Abstract
Soil acidification, primarily driven by intensive agricultural practices and excessive nitrogen fertilization, is a growing concern worldwide, severely affecting soil health and crop growth. To identify effective soil amendments for mitigating acidity, this study systematically evaluated the physicochemical characteristics and acid-neutralization capacity of biochar derived from different biomass and pyrolysis conditions. Maize straw was pyrolyzed at different temperature to determine the optimal preparation temperature, which was then applied to other biomass, including reed straw, soybean straw, and corn cobs, for comparative analysis. The objectives were to compare the structure, surface features, and acid-neutralization capacity of this biochar, specifically targeting the acidic soil in the black soil region of Northeast China, and to determine the optimal biochar for mitigating soil acidification. The results show that biochar from maize straw pyrolyzed at 700 °C (MBC700) exhibited the highest acid-neutralization efficiency, increasing the pH of acidic soil from 5.50 to 6.06 within seven days, with a long-term buffering capacity. In contrast, reed straw biochar (RBC) exhibited a higher intrinsic pH (9.96) and a larger specific surface area (42.3 m2∙g−1), but its weaker structural integrity limited its durability. Gradient addition experiments further demonstrated that MBC700 at a 0.5% dosage achieved superior acid-neutralization efficiency compared with RBC and other types of biochar at the same rate, indicating better reactivity and applicability. The findings suggest that biochar derived from crop residues can effectively mitigating soil acidification, offering a viable alternative to traditional lime application. This study provides a more sustainable and environmentally friendly alternative solution for soil acidification.
1. Introduction
Soil acidification, resulting from continuous agricultural cultivation, excessive application of chemical nitrogen fertilizers, and pollutant emissions from industrial activities, has become a major obstacle to sustainable agricultural production and ecosystem stability [1,2,3]. Soil acidification not only leads to pH decline, loss of alkaline cations, decreased acid–base buffering capacity, and structural deterioration, but also causes reduced nutrient availability and toxic metal activation, thereby seriously threatening agricultural productivity and ecological security [4]. Statistics indicate that approximately 30% of global farmland is affected by varying degrees of acidification, with the area of strongly acidic soil (pH < 5.5) exceeding 3.9 × 109 h [5]. From 1980 to 2010, the average pH of farmland in China decreased by 0.5 units [6,7]. As of 2023, the area of soil acidification reached 2.04 × 108 ha, accounting for approximately 22.7% of the country’s total land area [8,9]. Compared with the soil survey, the area of farmland with pH less than 5.0 increased by 30%, and that with pH 5.0–5.5 increased by 58%, indicating intensification of farmland soil acidification [10,11]. Acidified soil exhibits significantly elevated concentrations of aluminum ions (Al3+) and hydrogen ions (H+), coupled with substantial leaching of alkaline cations, such as Ca2+, Mg2+, and K+. This dual effect not only disrupts the soil aggregate structure but also leads to microbial community imbalance, ultimately resulting in diminished soil fertility and degraded ecological functions [12,13]. Therefore, preventing soil acidification and restoring soil health have become core tasks for ensuring sustainable agricultural development.
Traditional soil acidification mitigation measures primarily include lime application [14], organic fertilizer application [15,16], and adjustment of tillage systems [17]. Lime-based substances (mainly composed of CaCO3) are widely used due to the rapid neutralizing effect [18]. However, this method is susceptible to rebound effects [19]. Moreover, continuous or excessive application of lime can deteriorate soil structure, disturb cation balance [20], and inhibit microbial activity, ultimately reducing soil fertility and ecological stability [21]. In addition, variations in soil types, acidification intensity, and cropping systems across regions limit the applicability and universality of lime improvement techniques [22]. Therefore, it is necessary to develop a new soil acidification conditioner that combines long-term efficacy, environmental friendliness, and economic feasibility. In recent years, biochar, as a novel conditioning agent, has demonstrated unique advantages in acidic soil improvement [23]. Biochar is produced through pyrolysis of agricultural and forestry biomass under high-temperature and oxygen-limited conditions, possesses a high specific surface area, porous structure, and abundant surface functional group [24]. Its strong alkaline buffering capacity and nutrient retention ability make it superior in enhancing soil pH, improving soil structure, increasing cation exchange capacity, and promoting microbial diversity [25]. The biochar surface contains alkaline functional groups such as carboxyl groups, hydroxyl groups, and ether bonds, which can effectively adsorb and neutralize H+ ions in soil solutions, thereby increasing pH and slowing down the acidification process [26,27]. Meanwhile, the mineral elements contained in biochar, such as Ca, Mg, and K, can be slowly released, providing essential nutrients for plants [28]. Its porous structure not only enhances soil’s water holding and nutrient retention capacity but also provides a suitable habitat for beneficial microorganisms, promoting the remediation and functional reconstruction of soil ecosystems [29].
Existing studies have shown that the physicochemical properties of biochar are regulated by both pyrolysis conditions and the type of raw materials [30,31]. During pyrolysis, increasing temperature promotes the release of volatile components from the biomass and reorganization of carbon structures, significantly influencing key properties such as the specific surface area, porosity, surface functional group, and pH [28]. Generally, high-temperature pyrolysis can increase the carbon content and aromaticity of biochar and enhance its chemical stability, while also causing loss of nitrogen, hydrogen, and oxygen elements, weakening its cation exchange capacity [32]. On the contrary, low-temperature pyrolysis retains more active functional groups and uncondensed carbon structures, endowing biochar with higher surface reactivity and conditioning potential [33]. Additionally, the difference in raw material types significantly affects the performance and ecological effects of biochar. Lignocellulosic biochar (e.g., maize straw biochar) exhibits strong alkalinity and high structural stability, making it more suitable for long-term regulation, whereas herbaceous or manure-derived biochar is rich in soluble nutrients and organic matter, ideal for short-term soil fertility improvement [34]. Therefore, systematically comparing and screening the mitigating capabilities of biochar from different biomass based on their raw material characteristics, are crucial for targeted alleviation of soil acidification and the sustainable regulation of soils with varying acidity.
In summary, soil acidification has become a prominent limiting factor for sustainable agricultural development, while the development and application of biochar provide new approaches and technical methods for mitigating acidified soils. Although studies on the physicochemical properties of different biochar have been conducted, due to the diversity of pyrolysis processes and raw materials, the improvement mechanisms and effects still have considerable uncertainty. The optimization of preparation conditions for biochar based on the characteristics of acidified soil and clarification of its acid-neutralization effect and regulatory mechanisms remain critical scientific challenges that need to be urgently solved in this field. This study uses typical agricultural biomass, such as maize straw, reed straw, corn cobs, and soybean straw, as materials to systematically investigate the effects of different pyrolysis temperatures and raw material types on the physicochemical properties and acid-neutralization capabilities of biochar, aiming to reveal the efficacy and mechanisms of biochar from different biomass sources in mitigating soil acidification, and providing theoretical foundations and technical support for the remediation of acidified soil and sustainable agricultural development.
2. Materials and Methods
2.1. Raw Material Preparation and Soil Collection
The maize straw, corn cobs, reed straw, and soybean straw used in this study were collected from Da’an City, Jilin Province, China (45°30′ N, 124°18′ E). After natural air-drying at ambient temperature, all materials were ground and sieved through a 100-mesh sieve to ensure uniform particle size for pyrolysis. The test soil was collected from the surface layer (0–20 cm) of maize fields in Beian City, Heilongjiang Province, China (48°31′ N, 126°33′ E), with an initial pH of 5.50.
2.2. Preparation of Straw-Derived Biochar
To determine the optimal pyrolysis temperature for biochar production, maize straw was pyrolyzed at four different temperatures (300 °C, 500 °C, 700 °C, and 900 °C) using a vacuum-controlled muffle furnace (SA2-9-14TP, Zhuodi Instrument Equipment Co., Ltd., Shanghai, China). The heating rate was set to 10 °C per minute, the pyrolysis temperature was maintained for 2 h, and the entire process was carried out in an oxygen-limited environment continuously supplied by high-purity argon gas. The generated biochar was designated as MBC300, MBC500, MBC700, and MBC900. Through a comprehensive assessment of physical and chemical properties and acid-neutralization performance, the best pyrolysis temperature was ultimately determined. Based on this, the best pyrolysis temperature was used to prepare biochar out of reed straws, soybean straws, and corn cobs, respectively, designated as RBC (reed straw biochar), SBC (soybean straw biochar), and CBC (corn cob biochar), to investigate the acid-neutralization ability of different types of biochar at the same temperature.
2.3. Characterization of Biochar
2.3.1. pH Measure
The pH of biochar was measured using a pH meter (PB-30I) with a solid-to-liquid ratio of 1:20 (m/v) [35]. ∆pH represents the difference between the final pH and the initial pH.
2.3.2. Scanning Electron Microscopy (SEM) Analysis
The surface morphology of biochar was fixed on aluminum stubs with conductive adhesive and gold-coated using an ion sputtering apparatus. The surface was then observed using scanning electron microscopy (SEM, GEMINI 300, CARL ZEISS AG, Oberkochen, Germany).
2.3.3. Brunauer–Emmett–Teller (BET) Analysis
The specific surface area, pore size distribution, and pore volume were determined using a nitrogen adsorption–desorption analyzer (Quadrasorb Evo, Anton Paar GmbH, Graz, Austria) at liquid nitrogen temperature (77 K). The measurements were analyzed using the Brunauer–Emmett–Teller (BET) model based on nitrogen adsorption–desorption isotherms.
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The surface functional groups were identified by Nicolet NEXUS 670 (Thermo Fisher Scientific, Waltham, MA, USA), with spectra collected in the range of 400–4000 cm−1 at a resolution of 4 cm−1.
2.3.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
The chemical composition and binding energy of biochar were analyzed using X-ray photoelectron spectroscopy (XPS, EscaLab-250xi, Thermo Fisher Scientific, Waltham, MA, USA). The XPS measurements were carried out with an Al Kα X-ray source (1486.6 eV) and a detection depth of 5–10 nm, with C 1s (284.8 eV) as the calibration reference.
2.3.6. Elemental Analysis
The contents of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) were determined using an elemental analyzer (Vario Micro Cube, Elementar Analysensysteme GmbH, Langenselbold, Germany), and the oxygen content was calculated using the following formula: O% = 1 − ash% − C% − H% − N% − S% [36].
2.4. Assessment of the Acid-Neutralization Capacity of Biochar
The air-dried soil (2 mm) was mixed with deionized water at a ratio of 1:2.5 (w/v) to prepare the soil extract. The suspension was shaken for 30 min at 25 ± 1 °C (180 rpm), allowed to settle for 1 h, and then filtered through a 0.45 μm membrane. The initial pH of the extract was adjusted to 5.5 ± 0.5 using 0.01 mol∙L−1 HNO3 or NaOH.
Twenty-four milliliters of the adjusted soil extract was placed in 250 mL Erlenmeyer flasks. Biochar from each treatment (MBC300, MBC500, MBC700, MBC900, RBC, SBC and CBC) were added to the soil extract at a concentration of 1%(w/v), along with a control without biochar (CK). All experiments were repeated three times and incubated in the dark at 25 °C. The pH value was measured at 0, 0.1, 0.2, 0.3, 0.4, 0.5, 2, 4, 8, 12, 24, 48, 96, and 168 h, respectively (the pH values beyond the 168 h period can be found in Table S1).
Based on the results of this preliminary test, the most effective biochar (based on acid-neutralization performance) was selected for further testing at application dosages of 0.3%, 0.5%, and 1.0% (w/w). The pH of the soil extract was monitored over 7 days to assess the acid-neutralization capacity and identify the optimal biochar dosage.
2.5. Statistical Analysis
The data were analyzed using one-way analysis of variance (ANOVA), followed by Duncan’s test to determine significant differences between the treatments. Statistical significance was considered at a p-value < 0.05. All statistical analyses were performed using SPSS software (Version 26, IBM Corp., Armonk, NY, USA).
3. Results and Discussion
3.1. Effects of Preparation Temperature on Biochar Properties
3.1.1. Elemental Composition and Functional Group Characteristics
The pyrolysis temperature significantly influenced the elemental composition and functional group characteristics of maize straw biochar (p < 0.05) (Table 1). Specifically, as the temperature increased, the carbon content gradually increased from 37.02% to 68.00%. Simultaneously, the contents of hydrogen and oxygen decreased steadily from 300 °C to 900 °C. The hydrogen content decreased from 3.89% to 0.99%, while the oxygen content reduced from 51.48% to 12.75%. During pyrolysis, under the action of dehydrogenation and deoxygenation [37], an increase in temperature helps to enhance the stability and aromaticity of the carbon structure. As the pyrolysis temperature increased, ash content rose from 6.87% (300 °C) to 17.13% (900 °C), reflecting the combined effects of organic matter decomposition and mineral concentration. The van Krevelen diagram further illustrates the structural evolution of maize straw biochar with increasing pyrolysis temperature (Figure 1a). When the temperature increased from 300 °C to 900 °C, the ratios of H/C and O/C decreased from 0.11 to 0.01 and from 1.39 to 0.19, respectively. These results indicate that higher temperature pyrolysis enhanced the aromaticity and reduced the polar surface functional group, which is consistent with previous findings [38].

Table 1.
Elemental composition of maize straw-derived biochar preparation at different pyrolysis temperatures.
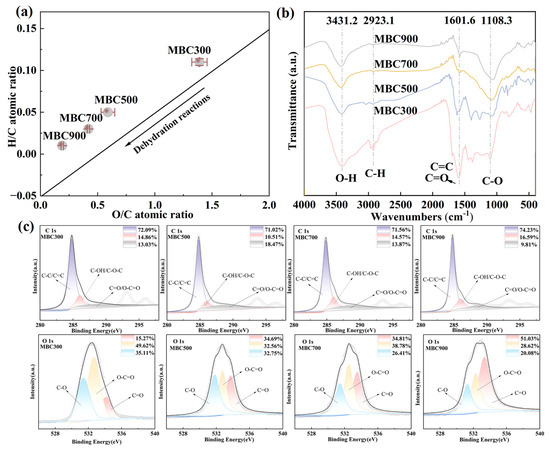
Figure 1.
(a) van Krevelen diagram of maize straw-derived biochar at different pyrolysis temperatures (bold lines indicate the direction of dehydration; single points represent mean values, n = 3; error bars denote standard deviation). (b) FTIR spectra of maize straw-derived biochar at different pyrolysis temperatures. (c) XPS spectra of maize straw-derived biochar at different pyrolysis temperatures.
The FTIR spectra revealed the evolution of a surface functional group with significant changes in the intensities of key peaks during pyrolysis (Figure 1b). At 300 °C, the O-H stretching vibration peak at 3431.2 cm−1 indicates incomplete carbonization of cellulose and hemicellulose [39], while the C-H and C-O vibration peaks suggest that the most of original biomass structure was retained [40]. At higher temperatures (500 °C to 900 °C), the weakening of these peaks and the intensification of the C=C stretching peak (1601.6 cm−1) signal the formation of an aromatic carbon structure [41,42]. These results demonstrated that as the pyrolysis temperature increased, the changes in the functional group content indicate that the generated biochar not only had a higher degree of graphitization and greater stability but also significantly enhanced its aromaticity.
XPS analysis further supported these findings, revealing that carbon (C 1s, 284.8 eV) and oxygen (O 1s, 531.8 eV) were the dominant elements on the surface of biochar (Figure 1c). Deconvolution of the C 1s spectra identified three distinct peaks: graphitic carbon (C-C/C=C, ~284.83 eV), oxygenated single bonds (C-OH/C-O-C, ~286.01 eV), and carbonyl/carboxyl groups (C=O/O-C=O, ~288.5 eV). As the pyrolysis temperature increased, the proportion of C-C/C=C content increased by 3.21%, while C-OH/C-O-C and C=O/O-C=O decreased significantly. These changes indicate that decarboxylation and dehydroxylation reactions caused the reorganization of the carbon structure and enhanced aromaticity [43]. The O 1s spectra exhibited a similar trend, with the C=O (~531.8 eV) and C-O (~534.1 eV) peaks weakening in MBC300. The weakening of these oxygen-related peaks aligns with the progressive loss of oxygen-containing functional groups and stabilization of the carbon matrix upon temperature increase [44]. Such surface chemical reconstruction enhanced the aromaticity index and altered the carbon structure [45].
3.1.2. Specific Surface Area and Surface Morphology of Maize Straw Biochar at Different Pyrolysis Temperatures
The SEM images show that the pyrolysis temperature significantly affected the microstructure of the biochar (Figure 2a and Figure S1). MBC300 exhibited a relatively smooth surface with a few irregular pores. MBC500 formed distinct tubular structures with diameters ranging from 2 to 8 μm and wall thickness from 0.5 to 1.2 μm, accompanied by visible micropores. This morphology correlated with reduced specific surface area and increased average pore size (Table 2). MBC700 displayed a smooth, intact surface with uniformly distributed pores (0.1–0.5 μm) and well-connected pore walls. Such structures could facilitate the release of alkaline minerals and enhance H+ diffusion and adsorption properties. In contrast, MBC900 exhibited collapsed pore walls and severe structural damage, with pore volume reducing to 0.0425 cm3∙g−1, suggesting that excessive temperature compromised structural stability and limited long-term utilization.
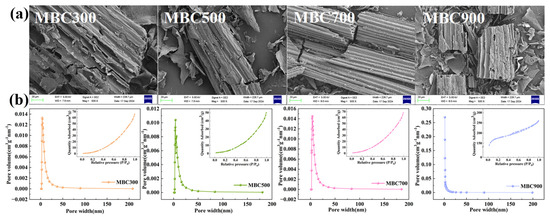
Figure 2.
(a) SEM images of maize straw-derived biochar at different pyrolysis temperatures; (b) N2 adsorption–desorption isotherms and pore size distributions.

Table 2.
Specific surface area and pore characteristics of maize straw-derived biochar at different pyrolysis temperatures.
The surface area followed a “V-shaped” pattern with increasing temperature (Table 2). Between 300 °C and 500 °C, the rapid decomposition of hemicellulose and cellulose led to pore collapse. From 500 °C to 700 °C, the gradual decomposition of lignin combined with aromatic ring rearrangement promoted micropore formation. At 900 °C, further graphitization reshaped the pore network. Among all biochar samples, MBC700 exhibited the largest pore volume (0.13 cm3∙g−1), while MBC500 had the largest average pore size (14.86 nm). In contrast, the average pore diameter of MBC900 was only 3.54 nm, indicating that the high-temperature environment caused mineral melting and pore blockage, resulting in pore contraction. The changes in the hysteresis loop of N2 adsorption–desorption isotherms (Figure 2b) indicate that pyrolysis temperature plays a crucial role in the pore structure and surface area of biochar, with high temperatures generally reducing surface area but enhancing structural stability to maximize biochar’s acid-neutralization and adsorption capabilities.
3.1.3. Yield and pH of Biochar at Different Pyrolysis Temperatures
The biochar yield showed a three-stage response to pyrolysis temperature (Figure 3a). Within the pyrolysis temperature range of 300 °C to 500 °C, the yield decreased significantly from 39.15% to 33.67% (p < 0.05), primarily due to the decomposition of hemicellulose (240–350 °C) and cellulose (300 –400 °C). As the pyrolysis temperature increased from 500 °C to 700 °C, the decomposition of lignin became the dominant process [46], and the yield of biochar decreased from 33.67% to 30.67%. The yield of biochar exhibited a significant decline from 30.67% to 18.79% as the pyrolysis temperature increased from 700 °C to 900 °C, indicating that aromatization and mineral concentration processes intensified, accelerating the rate of mass loss [47]. As expected, the pH of the biochar increased from 8.82 to 9.78 with temperature increasing from 300 °C to 900 °C, likely due to the enrichment of alkaline minerals such as Ca2+, Mg2+, and K+ during pyrolysis, contributing to the liming effect of biochar. This trend was particularly significant in the MBC900 sample, which had the highest ash content and pH, further confirming the role of ash in enhancing alkalinity [43].
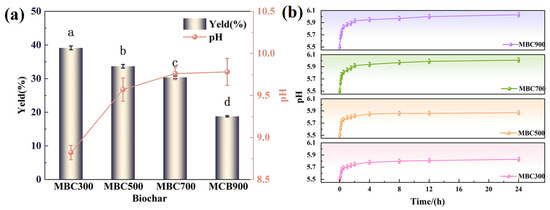
Figure 3.
(a) Yield and pH of maize straw-derived biochar at different pyrolysis temperatures; (b) acid-neutralization performance of biochar (24 h). Different lowercase letters (a–d) above the bars indicate significant differences among treatments at p < 0.05 according to Duncan’s test.
3.1.4. Acid-Neutralization Performance
The pH mitigation effect of biochar on the acidic extract varied significantly with pyrolysis temperature (Figure 3b). All treatment groups exhibited a rapid increase in pH within the initial 2 h, followed by stabilization. This trend reflects the rapid release of alkaline components and the subsequent equilibrium. After 24 h, the final pH values ranked as follows: MBC900 (6.03 ± 0.03) > MBC700 (6.01 ± 0.02) > MBC500 (5.87 ± 0.04) > MBC300 (5.83 ± 0.05). This indicates that higher pyrolysis temperatures facilitated the generation of alkaline phases (e.g., carbonates and metal oxides) in the biochar matrix, which played a dominant role in neutralizing acidic substances, thereby enhancing its overall acid-neutralizing performance. Although MBC900 released more alkalinity initially, its degraded pore structure and low stability constrained the long-term buffering capacity. In contrast, MBC700 maintained both a significant pH elevation (∆pH = 0.56) and structural integrity, with minimal variation over 168 h (p < 0.05; refer to Figure S2). Therefore, MBC700 demonstrated the best performance in terms of equilibrium behavior, functional group retention, and structural stability. By comprehensive consideration of physicochemical properties, morphology, and acid-neutralization capacity, MBC700 was identified as the optimal biochar.
3.2. Biochar Properties of Different Biomass Types
3.2.1. Elemental Composition and Surface Functional Group Characteristics
The elemental composition of biochar varied significantly with different biomass (p < 0.05) (Table 3). Generally, biochar derived from biomass with higher lignin content exhibited higher carbon content, following the order of CBC > RBC > SBC > MBC. The higher carbon content was conducive to the formation of aromatic carbon structures, thereby enhancing the stability of biochar during pyrolysis, which is consistent with the findings of Wang et al. (2022) [48]. In contrast, the oxygen content followed the opposite trend, with MBC having the highest oxygen content (24.89%) compared to CBC (3.87%). These functional characteristics enhanced the reactivity of biochar and played a crucial role in mitigating the acidic soil [30], which could neutralize H+ ions through proton exchange and fixed acidic Al3+ ions through coordination bonds, such as hydroxyl (-OH) and carboxyl (-COOH). Moreover, the atomic ratios of all biochar conformed to the stability criteria proposed by Schimmelpfennig and Glaser (2012) (i.e., O/C < 0.4 and H/C < 0.6), indicating a high degree of aromaticity and strong environmental persistence [49]. MBC exhibited moderate O/C and H/C ratios (0.42 and 0.03), suggesting that it maintained a balance between alkaline reactivity and structural stability, making it particularly suitable for long-term acid-neutralization applications.

Table 3.
Elemental composition of biochar derived from different raw materials at 700 °C.
3.2.2. FTIR Spectral Analysis of Functional Group Evolution
FTIR spectra of biochar prepared from different biomass showed distinct variations in the functional group present (Figure 4a). The O-H stretching vibration at 3434.5 cm−1 and the C-O absorption peak at 1091.9 cm−1 were the most pronounced in RBC, indicating abundant hydroxyl groups retained due to its higher cellulose/hemicellulose content and weaker dehydroxylation [39]. The weak C-H vibration peak at 2931.3 cm−1 in all biochar indicates complete degradation of aliphatic structures. At 1619.7 cm−1, RBC exhibits a strong C=C (aromatic skeleton) stretching vibration, indicating higher graphitization and aromaticity [48]. In contrast, MBC700 showed a more prominent C-O band, indicating the presence of an oxygen-containing functional group that could interact with protons (H+) and contribute to acid neutralization. The weakening of the O-H peak in MBC suggests a more thorough dehydroxylation reaction during the high-temperature pyrolysis process, resulting in lower surface polarity and a more graphite-like structure [37]. The combination of a higher O/C ratio and structural features demonstrated a balance between carbon structure stability and functional group reactivity. This structural evolution confers superior long-term buffering stability [50].
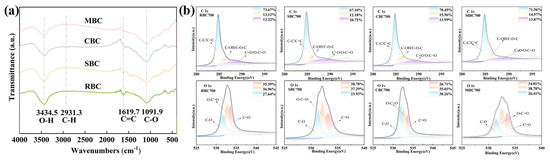
Figure 4.
(a) FTIR spectra of biochar derived from different biomass at 700 °C. (b) XPS spectra of biochar produced from varied biomass sources under consistent thermal conditions (700 °C), showing C 1s and O 1s core-level spectra with deconvoluted peaks.
XPS spectra revealed significant differences in the C 1s and O 1s chemical states for all biochar (Figure 4b). All biochar exhibited the characteristic C-C/C=C peak at 284.8 eV in the C 1s spectrum, with proportions ranked as RBC > CBC > SBC > MBC. This is highly consistent with its H/C ratios, confirming that RBC possessed a more stable aromatic carbon structure. The SBC exhibited the highest C-O and C=O content, while the XBC demonstrated the lowest C=O content. Combined with its low O/C ratio (0.05), CBC indicated a highly inert surface. In contrast, MBC (C-C/C=C 71.56%, oxygenated groups 28.44%) represented a balance between structural stability and surface activity. In the O 1s spectra, SBC was enriched in carboxyl (38.78%) and carbonyl (37.29%) species, while the lower C-O ratio in RBC (27.64%) reflected extensive dehydroxylation and enhanced surface inertness. Overall, RBC was highly stable but functionally deficient, SBC was functionalized but lacked aromaticity, whereas MBC achieved the best balance between graphitization and polar sites, conferring both stability and reactivity for effective buffering in acidic soil.
3.2.3. Specific Surface Area and Surface Morphology of Biochar Derived from Different Raw Materials
BET analysis showed that the type of biomass significantly impacted the pore structure (p < 0.05) (Table 4, Figure 5b). RBC exhibited the highest surface area (42.30 m2∙g−1) and pore volume (0.043 cm3∙g−1), with a predominant pore size of 3.54 nm, favoring adsorption of H+ and Al3+ ions [51]. CBC presented the largest average pore size (29.09 nm), providing advantages in mesopore/macropore diffusion, but lacking adequate micropores for rapid reactivity [52]. SBC exhibited an extremely low specific surface area (4.42 m2∙g−1) due to ash deposition, which blocked the pores. MBC showed a moderate specific surface area (35.34 m2∙g−1), dominated by mesopores (11.89 nm), with uniform pore distribution, enabling controlled release of alkaline substances and the diffusion of H+. From the pore structure–function relationship, the high specific surface area and abundant microporous structure of MBC and RBC provide sufficient surface-active sites, which were crucial for H+ adsorption and Al3+ fixation in acidic soil. Relevant research showed that biochar with a specific surface area of more than 30 m2∙g−1 could achieve a fixed capacity of Al3+ of 2.8–3.5 cmol∙kg−1 [45]. However, the macroporous characteristics of CBC reduced its specific surface area, which might indirectly mitigate soil acidification by promoting microbial colonization and organic matter transformation [53].

Table 4.
Specific surface area and pore characteristics of biochar derived from different biomass.
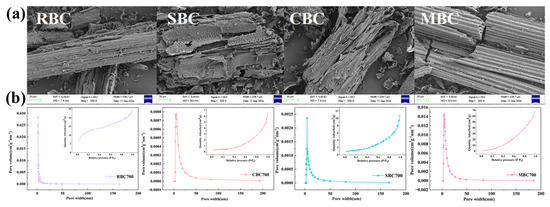
Figure 5.
(a) SEM images of biochar at 700 °C from different biomass; (b) N2 adsorption–desorption isotherm and pore size distribution.
SEM images revealed distinct morphological features of the different biochar (Figure 5a and Figure S3). Both RBC and MBC mainly exhibited mesopores pores ranging from 0.5 to 2.0 μm and micropores from 50 to 200 nm. Their vascular bundles were well-preserved, the layer spacings were uniform, and the pore system presented a layered structure. These morphological features could promote the gradual release of alkaline substances and the diffusion of H+ ions for efficient neutralization. The coexistence of mesopores and micropores also promoted the retention and migration of reactive ions, which was favorable for sustained buffering performance. In contrast, SBC showed rough surfaces with mineral deposits disrupting its layered structure and pore distribution. CBC exhibited a more collapsed structure with irregular pores, suggesting that its structural integrity and long-term buffering capacity might be affected.
3.2.4. Yield and pH of Different Biomass-Derived Biochar
The yield and pH exhibited significant variations among different biochar (p < 0.05) (Figure 6a). SBC showed the highest yield, owing to the material’s higher content of lignin and ash, coupled with high aromatic carbon and mineral conversion, whereas CBC showed the lowest yield, owing to the relatively high content of cellulose that underwent decomposition and volatilization [54]. RBC had the highest pH (9.96), which was attributed to alkaline ion enrichment and a high degree of graphitization [55], whereas its yield (29.08%) was slightly lower than that of SBC and MBC. MBC achieved synergistic optimization with a moderate yield (30.67%) and relatively high alkalinity (9.76), indicating that MBC could effectively retain alkaline components while maintaining functional group stability. Overall, the differences in biochar yields and pH reflect the control of alkaline-component retention and component pyrolysis by biomass composition. Specifically, biomass with greater ash and lignin tended to produce biochar with higher pH, whereas cellulose-rich biomass was more prone to decomposition and greater loss of base minerals [56].
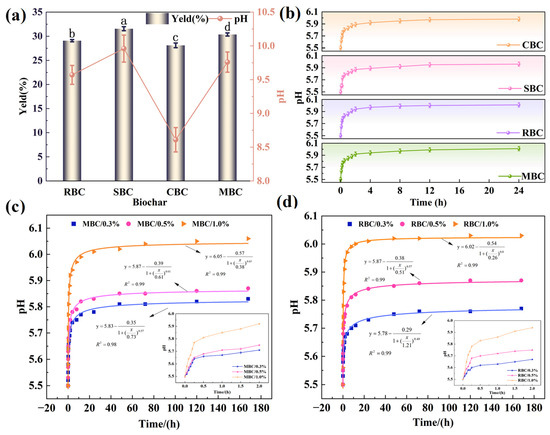
Figure 6.
(a) Yield and pH of biochar pyrolyzed at 700 °C; (b) acid-neutralization performance of biochar (24 h); (c,d) pH increase of MBC at and RBC at different application rates. Different lowercase letters (a–d) above the bars indicate significant differences among treatments at p < 0.05 according to Duncan’s test.
3.2.5. Acid-Neutralization Performance and Optimal Dosage
Significant differences were observed in the acid-neutralization capacities of the biochar (Figure 6b). RBC and MBC exhibited the highest efficacy, increasing the pH of soil extract from 5.50 to 6.01 and 5.99 within 7 days, respectively, which were significantly higher than the other biochar. RBC exhibited the fastest initial pH increase, with an increase of 0.44 units within 2 h, likely due to its microporous and hierarchical pore structure, which facilitated the rapid release of alkaline components [57]. In contrast, MBC displayed a sustained-release pattern, with an increase of 0.03 units after 24 h, highlighting the critical role of structural stability in long-term buffering. The effectiveness of SBC was constrained by ash deposition (13.10%), which blocked pores and limited the release of alkali minerals, while the low surface area of CBC (1.38 m2·g−1) restricted its reactivity and long-term buffering. The results confirm that the surface area and pore structure of biochar jointly determine the alkalinity release and H+ adsorption capacity, which directly influence its acid-neutralization performance [58]. This is the most effective amendment for moderately acidic soil (with pH ≈ 5.5–6.0), and its long-term buffering and stability are comparable to agricultural limestone.
In gradient addition experiments, both MBC and RBC improved pH more effectively at higher dosages, with a rapid increase in pH within the initial 2 h (Figure 6c,d). At a 1.0% dosage, MBC increased the pH from 5.50 to 6.06, and maintained this level within ±2.1% variation over 7 days. Notably, at a 0.5% dosage, MBC exhibited a ∆pH of 0.37, which was higher than that observed for RBC, indicating superior acid-neutralization efficiency. The results highlight the synergistic effect between MBC’s moderate surface area (35.34 m2·g−1), abundant oxygen-containing functional groups (e.g., carboxyl and hydroxyl), and well-developed pore structure, which promote OH− release and H+ adsorption and neutralization [48,57]. Although RBC provided the fastest initial neutralization, its effect declined over time, and its overall efficiency at equivalent dosages was inferior to that of MBC.
4. Conclusions
The types of biomass and pyrolysis temperature significantly affected the pore characteristics, surface morphology, elemental composition, functional group evolution, and yield changes in biochar. The MBC obtained through 700 °C pyrolysis featured a uniform pore distribution, distinct layered fiber structure, stable carbon skeleton, rich functional groups with high activity, and effective retention of alkaline components. The acid-neutralization results demonstrate that MBC700 could rapidly increase the pH of moderate acidic soil (initial pH = 5.50) to a near-neutral level at an application rate of 0.5% and exhibited long-lasting buffering performance, which demonstrated a synergistic effect between structure stability and function group activity. The novelty of this research lies in the systematic integration of temperature optimization tailored for moderate acidic soil and raw material optimization. Unlike lime, biochar derived from agricultural residues, especially MBC700 is a feasible, sustainable, sustained buffering and cost-effective alternative amendment suitable for the improvement of acidic black soil.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15112523/s1: Figure S1: SEM images of maize straw-derived biochar at different pyrolysis temperatures: (a–d) 300 °C, (e–h) 500 °C, (i–l) 700 °C, and (m–p) 900 °C; Table S1: Acid-neutralization performance of maize straw-derived biochar at different pyrolysis temperatures. Figure S2: Acid-neutralization performance of biochar (168 h); Table S2: Acid-neutralization performance of different biochar. Figure S3: SEM images of biochar at 700 °C with different biomass. Figure S4: Acid-neutralization performance of biochar (168 h). Figure S5: XPS spectra of maize straw-derived biochar at different pyrolysis temperatures (Figure 1c in manuscript). Figure S6: N2 adsorption–desorption isotherms and pore size distributions (Figure 3b in manuscript). Figure S7: XPS spectra of biochar produced from varied biomass sources under consistent thermal conditions (700 °C), showing C 1s and O 1s core-level spectra with deconvoluted peaks (Figure 5b in manuscript). Figure S8: N2 adsorption–desorption isotherm and pore size distribution (Figure 6b in manuscript).
Author Contributions
All authors contributed to the study′s conception and design. Material preparation was performed by N.W. Acidic soil collection was carried out by L.X. and J.M. The performance characterization of biochar was conducted by N.W. and Z.C. The first draft of the manuscript was written by N.W. and Z.C. and analyzed with constructive discussions by Y.W. and R.Y. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China Program, Grant No. 2023YFD1501003; the National Basic Science and Technology Resources Survey Project, Grant No. 2021FY100402; and the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDA28020102.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, L.; Hua, K.; Zhan, L.; He, C.; Wang, D.; Nagano, H.; Cheng, W.; Inubushi, K.; Guo, Z. Effect of Soil Acidification on Temperature Sensitivity of Soil Respiration. Agronomy 2024, 14, 1056. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, L.; Wang, Z.; Yang, C.; Dong, F.; Yang, D.; Xi, H.; Sun, Z.; Bol, R.; Awais, M.; et al. Effect of simulated acidification on soil properties and plant nutrient uptake of eggplant in greenhouse. Front. Plant Sci. 2025, 16, 1558458. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The key role of biochar in amending acidic soil: Reducing soil acidity and improving soil acid buffering capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Sci. Total Environ. 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Zhu, Q.; de Vries, W.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F. Enhanced acidification in Chinese croplands as derived from element budgets in the period 1980–2010. Sci. Total Environ. 2018, 618, 1497–1505. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Jiang, B.; Baoyin, B.; Cui, Z.; Wang, H.; Li, Q.; Cui, J. Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis. Microorganisms 2024, 12, 1683. [Google Scholar] [CrossRef]
- Fei, L.; Binzhe, L.; David, V.R.; Jan, M.; He, S.; Jingsheng, C.; Jingheng, G. Straw return exacerbates soil acidification in major Chinese croplands. Resour. Conserv. Recycl. 2023, 198, 107176. [Google Scholar] [CrossRef]
- Yang, F.; Jia, W.; Yang, N.; Li, W.-X.; Duan, Y.-H.; Hu, Y.; Cui, Y. Spatio-temporal variation of surface soil pH of farmland in different regions of China in the past 30 years. J. Plant Nutr. Fertil. 2023, 29, 1213–1227. [Google Scholar]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Acta Ecol. Sin. 2011, 31, 212–216. [Google Scholar] [CrossRef]
- Rahman, S.U.; Han, J.-C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A. Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Wang, Y.; Li, J.; Wang, G.; Ye, J.; Wang, H.; He, H. Soil metagenomic analysis on changes of functional genes and microorganisms involved in nitrogen-cycle processes of acidified tea soils. Front. Plant Sci. 2022, 13, 998178. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, W.; Li, F.; Li, S.; Han, Y.; Li, P. Effects of Tillage Depth and Lime Application on Acidification Reduction and Nutrient Availability in Vertisol Soil. Agric. Ecosyst. Environ. 2024, 14, 1728. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Jia, X.; Kang, J.; Lin, S.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.A.; Fontoura, S.M.; Ambrosini, V.G.; Pesini, G.; Flores, J.P.M.; Bayer, C.; Tiecher, T. Impacts of tillage and liming on crop yields and soil acidity correction: Insights from a 32-year experiment in Southern Brazil. Plant Soil 2025, 511, 1621–1640. [Google Scholar] [CrossRef]
- Enesi, R.O.; Dyck, M.; Chang, S.; Thilakarathna, M.S.; Fan, X.; Strelkov, S.; Gorim, L.Y. Liming remediates soil acidity and improves crop yield and profitability-a meta-analysis. Front. Agron. 2023, 5, 1194896. [Google Scholar] [CrossRef]
- Jouichat, H.; Khiari, L.; Gallichand, J.; Ismail, M. Modeling temporal variation of soil acidity after the application of liming materials. Soil Tillage Res. 2024, 240, 106050. [Google Scholar] [CrossRef]
- Saleem, M.H.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Wenyika, P.; Enesi, R.O.; Gorim, L.Y.; Dyck, M. Effects of liming on soil biota and related processes in agroecosystems: A review. Discov. Soil 2025, 2, 37. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: A global meta-analysis. J. Soils Sediments 2019, 19, 1393–1406. [Google Scholar] [CrossRef]
- Huang, K.; Li, M.; Li, R.; Rasul, F.; Shahzad, S.; Wu, C.; Shao, J.; Huang, G.; Li, R.; Almari, S. Soil acidification and salinity: The importance of biochar application to agricultural soils. Front. Plant Sci. 2023, 14, 1206820. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Meca, S.; Zhang, S.; Clarens, F.; Hu, X. Understanding the dependence of biochar properties on different types of biomass. Waste Manag. 2024, 182, 142–163. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar production under different pyrolysis temperatures with different types of agricultural wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Uroić Štefanko, A.; Leszczynska, D. Impact of biomass source and pyrolysis parameters on physicochemical properties of biochar manufactured for innovative applications. Front. Energy Res. 2020, 8, 138. [Google Scholar] [CrossRef]
- Barszcz, W.; Łożyńska, M.; Molenda, J. Impact of pyrolysis process conditions on the structure of biochar obtained from apple waste. Sci. Rep. 2024, 14, 10501. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo; Theoneste, Y. The impact of pyrolysis temperature on biochar properties and its effects on soil hydrological properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Viana, D.G.; Soares, M.B.; Alleoni, L.R.F.; Egreja Filho, F.B.; Duckworth, O.W.; Regitano, J.B. Sugarcane straw biochar: Effects of pyrolysis temperature on barite dissolution and Ba availability under flooded conditions. Biochar 2024, 6, 83. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Gascó, G.; Lado, M.; Méndez, A.; Paz-Ferreiro, J.; Management, A.P.-G.J.W. New insights into the production, characterization and potential uses of vineyard pruning waste biochars. Waste Manag. 2023, 171, 452–462. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokoowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The production of engineered biochars in a vertical auger pyrolysis reactor for carbon sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- An, X.; Zhu, Z.; Luo, X.; Chen, C.; Liu, T.; Zou, L.; Li, S.; Liu, Y. Effects of Raw Materials and Pyrolysis Temperatures on Physicochemical Properties of Biochars Derived from Hemp Stalks. Plants 2025, 14, 2564. [Google Scholar] [CrossRef]
- Hu, Y.; Li, P.-Y.; Yang, Y.-P.; Ling, M.; Li, X.-F. Preparation and Characterization of Biochar from Four Types of Waste Biomass under Matched Conditions. BioResources 2022, 17, 6464–6475. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-a critical review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Liang, G.; Song, D.; Zhang, X. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Qian, F.; Xi, Z.; Qin, L.; Yan, W.; Jie, C.; Wei, C.; Hui, C.; Lin, J.; Lin, C.; Bing, L. Properties of Eupatorium adenophora Spreng (Crofton Weed) Biochar Produced at Different Pyrolysis Temperatures. Environ. Eng. Sci. 2019, 36, 937–946. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Sharifian, M.; Kern, W.; Riess, G. Innovative approaches to hydrogen storage in vinyl aromatic polymers. Int. J. Hydrogen Energy 2025, 149, 149992. [Google Scholar] [CrossRef]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Lignin Contribution to Aliphatic Constituents of Humic Acids through Fungal Degradation. J. Environ. Qual. 2019, 48, 1565–1570. [Google Scholar] [CrossRef]
- Gianluca, G.; María, V.; Christian, D.S.; Elisabet, P.; Manyà, J.J. Importance of pyrolysis temperature and pressure in the concentration of polycyclic aromatic hydrocarbons in wood waste-derived biochars. J. Anal. Appl. Pyrolysis 2021, 159, 105337. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Q.; Wang, Y.; Han, Z.; Chen, Z.; Mu, Y. Biochar enhanced biological nitrobenzene reduction with a mixed culture in anaerobic systems: Short-term and long-term assessments. Chem. Eng. J. 2018, 351, 912–921. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Chen, Q.; Tong, H.; Gao, X.; Li, P.; Li, J.; Zhuang, H.; Wu, S. Preparation and Application of Wetland-Plant-Derived Biochar for Tetracycline Antibiotic Adsorption in Water. Sustainability 2025, 17, 6625. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.; Moni, C.; Dieguez-Alonso, A.; de la Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of properties of biochar produced from different types of lignocellulosic biomass by slow pyrolysis at 600 °C. Appl. Energy Combust. Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Dodla, S.K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 2016, 142, 4–13. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Wang, Z.; Yong, Q.; Peng, W.; Yao, F.; Luo, K.; Li, B.; Wang, D.; Li, X.; et al. Biological denitrification driven by hydrochar: Insight into the mechanism from intracellular and extracellular electron transfer. J. Environ. Chem. Eng. 2025, 13, 117167. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Ogbonnaya, U.O. Biochar porosity: A nature-based dependent parameter to deliver microorganisms to soils for land restoration. Environ. Sci. Pollut. Res. Int. 2021, 28, 46894–46909. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, L.; Song, W.; Jiang, H.; Liu, Y.; Chen, H. Predicting and refining acid modifications of biochar based on machine learning and bibliometric analysis: Specific surface area, average pore size, and total pore volume. Sci. Total Environ. 2024, 948, 174584. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Se-Eun, B.; Eun-Ju, L.; Jihyun, Y.; Da-Jung, L.; In-Seon, K.; Jae-Won, L. Role of cellulose and lignin on biochar characteristics and removal of diazinon from biochar with a controlled chemical composition. Ind. Crops Prod. 2023, 200, 116913. [Google Scholar]
- Lan, X.; Zhen, F.; Zhang, Q.; Li, H.; Zhang, Z.; Qu, B.; Wang, Y. Characterizations of high nitrogen-doped rice straw biogas residue biochars and their photocatalytic antifouling activity. Ind. Crops Prod. 2024, 222, 120073. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Bing, W.; Yuena, M.; Xinqing, L.; Pan, W.; Fang, L.; Xueyang, Z.; Ling, L.; Miao, C. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer. Sci. Total Environ. 2020, 758, 143664. [Google Scholar]
- Nanthi, B.; Sarmah, A.K.; Sanandam, B.; Shankar, B.; Lokesh, P.; Lukas, V.Z.; Prasanthi, S.; Ahmed, K.B.; Mahtab, A.; Zakaria, S.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).