nodal roots and the root/shoot ratio of maize. Plant Soil 1992, 147, 87–93. [Google Scholar] [CrossRef]
- Hebert, Y.; Guingo, E.; Loudet, O. The response of root/shoot partitioning and root morphology to light reduction in maize genotypes. Crop Sci. 2001, 41, 363–371. [Google Scholar] [CrossRef]
- Somma, F.; Hopmans, J.; Clausnitzer, V. Transient three-dimensional modeling of soil water and solute transport with simultaneous root growth, root water and nutrient uptake. Plant Soil 1998, 202, 281–293. [Google Scholar] [CrossRef]
- Reneau, J.W.; Khangura, R.S.; Stager, A.; Erndwein, L.; Weldekidan, T.; Cook, D.D.; Dilkes, B.P.; Sparks, E.E. Maize brace roots provide stalk anchorage. Plant Direct 2020, 4, e00284. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 2011, 156, 1190–1201. [Google Scholar] [CrossRef]
- Liedgens, M.; Richner, W. Minirhizotron observations of the spatial distribution of the maize root system. Agron. J. 2001, 93, 1097–1104. [Google Scholar] [CrossRef]
- Kuroda, H.; Chiba, K. Effect of planting density on root growth in “Starking delicious” apple [Malus pumila] trees grafted on dwarfing and semi-dwarfing rootstocks. J. Jpn. Soc. Hortic. Sci. 1999, 68, 312–320. [Google Scholar] [CrossRef][Green Version]
- Borden, K.A.; Thomas, S.C.; Isaac, M.E. Variation in fine root traits reveals nutrient-specific acquisition strategies in agroforestry systems. Plant Soil 2020, 453, 139–151. [Google Scholar] [CrossRef]
- Maltese, N.E.; Maddonni, G.A.; Melchiori, R.J.M.; Ferreyra, J.M.; Caviglia, O.P. Crop nitrogen status of early- and late-sown maize at different plant densities. Field Crops Res. 2020, 258, 107965. [Google Scholar] [CrossRef]
- Kuchenbuch, R.O.; Gerke, H.H.; Buczko, U. Spatial distribution of maize roots by complete 3D soil monolith sampling. Plant Soil 2009, 315, 297–314. [Google Scholar] [CrossRef]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can Changes in Canopy and/or Root System Architecture Explain Historical Maize Yield Trends in the U.S. Corn Belt? Crop Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Ma, B.; Dwyer, L.; Costa, C. Row spacing and fertilizer nitrogen effects on plant growth and grain yield of maize. Can. J. Plant Sci. 2003, 83, 241–247. [Google Scholar] [CrossRef]
- Jordan, M.; Picard, D. A simulation model of the three-dimensional architecture of the maize root system. Plant Soil 1989, 119, 147–154. [Google Scholar] [CrossRef]
- Feldman, L. The Maize Handbook; Springer: Berlin/Heidelberg, Germany, 1994; pp. 29–37. [Google Scholar]
- Gao, J.; Lei, M.; Yang, L.; Wang, P.; Tao, H.; Huang, S. Reduced row spacing improved yield by optimizing root distribution in maize. Eur. J. Agron. 2021, 127, 126291. [Google Scholar] [CrossRef]
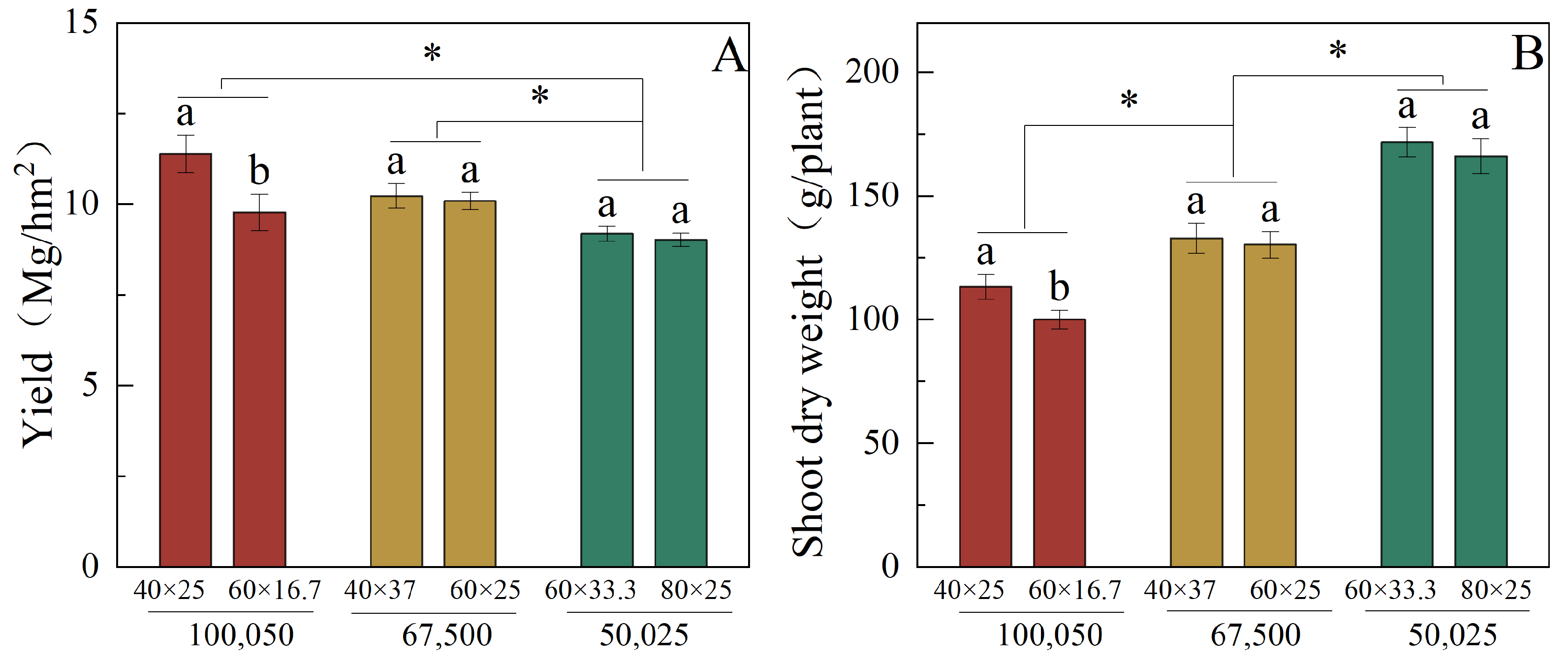
| Year | Month | Mean Daily Solar Radiation (MJ/m2/Day) | Mean Max Temperature (°C) | Mean Min Temperature (°C) | Precipitation (mm) |
|---|---|---|---|---|---|
| 2011 | 5 | 26.69 | 26.52 | 13.97 | 30.08 |
| 2011 | 6 | 23.90 | 31.35 | 19.90 | 112.38 |
| 2011 | 7 | 20.21 | 30.88 | 22.62 | 376.32 |
| 2011 | 8 | 18.84 | 30.45 | 22.05 | 103.93 |
| 2011 | 9 | 17.36 | 24.62 | 14.18 | 21.59 |
| 2011 | 10 | 13.85 | 19.42 | 8.03 | 7.85 |
| 2012 | 5 | 26.28 | 28.08 | 15.05 | 20.61 |
| 2012 | 6 | 22.87 | 29.67 | 19.49 | 95.62 |
| 2012 | 7 | 20.25 | 31.34 | 22.87 | 354.80 |
| 2012 | 8 | 20.01 | 30.51 | 21.08 | 52.65 |
| 2012 | 9 | 17.48 | 26.23 | 13.95 | 82.21 |
| 2012 | 10 | 14.71 | 20.72 | 6.84 | 23.10 |
| 2013 | 5 | 25.24 | 27.88 | 15.27 | 8.47 |
| 2013 | 6 | 20.73 | 28.24 | 20.02 | 101.08 |
| 2013 | 7 | 21.48 | 31.93 | 22.82 | 239.07 |
| 2013 | 8 | 19.85 | 31.40 | 21.87 | 109.5 |
| 2013 | 9 | 16.80 | 25.10 | 15.79 | 81.84 |
| 2013 | 10 | 14.76 | 19.17 | 6.48 | 15.36 |
| 2014 | 5 | 26.85 | 27.67 | 14.52 | 36.68 |
| 2014 | 6 | 24.51 | 30.89 | 19.37 | 108.72 |
| 2014 | 7 | 21.49 | 32.52 | 23.48 | 84.86 |
| 2014 | 8 | 21.07 | 31.42 | 20.57 | 100.18 |
| 2014 | 9 | 16.46 | 25.31 | 15.51 | 106.38 |
| 2014 | 10 | 13.43 | 18.80 | 8.12 | 12.76 |
| Treatments | Nitrogen (mg/Plant) | Phosphorus (mg/Plant) | Potassium (mg/Plant) |
|---|---|---|---|
| Year | |||
| 2011 | 2365.0 a | 466.2 a | 1940.5 a |
| 2013 | 1713.7 b | 473.5 a | 1775.2 a |
| Density (plants/ha) | |||
| D100050 | 1695.6 c | 373.2 c | 1538.3 b |
| D67500 | 2239.4 b | 454.4 b | 1692.0 b |
| D50025 | 2671.6 a | 584.5 a | 2354.9 a |
| Row space × plant space (cm) | |||
| 40 × 25 | 1769.2 a | 383.7 a | 1690.9 a |
| 60 × 16.7 | 1622.1 a | 359.2 a | 1334.8 b |
| 40 × 37 | 2313.4 a | 471.0 a | 1702.7 a |
| 60 × 25 | 2165.4 a | 457.8 a | 1681.3 a |
| 60 × 33.3 | 2723.1 a | 594.1 a | 2426.2 a |
| 80 × 25 | 2620.0 a | 575.0 a | 2283.7 a |
| Source of variation | |||
| Year | ** | NS | NS |
| Density | *** | *** | *** |
| Row space × plant space | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Z.; Liang, Z.; Yin, Y.; Huang, S.; Meng, Q.; Cui, Z.; Wang, P. Interactive Effects of Planting Density and Row Spacing on Maize Root Distribution and Yield. Agronomy 2025, 15, 2552. https://doi.org/10.3390/agronomy15112552
Wang J, Chen Z, Liang Z, Yin Y, Huang S, Meng Q, Cui Z, Wang P. Interactive Effects of Planting Density and Row Spacing on Maize Root Distribution and Yield. Agronomy. 2025; 15(11):2552. https://doi.org/10.3390/agronomy15112552
Chicago/Turabian StyleWang, Junhao, Zhong Chen, Zhengyuan Liang, Yulong Yin, Shoubing Huang, Qingfeng Meng, Zhenling Cui, and Pu Wang. 2025. "Interactive Effects of Planting Density and Row Spacing on Maize Root Distribution and Yield" Agronomy 15, no. 11: 2552. https://doi.org/10.3390/agronomy15112552
APA StyleWang, J., Chen, Z., Liang, Z., Yin, Y., Huang, S., Meng, Q., Cui, Z., & Wang, P. (2025). Interactive Effects of Planting Density and Row Spacing on Maize Root Distribution and Yield. Agronomy, 15(11), 2552. https://doi.org/10.3390/agronomy15112552





