Biological Control Potential of Entomopathogenic Fungi Against Aleurocanthus spiniferus: Field Trials on Citrus sinensis in Agroforestry Ecosystems
Abstract
1. Introduction
2. Materials and Methods
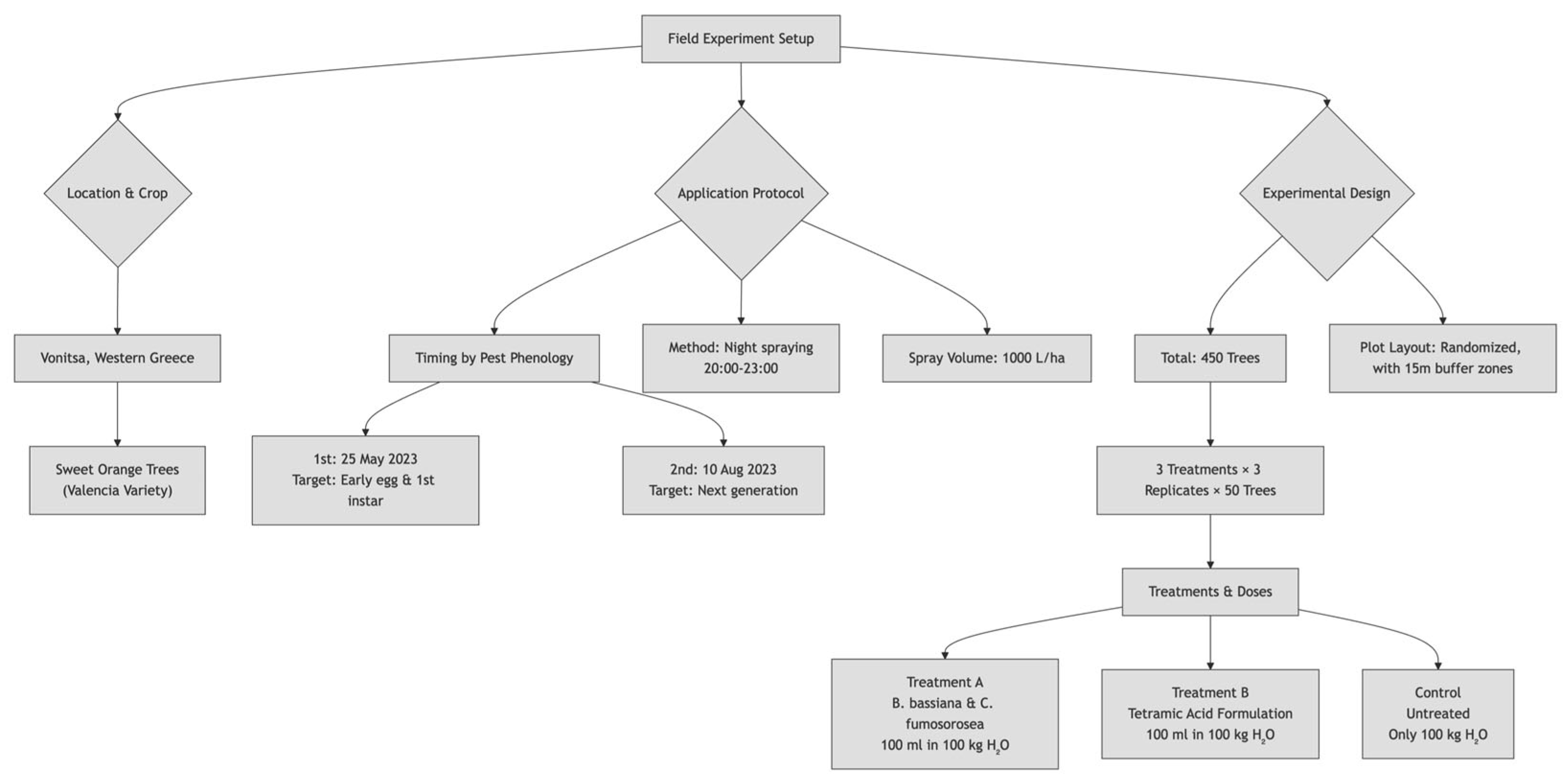
2.1. Field Site and Experimental Design
2.2. Monitoring of A. spiniferus
2.3. Mathematical Estimation
- -
- Actual (or “real”) mortality was calculated as , where represents the number of individuals dying during stage x and is the number of insects present at the start of the generation. This measure provides the cumulative proportion lost across all stages when summed over the entire life cycle.
- -
- Apparent mortality ( ) was defined as the proportion of individuals dying within a given stage relative to those alive at the beginning of that stage. These values can be partitioned into specific factors within each stage, but they can only be combined within that stage rather than across different stages.
- -
- Marginal mortality for a given factor B was obtained using the expression , where is the apparent mortality from factor B and is the cumulative apparent mortality from all other factors. Marginal stage-specific mortalities were further transformed into k-values according to , where is the marginal mortality rate of interest. k-values are additive across factors and stages, simplifying overall analysis. Mortality proportions can be back-calculated from k-values using the relation .
- -
- Irreplaceable mortality for a given factor was derived as the difference between the total proportional mortality and the proportional mortality recalculated after removing the k-value of that factor .
2.4. Statistical Analysis
3. Results
3.1. Observed, Apparent, and Marginal Mortality of A. spiniferus
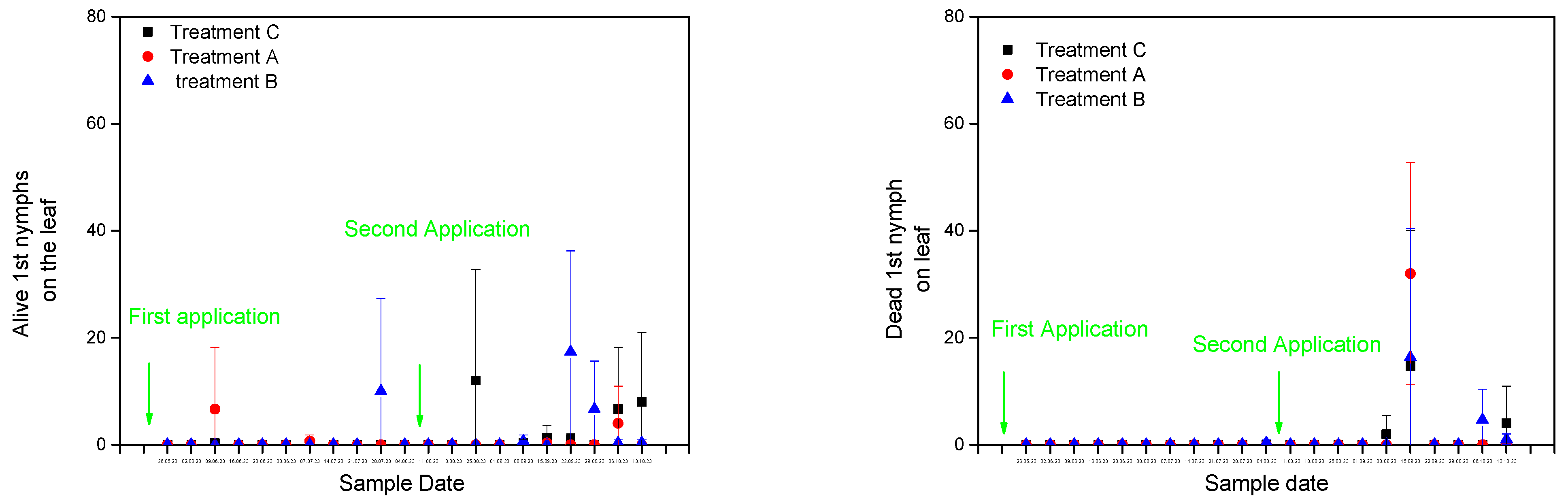
3.2. Alive and Dead Biological Stages of A. spiniferus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seminara, S.; Bennici, S.; Di Guardo, M.; Caruso, M.; Gentile, A.; La Malfa, S.; Distefano, G. Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives. Agriculture 2023, 13, 264. [Google Scholar] [CrossRef]
- Moore, S.D.; Duncan, L.W. Microbial Control of Insect and Mite Pests of Citrus. In Microbial Control of Insect and Mite Pests: From Theory to Practice; Academic Press: Cambridge, MA, USA, 2017; pp. 283–298. [Google Scholar] [CrossRef]
- Malumphy, C.; Radonjić, S.; Hrnčić, S.; Raičević, M. New Data on the Whiteflies (Insecta: Hemiptera: Aleyrodidae) of Montenegro, Including Three Species New For The Country. Acta Entomol. Serbica 2015, 20, 29–41. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest Categorisation of Aleurocanthus Spp. EFSA J. 2018, 16, e05436. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, S.; Hrnčić, S.; Malumphy, C. First Record of Aleurocanthus spiniferus (Quaintance) (Hemiptera Aleyrodidae) in Montenegro. Redia-G. Di Zool. 2014, 97, 141–145. [Google Scholar]
- Kapantaidaki, D.E.; Antonatos, S.; Kontodimas, D.; Milonas, P.; Papachristos, D.P. Presence of the Invasive Whitefly Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Greece. EPPO Bull. 2019, 49, 127–131. [Google Scholar] [CrossRef]
- Massimino Cocuzza, G.E.; Jovičić, I.; Frisenna, F.; Tumminelli, R.; Siscaro, G. Discovery of Serangium montazerii Fürsch (Coleoptera, Coccinellidae) as a Predator of Aleurocanthus spiniferus (Quaintance) (Hemiptera, Aleyrodidae) in Italy. EPPO Bull. 2023, 53, 376–386. [Google Scholar] [CrossRef]
- Jia, Z.F.; Cui, Y.G.; Liu, M.Y.; Kabissa, J.J.; Xu, Y.Y.; Kang, Z.W.; Chen, Z.Z. Brief Warm and Aldo-Keto Reductase Family AspiAKR1B1 Contribute to Cold Adaptation of Aleurocanthus spiniferus. Insects 2025, 16, 38. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Banazeer, A.; Serrao, J.E.; Rizwan, M.; Naeem, A.; Afzal, M.B.S.; Banazeer, A.; Serrao, J.E.; Rizwan, M.; Naeem, A. Ecology, Biology, Damage, and Management of Sucking and Chewing Insect Pests of Citrus. In Citrus Research—Horticultural and Human Health Aspects; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Davidson, E.W.; Segura, B.J.; Steele, T.; Hendrix, D.L. Microorganisms Influence the Composition of Honeydew Produced by the Silverleaf Whitefly, Bemisia argentifolii. J. Insect Physiol. 1994, 40, 1069–1076. [Google Scholar] [CrossRef]
- Šimala, M.; Masten Milek, T. First Record of Whitefly Quarantine Species Aleurocanthus spiniferus Quaintance, 1903 (Hemiptera: Aleyrodidae) in Croatia. Glas. Biljn. Zaštite 2013, 13, 425–433. [Google Scholar]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.M.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Some European Countries: Diffusion, Hosts, Molecular Characterization, and Natural Enemies. Insects 2020, 11, 42. [Google Scholar] [CrossRef]
- Mokrane, S.; Cavallo, G.; Tortorici, F.; Romero, E.; Fereres, A.; Djelouah, K.; Verrastro, V.; Cornara, D. Behavioral Effects Induced by Organic Insecticides Can Be Exploited for a Sustainable Control of the Orange Spiny Whitefly Aleurocanthus spiniferus. Sci. Rep. 2020, 10, 15746. [Google Scholar] [CrossRef]
- George, A.; Rao, C.N.; Mani, M. Pests of Citrus and Their Management. In Trends in Horticultural Entomology; Springer: Singapore, 2022; pp. 551–575. [Google Scholar] [CrossRef]
- Sani, I.; Ismail, S.I.; Abdullah, S.; Jalinas, J.; Jamian, S.; Saad, N. A Review of the Biology and Control of Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with Special Reference to Biological Control Using Entomopathogenic Fungi. Insects 2020, 11, 619. [Google Scholar] [CrossRef]
- Kambrekar, D.N.; Aruna, J. Screening for Endophytic Beauveria bassiana from Different Plants and Its Pathogenecity against Chickpea Pod Borer, Helicoverpa armigera (Hubner). J. Exp. Zool. 2018, 21, 727–731. [Google Scholar]
- Priyashantha, A.K.H.; Galappaththi, M.C.A.; Karunarathna, S.C.; Lumyong, S. Entomopathogenic Fungi: Bioweapons against Insect Pests. In The Role of Entomopathogenic Fungi in Agriculture; CRC Press: Boca Raton, FL, USA, 2024; pp. 91–116. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Effect of Seed Treatment Duration on Growth and Colonization of Vicia Faba by Endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control. 2016, 103, 187–195. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of Entomopathogenic Fungi in Integrated Pest Management. In Integrated Pest Management: Current Concepts and Ecological Perspective; Academic Press: San Diego, CA, USA, 2014; pp. 169–191. [Google Scholar] [CrossRef]
- Osborne, L.S.; Landa, Z. Biological Control of Whiteflies with Entomopathogenic Fungi. Fla. Entomol. 1992, 75, 456. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, N.; Shi, L.; Qin, Z.Y.; He, P.; Hu, D.Y.; Tan, X.F.; Chen, Z. Evaluation of the Attractive Effect of Coloured Sticky Traps for Aleurocanthus spiniferus (Quaintance) and Its Monitoring Method in Tea Garden in China. J. Entomol. Acarol. Res. 2015, 47, 86–90. [Google Scholar] [CrossRef]
- Stathas, G.J.; Kavallieratos, N.G.; Cheliotis, L.N.; Skouras, P.J.; Giakoumaki, M.V.; Milonas, P.G. New Data on the Parasitization of Aleurothrixus Fl Occosus (Maskell) (Hemiptera: Aleyrodidae) in Greece. Hell. Plant Prot. J. 2023, 16, 79–82. [Google Scholar] [CrossRef]
- Avery, P.B.; Kumar, V.; Francis, A.; McKenzie, C.L.; Osborne, L.S. Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida. Insects 2020, 11, 590. [Google Scholar] [CrossRef]
- Lima, B.M.F.V.; de Almeida, J.E.M.; Moreira, J.O.T.; dos Santos, L.C.; Bittencourt, M.A.L. Entomopathogenic Fungi Associated with Citrus Blackfly (Aleurocanthus woglumi Ashby) in Southern Bahia. Arq. Inst. Biol. 2018, 84, 1–4. [Google Scholar] [CrossRef][Green Version]
- Barbosa, L.F.S.; da Silva Santos, A.C.; Diniz, A.G.; Alves, A.L.; de Oliveira, A.F.M.; da Costa, A.F.; Tiago, P.V. Entomopathogenicity of Fungi in Combination with Ricinus Communis Extract for the Control of Aleurocanthus woglumi. Entomol. Exp. Appl. 2021, 169, 838–847. [Google Scholar] [CrossRef]
- Yoon, H.G.; Shin, T.Y.; Yu, M.R.; Lee, W.W.; Ko, S.H.; Bae, S.M.; Choi, J.B.; Woo, S.D. Characterization of Entomopathogenic Fungus from Trialeurodes vaporariorum and Evaluation as Insecticide. Korean J. Microbiol. 2013, 49, 64–70. [Google Scholar] [CrossRef]
- Pourtaghi, E.; Talaei-Hassanloui, R.; Nasibi, F.; Fotouhifar, K.-B. Endophytic Colonization of Tomato by Beauveria bassiana for Control of the Greenhouse Whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Acta Biol. 2020, 27, 149–160. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science: Oxford, UK, 2009; ISBN 1444312308. [Google Scholar]
- Quesada-Moraga, E.; Ruiz-García, A.; Santiago-Álvarez, C. Laboratory Evaluation of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae against Puparia and Adults of Ceratitis Capitata (Diptera: Tephritidae). J. Econ. Entomol. 2006, 99, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Carruthers, R.I.; Jaronski, S.T.; Bradley, C.A.; Garza, C.J.; Galaini-Wraight, S. Evaluation of the Entomopathogenic Fungi Beauveria bassiana and Paecilomyces Fumosoroseus for Microbial Control of the Silverleaf Whitefly, Bemisia argentifolii. Biol. Control. 2000, 17, 203–217. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular Enzyme Activity of Entomopathogenic Fungi, Beauveria bassiana and Metarhizium anisopliae and Their Pathogenicity Potential as a Bio-Control Agent against Whitefly Pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef]
- Maketon, M.; Orosz-Coghlan, P.; Hotaga, D. Laboratory and Field Evaluation of Beauveria bassiana for Controlling Mulberry Whitefly Pealius Mori Takahashi (Homoptera: Aleyrodidae) in Mulberry (Morus Alba Linn). J. Pest Sci. (2004) 2009, 82, 251–259. [Google Scholar] [CrossRef]
- Sun, T.; Shen, Z.; Shaukat, M.; Du, C.; Ali, S. Endophytic Isolates of Cordyceps fumosorosea to Enhance the Growth of Solanum Melongena and Reduce the Survival of Whitefly (Bemisia tabaci). Insects 2020, 11, 78. [Google Scholar] [CrossRef]
- Zafar, J.; Freed, S.; Khan, B.A.; Farooq, M. Effectiveness of Beauveria bassiana Against Cotton Whitefly, Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on Different Host Plants. Pakistan J. Zool. 2016, 48, 91–99. [Google Scholar]
- Bohatá, A.; Folorunso, E.A.; Lencová, J.; Osborne, L.S.; Mraz, J. Control of Sweet Potato Whitefly (Bemisia tabaci) Using Entomopathogenic Fungi under Optimal and Suboptimal Relative Humidity Conditions. Pest Manag. Sci. 2024, 80, 1065–1075. [Google Scholar] [CrossRef]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A Comprehensive List with Worldwide Coverage and International Classification of Formulation Types. Biol. Control. 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Li, Y.Y.; Xu, C.; Wu, Y.X.; Zhang, Y.R.; Liu, H. Endophytic Colonization by Beauveria bassiana Increases the Resistance of Tomatoes against Bemisia tabaci. Arthropod Plant Interact. 2020, 14, 289–300. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Hajek, A.E.; Leger, R.J.S. Interactions between Fungal Pathogens and Insect Hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Yun, H.G.; Kim, D.J.; Gwak, W.S.; Shin, T.Y.; Woo, S.D. Entomopathogenic Fungi as Dual Control Agents against Both the Pest Myzus Persicae and Phytopathogen Botrytis Cinerea. Mycobiology 2017, 45, 192–198. [Google Scholar] [CrossRef]
- Butt, T.M.; Jackson, C.; Magan, N. Fungi as Biocontrol Agents: Progress, Problems and Potential; CAB International: Wallingford, UK, 2001. [Google Scholar] [CrossRef]





| Stage/Factor | Biological Stage | Observed Mortality * | Apparent Mortality ** | Marginal Mortality *** | k-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Factor (lx) | Stage (dx) | Factor (dx) | Stage (dx/l0) | Factor (dx/l0) | Stage (qx) | Factor (qx) | |||
| Egg | 1000 | 10 | 0.010 | 0.010 | |||||
| Effect | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Unknown | 10 | 0.010 | 0.010 | 0.015 | 0.026 | ||||
| 1st Instar | 990 | 3 | 0.003 | 0.004 | |||||
| Effect | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Unknown | 3 | 0.003 | 0.008 | 0.009 | 0.018 | ||||
| 2nd Instar | 987 | 3 | 0.0005 | 0.128 | |||||
| Effect | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Unknown | 9 | 0.009 | 0.007 | 0.006 | 0.012 | ||||
| 3rd Instar | 984 | 0 | 0.000 | 0.000 | |||||
| Effect | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Unknown | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| 4th Instar | 984 | 0 | 0.000 | 0.000 | |||||
| Effect | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Unknown | 0 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Stage/Factor | Biological Stage | Observed Mortality * | Apparent Mortality ** | Marginal Mortality *** | k-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Factor (lx) | Stage (dx) | Factor (dx) | Stage (dx/l0) | Factor (dx/l0) | Stage (qx) | Factor (qx) | |||
| Egg | 1000 | 665 | 0.665 | 0.665 | |||||
| Effect | 524 | 0.524 | 0.524 | 0.588 | 0.801 | ||||
| Unknown | 141 | 0.141 | 0.141 | 0.374 | 0.426 | ||||
| 1st Instar | 335 | 72 | 0.072 | 0.495 | |||||
| Effect | 52 | 0.052 | 0.379 | 0.385 | 0.489 | ||||
| Unknown | 20 | 0.020 | 0.142 | 0.150 | 0.232 | ||||
| 2nd Instar | 263 | 101 | 0.101 | 0.548 | |||||
| Effect | 92 | 0.092 | 0.501 | 0.698 | 0.964 | ||||
| Unknown | 9 | 0.009 | 0.028 | 0.033 | 0.048 | ||||
| 3rd Instar | 162 | 98 | 0.99 | 0.668 | |||||
| Effect | 92 | 0.092 | 0.596 | 0.967 | 0.783 | ||||
| Unknown | 7 | 0.007 | 0.021 | 0.029 | 0.042 | ||||
| 4th Instar | 64 | 51 | 0.050 | 0.822 | |||||
| Effect | 50 | 0.050 | 0.470 | 0.511 | 0.711 | ||||
| Unknown | 1 | 0.001 | 0.003 | 0.004 | 0.013 | ||||
| Stage/Factor | Biological Stage | Observed Mortality * | Apparent Mortality ** | Marginal Mortality *** | k-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Factor (lx) | Stage (dx) | Factor (dx) | Stage (dx/l0) | Factor (dx/l0) | Stage (qx) | Factor (qx) | |||
| Egg | 1000 | 678 | 0.678 | 0.678 | |||||
| Effect | 570 | 0.570 | 0.570 | 0.666 | 0.828 | ||||
| Unknown | 108 | 0.108 | 0.108 | 0.274 | 0.226 | ||||
| 1st Instar | 322 | 58 | 0.258 | 0.547 | |||||
| Effect | 48 | 0.048 | 0.379 | 0.485 | 0.589 | ||||
| Unknown | 10 | 0.010 | 0.042 | 0.050 | 0.032 | ||||
| 2nd Instar | 264 | 92 | 0.092 | 0.628 | |||||
| Effect | 81 | 0.081 | 0.491 | 0.598 | 0.901 | ||||
| Unknown | 9 | 0.009 | 0.038 | 0.043 | 0.028 | ||||
| 3rd Instar | 172 | 103 | 0.103 | 0.709 | |||||
| Effect | 89 | 0.089 | 0.596 | 0.707 | 0.813 | ||||
| Unknown | 14 | 0.014 | 0.290 | 0.305 | 0.254 | ||||
| 4th Instar | 69 | 45 | 0.045 | 0.552 | |||||
| Effect | 42 | 0.042 | 0.374 | 0.478 | 0.674 | ||||
| Unknown | 3 | 0.003 | 0.058 | 0.073 | 0.093 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzoukas, S.; Papantzikos, V.; Sourouni, T.; Zarmakoupi, C.; Margaritis, A.; Eliopoulos, P.A.; Patakioutas, G. Biological Control Potential of Entomopathogenic Fungi Against Aleurocanthus spiniferus: Field Trials on Citrus sinensis in Agroforestry Ecosystems. Agronomy 2025, 15, 2488. https://doi.org/10.3390/agronomy15112488
Mantzoukas S, Papantzikos V, Sourouni T, Zarmakoupi C, Margaritis A, Eliopoulos PA, Patakioutas G. Biological Control Potential of Entomopathogenic Fungi Against Aleurocanthus spiniferus: Field Trials on Citrus sinensis in Agroforestry Ecosystems. Agronomy. 2025; 15(11):2488. https://doi.org/10.3390/agronomy15112488
Chicago/Turabian StyleMantzoukas, Spiridon, Vasileios Papantzikos, Thomais Sourouni, Chrysanthi Zarmakoupi, Alexandros Margaritis, Panagiotis A. Eliopoulos, and George Patakioutas. 2025. "Biological Control Potential of Entomopathogenic Fungi Against Aleurocanthus spiniferus: Field Trials on Citrus sinensis in Agroforestry Ecosystems" Agronomy 15, no. 11: 2488. https://doi.org/10.3390/agronomy15112488
APA StyleMantzoukas, S., Papantzikos, V., Sourouni, T., Zarmakoupi, C., Margaritis, A., Eliopoulos, P. A., & Patakioutas, G. (2025). Biological Control Potential of Entomopathogenic Fungi Against Aleurocanthus spiniferus: Field Trials on Citrus sinensis in Agroforestry Ecosystems. Agronomy, 15(11), 2488. https://doi.org/10.3390/agronomy15112488









