Impact of Artificial Humic Acid on the Migration and Transformation of Soil Phosphorus
Abstract
1. Introduction
2. Materials and Methods
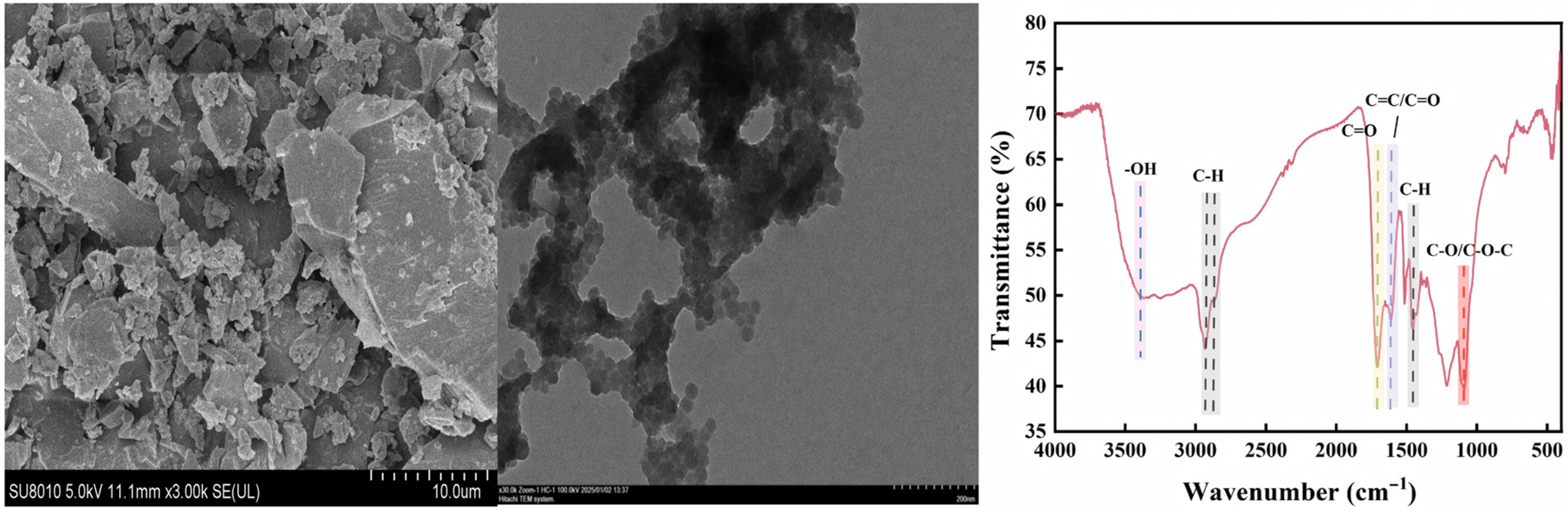
2.1. Experimental Materials
2.2. Column Experimental Design
2.3. Sample Collection and Measurement
2.4. Statistical Analyses
3. Results
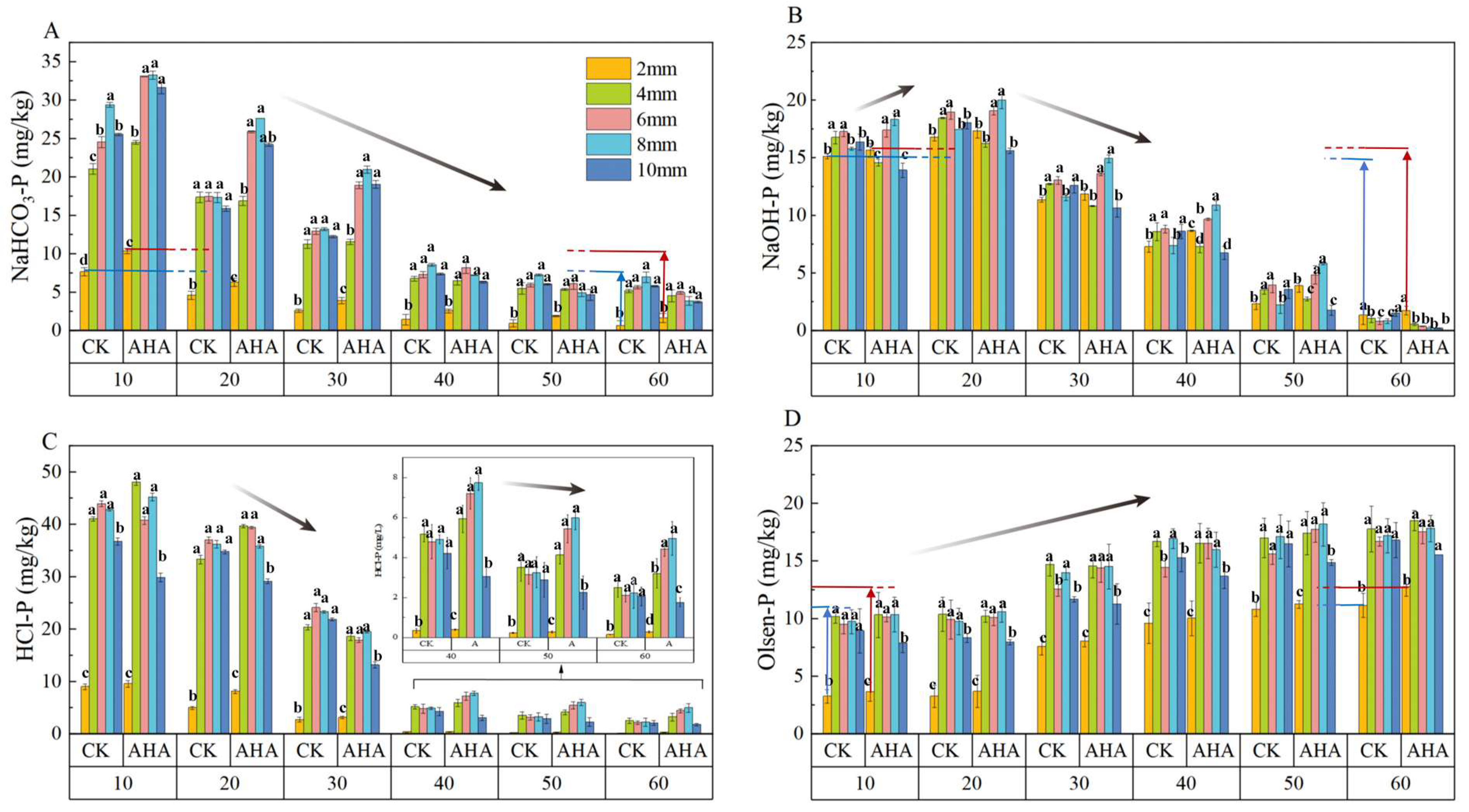
3.1. Transformation Pattern of Soil P with the Addition of AHA in Different Soil Particle Size Fractions
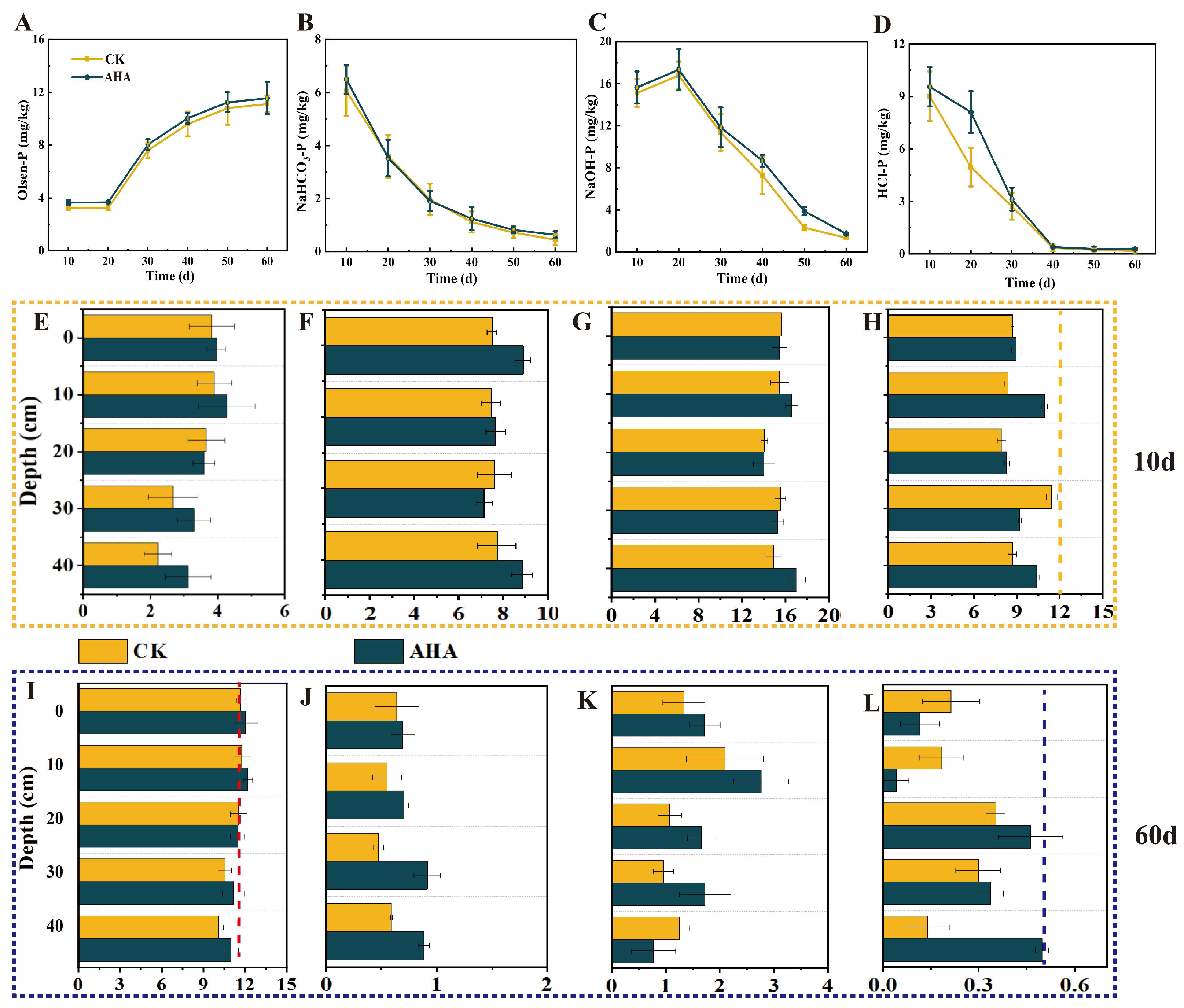
3.2. Migration Patterns of Soil P Influenced by AHA
3.3. Migration and Transformation Patterns of Soil P Under Different Humic Acid Addition Depths
3.4. Structural Equation Model of Factors Affecting Soil P
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, U.; Lin, J.C.-W.; Srivastava, G.; Djenouri, Y. A nutrient recommendation system for soil fertilization based on evolutionary computation. Comput. Electron. Agr. 2021, 189, 106407. [Google Scholar] [CrossRef]
- Wan, W.; Li, X.; Han, S.; Wang, L.; Luo, X.; Chen, W.; Huang, Q. Soil aggregate fractionation and phosphorus fraction driven by long-term fertilization regimes affect the abundance and composition of P-cycling-related bacteria. Soil Tillage Res. 2020, 196, 104475. [Google Scholar] [CrossRef]
- Li, H.; Han, Q.; Liu, Q.; Gan, Y.; Ren, C.; Rivera, W.; Zhao, Q.; Zhang, J. Roles of phosphate-solubilizing bacteria in mediating soil legacy phosphorus availability. Microbiol. Res. 2023, 272, 11. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, J.; Wang, Y.; He, S.; Xie, H.; Fu, H.; Feng, Y.; Shaheen, S.; Rinklebe, J.; Xue, L. Manure derived hydrochar reduced phosphorus loss risk via an alteration of phosphorus fractions and diversified microbial community in rice paddy soil. Sci. Total Environ. 2024, 918, 11. [Google Scholar] [CrossRef]
- Anne, F.C.; William, H.S. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Bowman, R.A.; Olsen, S.R.; Watanabe, F.S. Greenhouse evaluation of residual phosphate by four phosphorus methods in neutral and calcareous soils1. Soil Sci. Soc. Am. J. 1978, 42, 451–454. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wang, Z.; Wu, D.; Wang, M.; Torsten, M.; Zou, C.; Chen, X.; Zhang, W. Phosphorus fertilizer management for high yields in intensive winter wheat-summer maize rotation system: Integrating phosphorus budget and soil available phosphorus. Field Crops Res. 2024, 313, 109410. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, Y.; Ding, N. Spectroscopic analysis reveals that soil phosphorus availability and plant allocation strategies impact feedstock quality of nutrient-limited switchgrass. Commun. Biol. 2022, 5, 227. [Google Scholar] [CrossRef]
- Cong, W.; Suriyagoda, L.; Lambers, H. Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci. 2020, 25, 967–975. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Haygarth, P.M.; Pavinato, P.S.; Cherubin, M.R.; Teles, A.P.B.; Bordonal, R.O.; Carvalho, J.L.N.; Withers, P.J.A.; Martinelli, L.A. Long term sugarcane straw removal affects soil phosphorus dynamics-sciencedirect. Soil Till. Res. 2021, 208, 1048989. [Google Scholar] [CrossRef]
- Williams, A.; Kay, P.; Stirling, G.; Weng, X.; Bell, L. Impacts of reducing fallow periods on indicators of soil function in subtropical dryland farming systems. Agric. Ecosyst. Environ. 2022, 324, 107727. [Google Scholar] [CrossRef]
- Ma, R.; Dou, S.; Zhang, Y.; Wu, D.; Ndzelu, B.S.; Xie, S.; YaLiHong, D. Different soil particle size changes the 15N retention in soil and 15N utilization by maize. Sci. Total Environ. 2022, 843, 157133. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.; Yoon, H.; Kang, M.; Mina, L.; Yesol, B.; Ji, M.; Eun, J.; Kyoung, S.; Dong, W.; Ki, S.; et al. Ionic liquid-based extraction of fulvic-like substances from wood sawdust: Reproducing unique biological activities of fulvic acids using renewable natural sources. J. Agric. Food Chem. 2024, 72, 20981–20990. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, Y.; Cheng, K.; Wang, L.; Dong, W.; Liu, Z.; Yang, F. Artificial humic acid mediated migration of phosphorus in soil: Experiment and modelling. Catena 2024, 238, 107896. [Google Scholar] [CrossRef]
- Lu, M.; Yang, M.; Yang, Y.; Wang, D.; Sheng, L. Soil carbon and nutrient sequestration linking to soil aggregate in a temperate fen in Northeast China. Ecol. Indic. 2019, 98, 869–878. [Google Scholar] [CrossRef]
- Milić, S.; Ninkov, J.; Vasin, J.; Zeremski, T.; Jakšić, S.; Živanov, M.; Šeremešić, S.; Milić, D. Organic Phosphorus Fractions in Relation to Soil Aggregate Fractions of Black Soil. Agronomy 2024, 14, 1022. [Google Scholar] [CrossRef]
- Shi, X.Z.; Yu, D.S.; Warner, E.D.; Sun, W.X.; Petersen, G.W.; Gong, Z.T. Cross-Reference System for Translating Between Genetic Soil Classification of China and Soil Taxonomy. Soil Sci. Soc. Am. J 2006, 70, 78–83. [Google Scholar] [CrossRef]
- Antonio, G.; Oriol, O.; David, B.; Clara, M. Effects of prescribed burning on soil organic C, aggregate stability and water repellency in a subalpine shrubland: Variations among sieve fractions and depths. Catena 2018, 166, 68–77. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Jin, J.; Wu, G.; Bolan, N.S.; White, J.R.; Shaheen SMRinklebe, J.; Chen, Q. Incorporation of calcium cyanamide and straw reduces phosphorus leaching in a flooded agricultural soil. Geoderma 2022, 428, 116150. [Google Scholar] [CrossRef]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-use change affects phosphorus fractions in highly weathered tropical soils. Catena 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Schermelleh-Engel, K.; Moosbrugger, H.; Hans, M. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Int. J. Meth. Psych. Res. 2003, 8, 23–74. [Google Scholar]
- Yinglan, A.; Wang, G.; Liu, T.; Shrestha, S.; Tan, Z. Vertical variations of soil water and its controlling factors based on the structural equation model in a semi-arid grassland. Sci. Total Environ. 2019, 691, 1016–1026. [Google Scholar] [CrossRef]
- Urrutia, O.; Guardado, I.; Erro, J.; Mandado, M.; Garcia-Mina, J.M. Theoretical chemical characterization of phosphate-metal-humic complexes and relationships with their effects on both phosphorus soil fixation and phosphorus availability for plants. J. Sci. Food. Agr. 2013, 93, 293–303. [Google Scholar] [CrossRef]
- Yuan, Y.; Gai, S.; Tang, C.; Jin, Y.; Cheng, K.; Antonietti, M.; Yang, F. Artificial humic acid improves maize growth and soil phosphorus utilization efficiency. Appl. Soil Ecol. 2022, 179, 104587. [Google Scholar] [CrossRef]
- Beck, M.A.; Sanchez, P.A. Soil phosphorus fraction dynamics during 18 years of cultivation on a typic paleudult. Soil Sci. Soc. Am. J. 1994, 34, 1424–1431. [Google Scholar] [CrossRef]
- Gao, Y.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, Z.; Sardans, J.; Peuelas, J. Allocation of foliar-p fractions of alhagi sparsifolia and its relationship with soil-p fractions and soil properties in a hyperarid desert ecosystem. Geoderma 2022, 407, 115546. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Antelo, J.; Arce, F.; Avena, M.; Fiol, S.; Lopez, R.; Macias, F. Adsorption of a soil humic acid at the surface of goethite and its competitive interaction with phosphate. Geoderma 2007, 138, 12–19. [Google Scholar] [CrossRef]
- Xing, B.; Ouyang, M.; Graham, N.; Yu, W. Enhancement of phosphate adsorption during mineral trans-formation of natural siderite induced by humic acid: Mechanism and application. Chem. Eng. J. 2020, 393, 124730. [Google Scholar] [CrossRef]
- Datta, A.; Sanyal, S.K.; Saha, S. A study on natural and synthetic humic acids and their complexing ability towards cadmium. Plant Soil 2001, 235, 115–125. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Tao, J.; Yao, R.; Li, W.; Xie, W.; Wang, X. Effects of the combined application of biochar and humic substances on the improvement of saline cropland in the Yellow River Delta of China. Land Degrad. Dev. 2023, 34, 4793–4809. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Lin, Z.; Xiong, Q.; Zhao, B. Combining humic acid with phosphate fertilizer affects humic acid structure and its stimulating efficacy on the growth and nutrient uptake of maize seedlings. Sci. Rep. 2020, 10, 17502. [Google Scholar] [CrossRef]
- Jalali, M.; Ranjbar, F. Aging effects on phosphorus transformation rate and fractionation in some calcareous soils. Geoderma 2010, 155, 101–106. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, H.; Hu, Y.; Wei, X.; Chen, X.; Ge, T.; Wu, J.; Su, Y. Cellulose and lignin regulate partitioning of soil phosphorus fractions and alkaline phosphomonoesterase encoding bacterial community in phosphorus-deficient soils. Biol. Fert. Soils. 2019, 55, 31–42. [Google Scholar] [CrossRef]
- Boilard, G.; Bradley, R.L.; Paterson, E.; Sim, A.; Carubba, A. Interaction between root hairs and soil phosphorus on rhizosphere priming of soil organic matter. Soil Biol. Biochem. 2019, 135, 264–266. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, C.; Jin, Y.; Cheng, K.; Yang, F. Contribution of exogenous humic substances to phosphorus availability in soil-plant ecosystem: A review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1085–1102. [Google Scholar] [CrossRef]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the potential role of humic products in modifying biological properties of the soil-a review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef]


| CMIN/DF | GFI | CFI | NFI | RMSEA |
|---|---|---|---|---|
| 1.826 | 0.921 | 0.996 | 0.990 | 0.071 |
| pH | Conductivity | Olsen-P | Total Nitrogen | Organic Matter | Cation Exchange Capacity | Zeta | |
|---|---|---|---|---|---|---|---|
| μS/cm | mg/kg | g/kg | g/kg | mmolc/kg | mV | ||
| soil | 7.15 ±0.05 | 196.53 ±5.42 | 7.44 ±1.64 | 1.44 ±0.19 | 6.51 ±0.38 | 3.1 ±0.1 | −3.65 ±0.16 |
| df | F | p | ||
|---|---|---|---|---|
| Olsen-P | Application Method of AHA | 3 | 5.123 | 0.002 |
| Incubation Time | 5 | 44.907 | <0.001 | |
| Soil depth | 4 | 1.294 | 0.274 | |
| NaHCO3-P | Application Method of AHA | 3 | 3.140 | 0.026 |
| Incubation Time | 5 | 48.492 | <0.001 | |
| Soil depth | 4 | 0.547 | 0.702 | |
| NaOH-P | Application Method of AHA | 3 | 2.332 | 0.050 |
| Incubation Time | 5 | 50.056 | <0.001 | |
| Soil depth | 4 | 1.148 | 0.335 | |
| HCl-P | Application Method of AHA | 3 | 9.673 | <0.001 |
| Incubation Time | 5 | 87.084 | <0.001 | |
| Soil depth | 4 | 6.034 | <0.001 | |
| Application Method of AHA—Soil depth | 10 | 4.107 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Hao, Y.; Antonietti, M.; Zhao, Y.; Yang, F.; Liu, Z. Impact of Artificial Humic Acid on the Migration and Transformation of Soil Phosphorus. Agronomy 2025, 15, 2482. https://doi.org/10.3390/agronomy15112482
Zhao L, Hao Y, Antonietti M, Zhao Y, Yang F, Liu Z. Impact of Artificial Humic Acid on the Migration and Transformation of Soil Phosphorus. Agronomy. 2025; 15(11):2482. https://doi.org/10.3390/agronomy15112482
Chicago/Turabian StyleZhao, Lin, Yun Hao, Markus Antonietti, Ying Zhao, Fan Yang, and Zhuqing Liu. 2025. "Impact of Artificial Humic Acid on the Migration and Transformation of Soil Phosphorus" Agronomy 15, no. 11: 2482. https://doi.org/10.3390/agronomy15112482
APA StyleZhao, L., Hao, Y., Antonietti, M., Zhao, Y., Yang, F., & Liu, Z. (2025). Impact of Artificial Humic Acid on the Migration and Transformation of Soil Phosphorus. Agronomy, 15(11), 2482. https://doi.org/10.3390/agronomy15112482








