Abstract
Soil-borne diseases of banana severely threaten the sustainable development of the banana industry. In the pineapple–banana rotation system, using rhizosphere microorganisms to control banana Fusarium wilt via pineapple root exudates is a promising green control strategy. However, the role of volatile organic compounds (VOCs) in mediating disease suppression remains unclear. To explore the disease-inhibiting mechanisms, this study employed in vitro assays and high-throughput sequencing to evaluate the effects of three pineapple-root-derived VOCs (decanal, nonanal, octanol). The results showed the following: (1) All three VOCs strongly inhibited the mycelial growth of Fusarium, with octanol exhibiting the highest inhibition. (2) Each VOC promoted Arabidopsis thaliana growth, and decanal was the most effective. (3) In pot experiments, these VOCs significantly altered the banana rhizosphere microbial community, facilitating the colonization of beneficial genera—characterized by reduced microbial diversity and increased beneficial genera abundance. These results delineate a VOC-mediated rhizosphere microbe–Fusarium–plant interaction network, offering a novel theoretical foundation for the ecological control of banana diseases via the rhizosphere microbiome. In conclusion, this study elucidates a new mechanism for banana disease inhibition via VOCs, highlighting the positive impacts on plant growth and rhizosphere soil health through microbiota modulation.
1. Introduction
Banana is globally recognized as one of the most economically significant crops in agricultural production and international fruit trade [1]. However, banana Fusarium wilt, caused by soil-borne pathogens, remains a major constraint to the sustainable development of the global banana industry [2].
Field experiments have shown that the implementation of a banana–pineapple rotation system in regions with high Fusarium wilt incidence significantly reduces disease occurrence in subsequent banana planting seasons [3]. High-throughput sequencing further confirmed that crop rotation enhances beneficial microbial populations, such as Bacillus and Aspergillus [4]. The mechanisms underlying this disease suppression may also involve the production of volatile organic compounds (VOCs) [5]. However, research on the effects of VOCs emitted by pineapple root microorganisms in controlling banana Fusarium wilt and their impact on banana soil microbial communities remains scarce—existing studies only propose a potential link between VOCs and disease suppression but lack analysis of pineapple root VOCs, leaving the specific regulatory process unclear.
In addition to mechanisms such as nutrient competition and antibiotic production, plant roots can interact with Fusarium, indigenous microorganisms, and host plants by emitting VOCs [6]. These compounds enhance root adaptability in the rhizosphere and contribute to disease suppression [7]. VOCs have attracted increasing attention in rhizosphere immunity research due to their distinctive properties, including low active concentrations, ability to migrate freely across water–soil–air interfaces, and long-distance transmission capacity [8]. For example, Yuan et al. demonstrated that VOCs produced by Bacillus amyloliquefaciens NJN-6 significantly increased the relative abundance of Burkholderia bacteria, known antagonists of pathogenic microorganisms [9]. Additionally, Xiaojuan Li et al. showed that VOCs exhibited strong antifungal activity against strawberry anthracnose, inhibiting mycelial growth and spore germination of Colletotrichum gloeosporioides [10]. These findings suggest that VOCs can enhance the inhibitory effects of microbial communities on pathogens by boosting the abundance of antagonistic genera. Nevertheless, these studies focus on VOCs from non-pineapple root, failing to address how pineapple root VOCs specifically act on banana Fusarium wilt and soil microbial community, resulting in a critical gap in the research on banana–pineapple rotation systems.
The release and accumulation of volatile substances from pineapple roots in the soil may influence the microbial community structure and the physical and chemical properties of the soil, thus affecting both the growth environment and the agricultural ecological balance. Therefore, studying these VOCs can provide technical support and a decision-making basis for the protection of agricultural ecosystems. Current research on VOC-induced improvements in plant performance primarily focuses on growth, reproduction, and the enhancement of plant immunity [11,12,13]. However, systematic studies on whether VOCs can induce plants to inhibit the growth and colonization of Fusarium and regulate the rhizosphere microbial community remain scarce; in particular, for pineapple root microbial VOCs in the context of banana cultivation, there is no integrated research combining VOCs identification, in vitro functional verification, and soil microbial regulation analysis.
In this study, to fill the aforementioned research gaps and clarify the unique role of pineapple root microbial VOCs in banana Fusarium wilt control and soil microecology regulation, a solid-phase microextraction (SPME) device was used to collect VOCs from pineapple roots, and gas chromatography–mass spectrometry (GC-MS) was employed to identify their constituents. The inhibitory effects of VOCs on Fusarium growth and their growth-promoting effects on Arabidopsis thaliana were analyzed in vitro. Pot experiments were conducted, and high-throughput sequencing was applied to investigate the regulation of soil microbiota by VOCs. The aim was to uncover the mechanisms through which rhizosphere microorganisms, mediated by VOCs in the rotation system, contribute to banana cultivation health, offering novel approaches and methods for improving its sustainability.
2. Materials and Methods
2.1. SPME and GC-MS Were Used in Combination to Collect and Identify Volatile Organic Compounds from Pineapple Roots
Soil from banana plantations that had been continuously cultivated for more than ten years was collected, passed through a 2 mm sieve, and divided into plastic pots. Each pot contained 1 kg of soil, into which pineapple seedlings were transplanted. The pots were watered with sterile water every three days. For detailed procedures, refer to Yin et al. with appropriate modifications [14]. Two weeks after transplanting, when the pineapple had developed new roots, the plastic pots and pineapple seedlings were placed into sealed bags. The seedlings were carefully removed and suspended, and a DVB/CAR/PDMS extraction head was inserted near the root system. VOCs were collected for 50 min at room temperature. After extraction, the fibers were inserted into the sampler port of a GC-MS system (7890B-7000B 2024, Agilent Technologies, Santa Clara, CA, USA) for desorption over 3 min. The extraction head was then conditioned at 250 °C for 30 min before sampling. VOCs were analyzed using gas chromatography, with helium as the carrier gas, on an HP-5 ms column (30 m × 250 μm × 0.25 μm) at a flow rate of 1 mL/min. The temperature program for the GC was as follows: 2 min at 40 °C, increased to 100 °C at 2 °C/min, then raised to 150 °C at 4 °C/min, held for 2 min, and finally increased to 280 °C at 20 °C/min for the remainder of the analysis. The mass spectrometer operated in 70 eV EI ionization mode with an ion source temperature of 250 °C and a quadrupole temperature of 150 °C. The scanning range was 29–450 m/z [15]. Data collection and analysis were performed using the Agilent Mass Hunter Qualitative Analysis platform, and the mass spectra were matched against the NIST 14 Mass Spectrum Library.
2.2. The Effects of Three VOCs on the Mycelium Growth of the Fusarium
The Fusarium used in this experiment is Fusarium oxysporum f. sp. cubense, and it was sourced from the rhizosphere soil of diseased banana plants. For microbial culture, PDA medium was poured onto one side of a 90 mm two-compartment Petri dish, and three VOCs were placed on sterilized filter paper on the other side. The dish was sealed with parafilm and incubated at 28 °C for 3 days. The inhibitory effects of the three VOCs on the mycelial growth of Fusarium were observed, and each experimental group was repeated five times. The growth inhibition rate of Fusarium oxysporum mycelium was calculated as follows: growth inhibition rate (%) = [(diameter of control mycelium − diameter of treated mycelium)/diameter of control mycelium] × 100%. The VOCs used were synthetic standards.
2.3. Growth Promotion Experiments in a Model Crop (Arabidopsis thaliana) Treated with VOCs
Plant Material: Arabidopsis wild-type Col-0 (Arabidopsis thaliana).
On one side of a 90 mm Petri dish, MS medium was added for culturing Arabidopsis thaliana seedlings, while the other side was left empty. The treatment method for Arabidopsis thaliana seeds followed the procedure outlined by Leschevin et al. [16]. The seeds were placed in a centrifuge tube and treated with an 8% sodium hypochlorite solution for 5 min, followed by thorough mixing with a pipette. Sterile water was then added, and the residual sodium hypochlorite was thoroughly rinsed off by gently blowing and washing the seeds. This rinsing process was repeated five times. The seeds were then suspended in an 8% agar solution. Sterilized seeds were evenly distributed on one side of the MS medium plate, with 5 seeds per plate. To the other side of the plate, 100 μL /mL, 10 μL of the VOC solution was added onto sterilized filter paper and sealed. The plates were vernalized in a 4 °C refrigerator for three days and then transferred to an artificial climate chamber (16 h of light and 8 h of darkness) at 23 °C for 10 days. The growth of each experimental group was measured. For root treatment, a press spoon was gently used to crush the MS medium. The roots were carefully laid down, and excess water and medium were absorbed with paper towels to maintain root integrity. The control group received the same amount of sterile water, and each experimental group was repeated nine times. The VOCs used were synthetic standards.
2.4. A Pot Experiment Design for Adding VOCs to the Soil of Banana Seedlings
The experimental setup is outlined in Table 1. Latosol was collected from banana plantations that had been continuously cultivated in Chengmai County, Hainan Province, China (19°45′7″ N, 110°0′57″ E), for over ten years. The physicochemical properties of latosol are shown in Table S1. Different VOC treatment groups were set up in the pot experiments, with each plastic pot containing 1 kg of soil. Each treatment was repeated five times. The VOCs used were synthetic standards. Referring to previous research, we selected 10 μg/g and 100 μg/g for our experiments [17,18,19]. Additionally, the carrier solvent for the VOCs was sterile water, and the VOCs were released under natural conditions after being added to the pots.

Table 1.
Pot experiment design.
Two months after the banana plants were planted from 10 November 2024 to 10 January 2025, samples were collected, and biomass as well as soil physical and chemical properties were measured. Five replicates of rhizosphere soil DNA were extracted for each treatment and sent to a third-party platform for high-throughput sequencing of bacterial 16S rRNA and fungal ITS regions to analyze the soil microbial community structure. The changes in the microbial community induced by VOCs and their impact on the assembly of rhizosphere microorganisms were investigated.
2.5. Data Analysis
Data analysis and visualization were performed using Excel 2021 and Origin 2022 for basic processing, while one-way analysis of variance was conducted with IBM SPSS Statistics 26. For data visualization, R 4.5.1 (including packages such as vegan and ggplot2) was used to generate Alpha diversity (Chao, ACE, and Richness), PCoA plots, LDA analysis, collinearity networks, and correlation analyses.
3. Results
3.1. Collection and Component Identification of Volatile Substances from Pineapple Root Systems
After an analysis of the mass spectrometry data of VOCs from pineapple roots, fourteen types of VOCs were identified, as shown in Table 2. Hexamethyl-cyclotrisiloxane, Octamethyl-cyclopentasiloxane, Decamethyl-cyclopentasiloxane, Dodecamethyl-cyclohexasiloxane, and Tris (1-chloropropan-2-yl) phosphate are also present in CK, which may be an artifact caused by environmental pollution.

Table 2.
VOCs’ type and peak area percentage.
A review of the literature revealed that decanal inhibits the growth of fungal pathogens [20]. Qian Li et al. found that both decanal and nonanal can suppress the germination of aflatoxin spores and mycelial growth [21]. May Khaing et al. demonstrated the effectiveness of octanol in controlling the mycelial growth of Fusarium sp. lycopersici [22]. Based on these findings, three VOCs—octanol, nonanal, and decanal—were identified as potential candidates for further investigation. The subsequent experiments will focus on these three substances.
3.2. The Effects of Three Types of VOCs on the Mycelial Growth of Fusarium
As shown in Figure 1, after the Fusarium were cultured in a constant–temperature incubator (28 °C) for 3 days, compared to the CK treatment, all three VOCs exhibited significant inhibitory effects on the mycelial growth of Fusarium. Among them, octanol was the most effective, with an inhibition rate of 81.55%. The inhibition rates for decanal and nonanal were 50.45% and 38.04%, respectively.
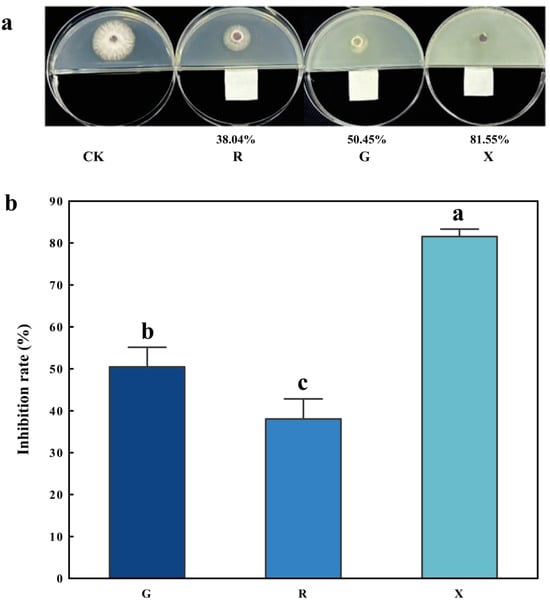
Figure 1.
Analysis of the effects and inhibition rates of three VOCs on the mycelial growth of Fusarium. (a) Plate display of the inhibitory rates of three VOCs against the Fusarium; the percentages below the plates represent the inhibitory rates. (b) Analysis of the inhibitory rates of three VOCs against the Fusarium. CK: blank treatment; G: add decanal; R: add nonanal, X: add octanol. The error bars represent the average ± s.d. (n = 6). Significant differences are indicated by different letters in the bar charts, p < 0.05. The 95% CIs are as follows: G and R (2.5197, 22.3079), G and X (−40.9942, −21.2061), R and X (−53.4080, −33.6199).
3.3. Study on the Promoting Effects of Different VOCs on Model Crops—Arabidopsis thaliana
As shown in Figure 2, when 10 μL aliquots of different VOCs were added to one side of a two-compartment medium, the biomass of Arabidopsis thaliana increased significantly in all aspects, including plant height, biomass, number of leaves, and root length. One-way ANOVA analysis revealed that, compared to the CK, all three VOCs significantly enhanced the biomass of Arabidopsis thaliana. This indicates that all three VOCs promoted growth, with decanal showing the most pronounced growth-promoting effect.
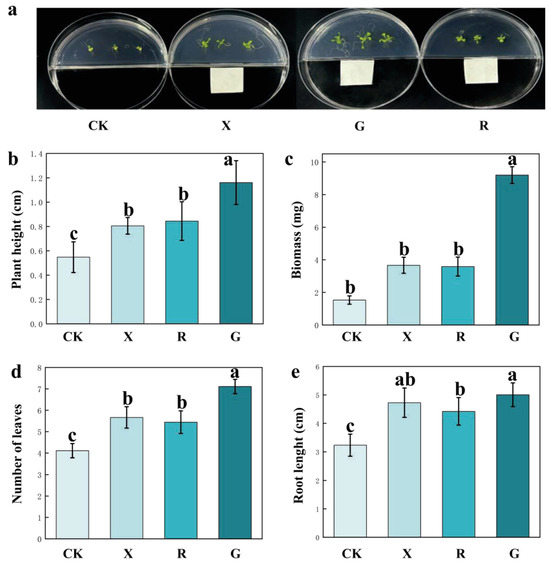
Figure 2.
Effects of different VOCs on the biomass of Arabidopsis thaliana. (a) Plate display of the growth-promoting effects of three VOCs on Arabidopsis thaliana. (b) Plant height, (c) biomass, (d) number of leaves, and (e) root length after VOC treatment. CK: blank treatment; G: add decanal; R: add nonanal, X: add octanol. The error bars represent the average ± s.d. (n = 9). Significant differences are indicated by different letters in the bar charts, p < 0.05.
3.4. The Influence of Different VOCs Added on the Microbial Community of Banana Rhizosphere
3.4.1. The Influence of VOCs on the Alpha Diversity of the Microbial Community in the Rhizosphere of Banana
As shown in Figure 3, in the fungal community, all VOC treatments, except for the R10 treatment, showed significantly lower Chao, ACE, and Richness indices compared to the CK group. Similarly, in the bacterial community, all VOC treatments, except for the R10 treatment, resulted in a significant reduction in Chao, ACE, and Richness indices relative to the CK group. Notably, the decanal treatments (G10 and G100) exhibited the most significant decline. This suggests that the addition of VOCs reduced the richness and diversity of both fungal and bacterial communities in the rhizosphere of bananas. A decrease in Alpha diversity may represent a “beneficial microbial community enrichment” state, which is selected after the addition of root VOCs. Bananas further “support” these beneficial microbial communities and inhibit other irrelevant microbial communities, thereby more accurately meeting the plant’s need for microbial services during growth. This process ultimately leads to a reduction in the Alpha diversity of rhizosphere microorganisms.
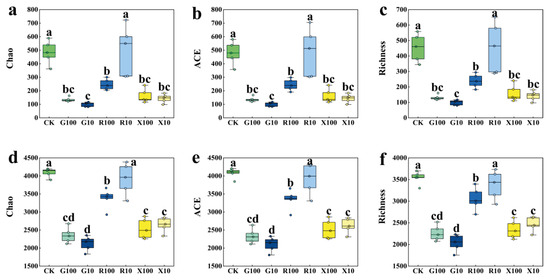
Figure 3.
Alpha diversity analysis of microorganisms in the rhizosphere of bananas. Panels (a–c) represent the Alpha diversity indices for the fungal community, while panels (d–f) correspond to the bacterial community. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol. Box plots of different colors represent different treatments, and box plots with different letters indicate significant differences, p < 0.05, n = 5. The circles represent the data distribution.
3.4.2. Analysis of the Beta Diversity of Microbial Communities in the Rhizosphere of Banana
Community structure analysis revealed significant differences in the microbial community compositions across the various treatments following the addition of VOCs. In the fungal community, the G10 treatment was distinctly separated from the CK, R10, and X10 treatments on the first principal component, while the CK treatment was significantly separated from the G10 and R10 treatments on the second principal component (Figure 4a). The CK treatment was also significantly separated from the G100, R100, and X100 treatments on the first principal component and from the G100 treatment on the second principal component (Figure 4b). In the bacterial community, both the G10 and X10 treatments were significantly separated from the CK treatment along the first principal component. The CK treatment also showed significant separation from the G10 and R10 treatments on the second principal component (Figure 4c). Moreover, the CK treatment was distinctly separated from the other three treatments on both the first and second principal components (Figure 4d). These results indicate that the addition of VOCs significantly altered the community structure of both fungi and bacteria in the rhizosphere of bananas.
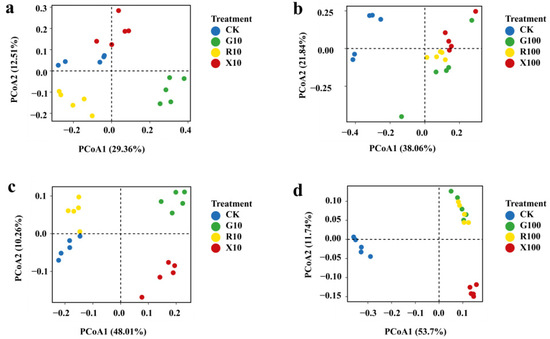
Figure 4.
Principal Coordinate Analysis (PCoA) of the microbial community in the rhizosphere of bananas after VOC addition. (a,b) PCoA of fungal communities; (c,d): PCoA of bacterial communities. n = 5. pseudo-F: 4.9627–12.924, R2: 0.482–0.70787, p-value < 0.05, permutations: 9999 times. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol.
3.4.3. Analysis of the Microbial Community Composition in the Rhizosphere of Banana
In the fungal community, as shown in Figure 5, the addition of low concentrations of VOCs led to a significant reduction in the relative abundance of Zygomycota compared to the CK treatment, while the relative abundance of Ascomycota increased significantly. Conversely, with high concentrations of VOCs, the relative abundance of Ascomycota decreased significantly, while Chytridiomycota became more abundant. In the bacterial community, after the addition of low concentrations of VOCs, the relative abundance of Proteobacteria in the X10 and G10 treatments was significantly lower than in the CK treatment, while Acidobacteria abundance increased significantly. After the addition of high concentrations of VOCs, the relative abundance of Acidobacteria further increased, while Proteobacteria abundance significantly decreased.
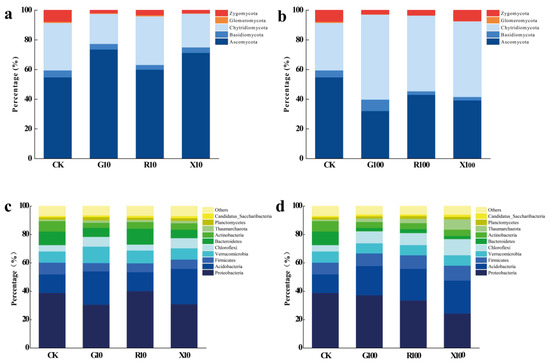
Figure 5.
Analysis of phylum-level composition of the microbial community in the rhizosphere of bananas after VOC addition. (a,b) Fungal phylum level; (c,d) bacterial phylum level. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol. Different color blocks represent different microbial phyla.
3.4.4. LEfSe Analysis of the Microbial Community in the Rhizosphere of Banana
LEfSe analysis was performed on the bacterial and fungal genera with relative abundance in the top 20%. A total of 15 differential communities were identified in the fungal community, with the CK, G10, G100, R10, R100, X10, and X100 treatments showing 3, 4, 2, 3, 1, 1, and 1 differential communities, respectively. Notably, the beneficial fungus Aspergillus was enriched in the G10 treatment (Figure 6a).
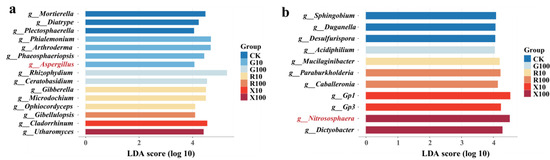
Figure 6.
Bar chart for LDA discrimination of microbial communities in the banana rhizosphere based on LEfSe analysis (LDA > 4, p < 0.05, n = 5). (a) LEfSe analysis of fungal communities; (b) LEfSe analysis of bacterial communities. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol.
In the bacterial community, 11 differential communities were identified, with the CK, G100, G10, R100, and X100 treatments exhibiting 3, 1, 1, 2, 2, and 2 differential communities, respectively. In the X100 treatment, Nitrososphaera, which promotes plant nitrogen supply, was enriched.
3.4.5. Analysis of the Relative Abundance of Three Beneficial Genera in the Microbial Community of the Banana Rhizosphere
After the addition of the three VOCs, the relative abundance of Aspergillus was significantly increased in the G10 and X10 treatments compared to the CK treatment (Figure 7a), and the relative abundance of Bacillus was significantly increased in the G100, R100, and X100 treatments compared to CK (Figure 7b). The relative abundance of Pseudomonas was significantly elevated in the G100, R100, X100, and X10 treatments compared to CK (Figure 7c). Overall, Aspergillus was enriched in the G10, G100, and X10 treatments, Pseudomonas was enriched in the X100 treatment, and Fusarium was relatively abundant in the R100 treatment. This suggests that, with the exception of the R100 treatment, VOCs play a positive role in promoting the enrichment of beneficial genera in the banana rhizosphere (Figure 7e).
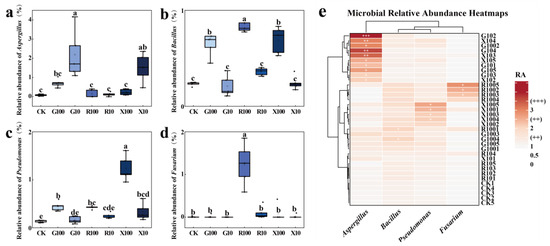
Figure 7.
Box plots of the relative abundance of three identified beneficial genera and Fusarium. (a) Relative abundance of Aspergillus; (b) relative abundance of Bacillus; (c) relative abundance of Pseudomonas; (d) relative abundance of Fusarium; (e) heat map of Fusarium and the three beneficial genera. RA (%) > 1 is marked as “+”, >2 as “++”, and >3 as “+++”. Significant differences are indicated by different letters in the bar charts, p < 0.05, n = 5. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol. The rhombus represent the data distribution. The squares represent the mean.
3.4.6. Collinear Network Analysis of Microbial Communities in the Rhizosphere of Banana Roots
The collinear network analysis of the fungal and bacterial communities in the rhizosphere of bananas after the addition of different VOCs is presented in Figure 8a,b.
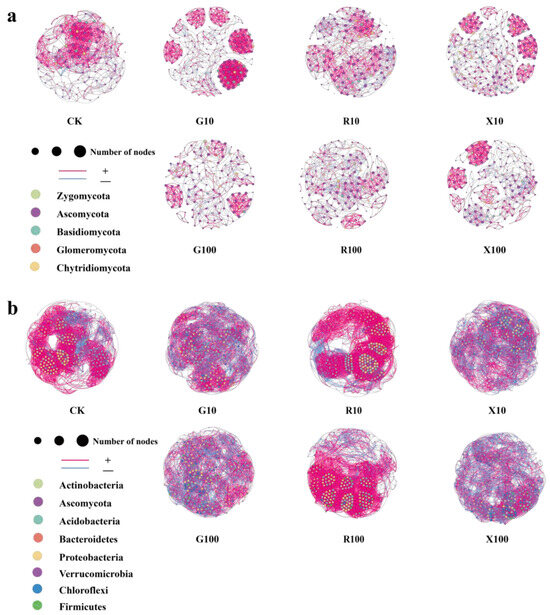
Figure 8.
Collinear network analysis of the rhizosphere community in bananas after the addition of VOCs. (a) Collinear network analysis of the rhizosphere fungal community; (b) collinear network analysis of the rhizosphere bacterial community. Each dot, representing an OTU species, is colored and sized differently. In the network, red lines indicate positive correlations between species, while blue lines represent negative correlations. n = 5. CK: add equal volume of sterile water; G100: add 100 μg/g decanal; G10: add 10 μg/g decanal; R100: add 100 μg/g nonanal; R10: add 10 μg/g nonanal; X100: add 100 μg/g octanol; X10: add 10 μg/g octanol.
The topological analysis results of the banana rhizosphere fungal network after the addition of VOCs are shown in Table 3. With the exception of the CK, the modularity index values of all other treatments were higher than 0.6, indicating that the network exhibits a typical modular structure. Modularity increased in all treatments, except for R100. After the addition of VOCs, the number of network nodes and the average path length both increased in each treatment compared to the CK. Specifically, the number of edges, average weight degree, and average path length in G10 were higher than in CK. The topological analysis results for the banana rhizosphere bacterial network after the addition of VOCs are presented in Table 4. Following the addition of VOCs, the average weight degree and network diameter decreased in each treatment. Except for the R10 and R100 treatments, the number of edges and network density decreased in all treatments.

Table 3.
Analysis of topological properties of the rhizosphere fungi network in banana.

Table 4.
Analysis of topological properties of bacteria network in the rhizosphere of banana.
The indices in the table correspond to the participation degree of biological entities in banana soil, interaction mode, network association strength and structure, signal transmission efficiency, functional module differentiation, etc. In general, low-concentration treatments (G10, R10, X10) mostly show activation of the network, which increases the association strength, promotes module aggregation, and optimizes functional differentiation, while high-concentration treatments (G100, R100, X100) inhibit the network, reducing associations, decreasing accessibility, and weakening module structure.
3.4.7. Linear Models (LMs) for the Relationship of Microbial Indicators with Relative Abundance of Fusarium and the Relative Importance of Each Predictor
An LM analysis was conducted, as shown in Table 5 using the relative abundance (RA) of Aspergillus, Pseudomonas, Bacillus, Talaromyces, Penicillium, bacterial richness (bac_Richness), bacterial Chao index (bac_Chao), bacterial Ace index (bac_Ace), fungal richness (fun_Richness), fungal Chao index (fun_Chao), and fungal Ace index (fun_Ace) as microbial indicators in relation to the relative abundance of Fusarium. After the exclusion of inappropriate indicators, the optimized model was obtained, as shown in Table 5. The analysis revealed that the key indicators in this model were as follows: RA of Pseudomonas (F = 0.517, p = 0.487, relative importance = 8.85%); RA of Bacillus (F = 20.694, p < 0.001, relative importance = 24.38%); bac_Chao (F = 1.504, p = 0.246, relative importance = 13.03%); fun_Ace (F = 17.07, p < 0.001, relative importance = 15.53%). These factors, either directly or indirectly, influence the relative abundance of Fusarium, thereby promoting the healthy growth of bananas.

Table 5.
Linear models (LMs) for the relationship of microbial indicators with the relative abundance of Fusarium and the relative importance of each of the predictors in the model.
4. Discussion
VOCs serve as a chemical communication language among microorganisms and play a pivotal role in plant–Fusarium interactions [23], influencing crop growth and enhancing plant resistance [24,25]. VOCs are also closely linked to microbial regulation and root communication [26].
In this study, a combination of SPME and GC-MS was employed to collect and identify the VOCs produced by the root system of pineapples. Three potential VOCs were selected based on their known effects on Fusarium. Research has documented their role in either inhibiting Fusarium or promoting plant growth [27,28], as well as the influence of similar substances [29]. For example, volatile components from fresh tea leaves exhibit a fumigation inhibitory effect on tea plant pathogens [30]. Arabidopsis thaliana seedlings exposed to Trichoderma VOCs showed greater leaf surface area and more lateral roots compared to control plants [31]. Paraburkholderia phytofirmans PsJN is known to promote growth in various plant hosts and enhance their tolerance to stressors such as cold, drought, and salinity [32]. Our experiment yielded similar results. Under in vitro conditions, all three VOCs significantly inhibited the mycelial growth of Fusarium, with octanol achieving an inhibition rate of 81.55% (Figure 1). Furthermore, these VOCs promoted plant height, biomass, leaf number, and root length in Arabidopsis thaliana (Figure 2).
Root secretions from plant roots and soil microorganisms can influence the composition and structure of soil microbial communities [33,34]. VOCs from potatoes and onions can reduce the diversity of the rhizosphere soil in tomatoes [19]. Similarly, root volatiles released by P. americana under Mn treatment were found to decrease the Alpha diversity of its bacterial community, with Shannon and Simpson indices being significantly reduced by 15% and 16%, respectively [35]. Research by Ning Ling et al. confirmed that reducing fungal diversity can help decrease fungal diseases [36]. Consistent with these findings, after the addition of VOCs to the pot soil as part of root secretions, the richness and diversity of the fungal and bacterial communities in the rhizosphere of bananas decreased. This reduction in diversity is likely driven specifically by VOCs rather than being a random decline. VOCs may act as a chemical inducer for beneficial genera while inhibiting other less adaptable ones, which directly contributes to Fusarium suppression (Figure 3). This may contribute to VOCs’ beneficial effect on soil health. Principal Coordinate Analysis (PCoA) further revealed significant separation of microbial communities along the coordinate axes (Figure 4), and changes in the microbial composition of communities such as Ascomycota, Chytridiomycota, Proteobacteria, and Acidobacteria were noted (Figure 5). LEfSe analysis of fungal and bacterial communities identified differential microbial communities in each treatment (Figure 6). Anwer and Md Arshad et al. found that Aspergillus niger can act as a quality enhancer and plant health promoter for tomato fruits (Lycopersicum esculentum Mill) [37]. Nitrososphaera, an ammonia-oxidizing archaeon involved in soil nitrification, converts ammonia to nitrite, providing essential nitrogen nutrients for plants and playing a key role in the nitrogen cycle and plant nitrogen supply [38].
Plant roots interact with rhizosphere microorganisms through VOCs, inhibiting competitors or recruiting beneficial genera to accumulate in the rhizosphere, thereby promoting a healthy rhizosphere microecology [39]. Further analysis of the relative abundance of Aspergillus, Pseudomonas, and Bacillus—microorganisms known for their antagonistic effects on Fusarium—showed significant increases in their abundance in the rhizosphere soil. However, except for the R10 and R100 treatments, no significant effect on the relative abundance of Fusarium was observed (Figure 7). Collinear network analysis of fungi and bacteria revealed that the strengthening of cooperative interactions within the community in the low-concentration treatment group was more active than in the high-concentration group. This may be due to high-concentration VOC treatments inhibiting the growth of certain microorganisms, leading to a lower microbial diversity than in the low-concentration group. Meanwhile, it revealed that low-concentration treatments (G10, R10, X10) increased the association strength of the microbial network and optimized functional differentiation, whereas high-concentration treatments (G100, R100, X100) inhibited the network and reduced associations. This indicates that the addition of low-concentration VOCs is more beneficial to banana growth than high-concentration ones (Figure 8). LMs analyzing the relationship between microbial indicators and the relative abundance of Fusarium, along with the relative importance of each predictor, identified the relative abundance of Pseudomonas, Bacillus, bac_Richness, and bac_ACE as important indicators (Table 5). This suggests that the bacterial community plays an important role in the system. VOCs may induce changes in the root secretions of bananas, recruiting beneficial bacteria genera like Pseudomonas and Bacillus to accumulate in the rhizosphere, thereby promoting plant growth.
Numerous studies have demonstrated that VOCs play multiple roles in the soil microecological environment. They participate in the transformation and circulation of substances within the soil [40], influencing the growth, reproduction, and metabolic activities of soil microorganisms [41]. Thus, through these mechanisms, VOCs contribute to the development of a more stable and healthy soil microecosystem, providing a solid environmental foundation for banana growth. The three VOCs selected in this study, all derived from the root system of pineapples, exhibited significant growth-promoting effects on Arabidopsis thaliana and inhibitory impacts on Fusarium. This suggests that these VOCs likely contribute to the disease-suppressing mechanisms in bananas. In actual banana cultivation, the rational use of these VOCs, obtained from pineapple roots, could be considered. Through indirect microbial mediation, VOCs act as stimulants for beneficial microorganisms, driving a targeted reduction in soil biodiversity and an enrichment of beneficial microorganisms. In this way, VOCs reshape the rhizosphere environment. This selective filtering alters the composition and structure of microbial communities, which in turn modulates soil properties to foster a more stable soil microecology. The optimization of soil microecology through VOCs offers valuable guidance for the future healthy production of bananas.
As eco-friendly chemical agents, VOCs are poised to play a significant role in green and sustainable agriculture [23]. They could be added to bio-organic fertilizers or soil conditioners, acting as “microbial regulators” to enrich beneficial genera in the soil of degraded banana plantations and address continuous cropping obstacles caused by microecological imbalance. Alternatively, bananas can be intercropped with cover crops to enable continuous in situ release of VOCs in the field. However, their effects are concentration-dependent and species-specific, and challenges such as poor field stability and difficulties in large-scale production remain [42]. The properties of VOCs mean that their effective duration in field soils is far shorter than the growth cycle of bananas. In the future, VOCs could be encapsulated in biodegradable carriers to extend their release period. Therefore, further research combining metabolomics is needed to develop sustained-release carriers or engineered strains for more efficient use of VOCs.
5. Conclusions
The inhibitory effects of the three VOCs on Fusarium and their growth-promoting effects on Arabidopsis thaliana were confirmed through in vitro inhibition experiments using two-compartment culture dishes. Our pot experiment results further revealed that VOCs altered soil properties, affecting the composition and structure of the rhizosphere microbial community, reducing soil biodiversity, and increasing the relative abundance of beneficial genera, thereby promoting a healthier rhizosphere microecology for bananas. These findings also provide directions for investigating VOC-mediated assembly characteristics of rhizosphere microorganisms in banana cultivation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15112520/s1: Table S1: The physicochemical properties of pot soil (latosol).
Author Contributions
X.C.: Conceptualization, Methodology, Software, Visualization, Writing—Review and Editing. Y.L.: Resources, Supervision. T.J.: Resources, Supervision. P.L.: Resources, Supervision. X.D.: Resources, Supervision. J.Y.: Resources, Supervision. B.W.: Investigation, Conceptualization, Methodology, Writing—Review and Editing, Supervision, Funding Acquisition. R.L.: Resources, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Program of China (2023YFD1901402), the National Natural Science Foundation of China (42367015, 42507408), and the Key Research and Development Project of Hainan Province (ZDYF2025XDNY087).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available. The sequencing data have been submitted to the NCBI Sequence Read Archive database (SRR35566461-SRR35566500). BioProject ID: PRJNA1332960. BioSample accessions: SAMN51784880-SAMN51784919.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sawarkar, A.N.; Kirti, N.; Tagade, A.; Tekade, S.P. Bioethanol from various types of banana waste: A review. Bioresour. Technol. Rep. 2022, 18, 101092. [Google Scholar] [CrossRef]
- Tamang, P.; Kumar, P.; Chauhan, A.; Rastogi, S.; Srivastava, S.; Jena, S.N. Molecular insights into the variability and pathogenicity of Fusarium odoratissimum, the causal agent of Panama wilt disease in banana. Microb. Pathog. 2024, 190, 106594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Q.; Wang, Y.; Chen, X.; Gao, W.; Zhao, Y.; Wang, B.; Ruan, Y. Suppression of banana fusarium wilt disease with soil microbial mechanisms via pineapple rotation and residue amendment. Agronomy 2023, 13, 377. [Google Scholar] [CrossRef]
- Yang, J.; Ren, X.; Liu, M.; Fan, P.; Ruan, Y.; Zhao, Y.; Wang, B. Suppressing soil-borne Fusarium pathogens of bananas by planting different cultivars of pineapples, with comparisons of the resulting bacterial and fungal communities. Appl. Soil Ecol. 2022, 169, 104211. [Google Scholar] [CrossRef]
- Gong, A.D.; Li, H.P.; Shen, L.; Zhang, J.B.; Wu, A.B.; He, W.J.; Yuan, Q.S.; He, J.D.; Liao, Y.C. The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front. Microbiol. 2015, 6, 1091. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. J. Plant Interact. 2022, 17, 685–694. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X. Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The chemistry of stress: Understanding the ‘cry for help’ of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, M.; Li, R.; Huang, Q.; Raza, W.; Rensing, C.; Shen, Q. Microbial volatile compounds alter the soil microbial community. Environ. Sci. Pollut. Res. 2017, 24, 22485–22493. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhao, Y.; Feng, J.; Chen, Y.; Li, K.; Zhang, M.; Qi, D.; Zhou, D.; Wei, Y.; et al. Biocontrol potential of volatile organic compounds produced by Streptomyces corchorusii CG-G2 to strawberry anthracnose caused by Colletotrichum gloeosporioides. Food Chem. 2024, 437, 137938. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Chen, C.; Zhou, Y.; Xiao, F.; Wang, Y.; Li, Q. Effect of plant VOCs and light intensity on growth and reproduction performance of an invasive and a native Phytolacca species in China. Ecol. Evol. 2022, 12, e8522. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, V.; Marcianò, D.; Sargolzaei, M.; Maddalena, G.; Maghradze, D.; Tirelli, A.; Casati, P.; Bianco, P.A.; Failla, O.; Fracassetti, D.; et al. From plant resistance response to the discovery of antimicrobial compounds: The role of volatile organic compounds (VOCs) in grapevine downy mildew infection. Plant Physiol. Biochem. 2021, 160, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Huang, J.A.; Huang, J.; Wu, W.; Tong, T.; Liu, S.; Zhou, L.; Liu, Z.; Zhang, S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. Lwt-Food. Sci. Technol. 2022, 164, 113656. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Xiao, L.; Zhang, X.; Yang, C.; Li, Z.; Zhu, M.; Liu, Z.; Wang, Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS–SPME/GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef]
- Leschevin, M.; Ismael, M.; Quero, A.; San Clemente, H.; Roulard, R.; Bassard, S.; Maicelo, P.; Pageau, K.; Elisabeth, J.; Rayon, C. Physiological and biochemical traits of two major Arabidopsis accessions, Col-0 and Ws, under salinity. Front. Plant Sci. 2021, 12, 639154. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.T.; Chung, F.Y.; Moyle, P.M.; Eltanahy, E.G.; Schenk, P.M. Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth. Sci. Total Environ. 2019, 692, 267–280. [Google Scholar] [CrossRef]
- Ji, X.Y.; Ye, C.; Kang, W.; Luan, W.; Liu, Y.; He, X.; Yang, M.; Sun, L.; Sun, W.; Huang, H.; et al. Interspecific allelopathic interaction primes direct and indirect resistance in neighboring plants within agroforestry systems. Plant Commun. 2025, 6, 1. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Shi, J.; Khashi u Rahman, M.; Liu, H.; Wei, Z.; Wu, F.; Dini-Andreote, F. Volatile-Mediated Interspecific Plant Interaction Promotes Root Colonization by Beneficial Bacteria via Induced Shifts in Root Exudation. Microbiome 2024, 12, 207. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Ye, B.; Shi, L.; Bai, X.; Lai, T. Effects of essential oil decanal on growth and transcriptome of the postharvest fungal pathogen Penicillium expansum. Postharvest Biol. Technol. 2018, 145, 203–212. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal properties and mechanisms of three volatile aldehydes (octanal, nonanal and decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Hpoo, M.K.; Mishyna, M.; Prokhorov, V.; Arie, T.; Takano, A.; Oikawa, Y.; Fujii, Y. Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici. Sustainabllity 2020, 12, 9334. [Google Scholar] [CrossRef]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.G.; Erb, M. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial volatile organic compounds: An alternative for chemical fertilizers in sustainable agriculture development. Microorganisms 2022, 11, 42. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Tyagi, S.; Mulla, S.I.; Lee, K.J.; Chae, J.C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- de Boer, W.; Li, X.; Meisner, A.; Garbeva, P. Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol. Ecol. 2019, 95, fiz105. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Li, Y.B.; Qi, L.; Wan, X.C. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J. Agric. Food Chem. 2006, 54, 3936–3940. [Google Scholar] [CrossRef]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E. Brettell. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 2019, 24, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lin, T.; Liu, D.; Wang, Y.; Xu, X.; Xu, Y.; Siemann, E.; Li, B. Changes in Soil Microbiome Mediated by Root Volatiles Enhanced Manganese Tolerance of an Invasive Plant Species. Plant Cell Environ. 2025, 48, 6605–6617. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Deng, K.; Song, Y.; Wu, Y.; Zhao, J.; Raza, W.; Huang, Q.; Shen, Q. Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Microbiol. Res. 2014, 169, 570–578. [Google Scholar] [CrossRef]
- Anwer, M.A.; Khan, M.R. Aspergillus niger as tomato fruit (Lycopersicum esculentum Mill.) quality enhancer and plant health promoter. J. Postharvest Technol. 2013, 1, 36–51. [Google Scholar]
- Bei, Q.; Reitz, T.; Schädler, M.; Hodgskiss, L.H.; Peng, J.; Schnabel, B.; Buscot, F.; Eisenhauer, N.; Schleper, C.; Heintz-Buschart, A. Metabolic potential of Nitrososphaera-associated clades. ISME J. 2024, 18, wrae086. [Google Scholar] [CrossRef]
- Liu, R.; Li, R.; Li, Y.; Li, M.; Ma, W.; Zheng, L.; Wang, C.; Zhang, K.; Tong, Y.; Huang, G.; et al. Benzoic acid facilitates ANF in monocot crops by recruiting nitrogen-fixing Paraburkholderia. ISME J. 2024, 18, wrae210. [Google Scholar] [CrossRef]
- Asensio, D.; Yuste, J.C.; Mattana, S.; Ribas, À.; Llusià, J.; Peñuelas, J. Litter VOCs induce changes in soil microbial biomass C and N and largely increase soil CO2 efflux. Plant Soil 2012, 360, 163–174. [Google Scholar] [CrossRef]
- Werner, S.; Polle, A.; Brinkmann, N. Belowground communication: Impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 2016, 100, 8651–8665. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Mooventhan, P.; Das, D.; Dixit, A.; Sharma, K.C.; Senthil-Nathan, S.; Kaushal, P.; Ghosh, P.K. The future of plant volatile organic compounds (pVOCs) research: Advances and applications for sustainable agriculture. Environ. Exp. Bot. 2022, 200, 104912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).