Nematode Detection and Classification Using Machine Learning Techniques: A Review
Abstract
1. Introduction
- (I)
- This survey systematically explores the nematode identification and counting using traditional and emerging techniques.
- (II)
- This survey compares and contrasts the most effective ML and DL models used in nematode identification, providing a performance-based comparison, particularly regarding detection accuracy, and their applicability.
2. Backgrounds and Research Questions
2.1. Microscopy Image and Nematodes
2.2. Remote Sensing Image Analysis and Nematodes
2.3. ML and DL Models for Nematode Detection and Monitoring
2.4. Research Questions
3. Survey Method
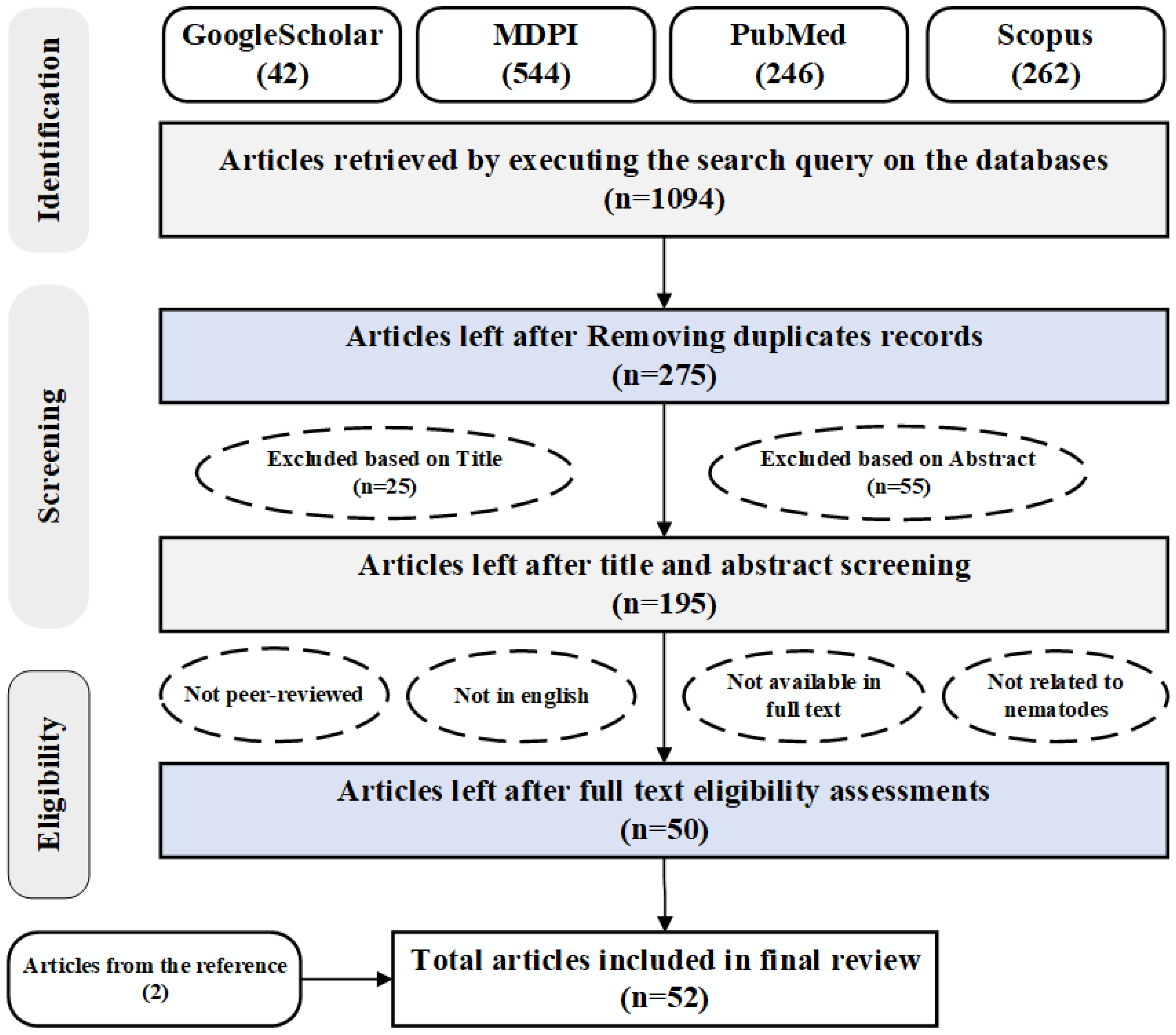
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
- (a)
- Publications that are not related to automated or semi-automated detection of nematodes;
- (b)
- Articles that are not peer-reviewed and not available in full;
- (c)
- Articles that are written in a language other than English.
3.3. Data Item Extraction
- (i)
- Meta information such as publisher, title, publication date and year, etc.
- (ii)
- Study area, nematode types, and data acquisition modalities.
- (iii)
- Nematode identification task, such as classification, segmentation, and detection.
- (iv)
- ML and DL models and their performance metrics.
4. Result and Discussion
4.1. Traditional Image Analysis Methods
4.2. ML-Based Methods
4.3. DL-Based Methods
4.3.1. Object-Level Classification Methods
4.3.2. Pixel-Based Segmentation Methods
4.3.3. Object Detection-Based Methods
5. Summary of Findings
5.1. Nematode Identification Using Image Analysis Methods
5.2. Recent Advances in Nematode Identification Using ML and DL
5.3. Challenges and Future Directions
- (i)
- The quality of image acquired with microscope relies on factors such as specimen preparation, microscope configuration, and sensor characteristics. Additionally, the skill and expertise of operators to utilise the optimal setting of the microscope plays a crucial role in controlling image resolution, lighting, and noise. These dependencies pose challenges for achieving consistent quality across datasets. Furthermore, the overlapping of various nematode species on the images further complicates the detection process, and many methods struggle to handle such complications. There is demand for developing more robust object detection and segmentation methods capable of handling such complex structure of a nematode.
- (ii)
- There are a few nematode datasets publicly available for benchmarking the performance of ML and DL models on the nematode detection task. Data labelling is always a labour-intensive and costly task that demands nematology experts to create such a dataset. Despite the scarcity of large, annotated datasets for training deep learning models that hinder the performance, there are opportunities to automate the data labelling process with advanced semi-supervised or foundational AI models such as Segment Anything (SAM) [91].
- (iii)
- The promising result of ML and DL models in nematode detection has paved the way towards the widespread use of such emerging technologies in the near future. However, these methods should be rigorously tested before implementing them in agricultural practice as the different nematode species have different characteristics at their different growth stage, which further complicate the detection process. For instance, the ML and DL models tested on the juvenile stage may not perform well in detecting nematodes in the adult stage. This brings the opportunities to develop the multi-stage multi-modal AI methods that can leverage the information from all growth stages of nematode from multiple input modalities, such as image and other environmental factors.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ANOVA | Analysis of Variance |
| ANN | Artificial Neural Network |
| CA | Contour Arc |
| CSAE | Convolutional Selective Autoencoder |
| CNN | Convolutional Neural Networks |
| COCO | Common Objects in Context |
| DIC | Differential Interference Contrast |
| DT | Decision Tree |
| DL | Deep Learning |
| DNA | Deoxyribonucleic Acid |
| EP | Extreme Point |
| EPN | Entomopathogenic Nematode |
| LMBI | Local Maximum of Boundary Intensity |
| LR | Logistic Regression |
| ML | Machine Learning |
| MLP | Multi-Layer Perceprtron |
| NB | Naive Bayes |
| PPN | Plant Parasite Nematode |
| PCN | Potato Cyst Nematode |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PWN | Pine Wood Nematode |
| R-CNN | Region-based Convolutional Neural Network |
| ResNet | Residual Network |
| RF | Random Forest |
| RKN | Root-Knot Nematode |
| RS | Remote Sensing |
| SAE | Selective Autoencoder |
| SBN | Sugar Beet Nematode |
| SCANet | Spatial-Context Attention Network |
| SCN | Soybean Cyst Nematode |
| SEM | Scanning Electron Microscopy |
| SGBoost | Stochastic Gradient Boosting |
| SS | Skeleton Structure |
| SSD | Single Shot Detector |
| SVM | Support Vector Machine |
| SAE | Selective Autoencoder |
| UAV | Unmanned Aerial Vehicle |
| VGG | Visual Geometry Group |
| YOLO | You Only Look Once |
Appendix A
| Ref. | Nematode | Input Type | Task Type | AI Method | Dataset Size | # Classes | Eval. Metrics | Public Avail. |
|---|---|---|---|---|---|---|---|---|
| [31] | Nematode | Drone | Classification | ML | - | - | Acc. | No |
| [62] | Marine nematode | Microscope | Classification | ML | 260 | - | Acc. | No |
| [63] | Cyst nematode | Microscope | Classification | ML | 435 | 2 | Prec. | No |
| [64] | Cyst nematode | Microscope | Classification | ML | - | - | Acc. | No |
| [65] | Nematode | Scanner | Classification | ML | 40,394 | 2 | Acc. | No |
| [41] | RKN | HS spectra | Classification | ML | - | 8 | Acc. | No |
| [67] | RLN | Proximal sensor | Classification | ML | - | 4 | Acc. | No |
| [68] | PPN | Microscope | Classification | DL | 957 | 11 | Acc. | Yes |
| [69] | EPN | Microscope | Classification | DL | 188 | 3 | Acc. | No |
| [70] | Phytoparasitic | Microscope | Classification | DL | 3063 | 5 | Acc. | - |
| [26] | Nematode | Microscope | Classification | DL | 2769 | 19 | Acc. | - |
| [71] | Nematode | Microscope | Classification | DL | 9215 | 40 | Acc. | Yes |
| [72] | Cyst-nematode | Microscope | Classification | DL | - | - | Acc. | No |
| [46] | Nematode | Microscope | Classification | DL | 513 | 5 | Acc. | No |
| [76] | PWN | Drone | Segmentation | DL | - | - | Prec., Rec., Acc. | No |
| [77] | PWN | Drone | Segmentation | DL | - | - | IoU, MPA, Acc. | No |
| [74] | Nematode | Microscope | Segmentation | 4000 | - | Acc. | No | |
| [78] | Nematode pest | Drone | Segmentation | DL | - | - | Acc. | No |
| [73] | C. elegans | microscope | Segmentation | DL | 1908 | 1 | Prec., Rec., F-score | Yes |
| [84] | SCN eggs | Microscope | Detection | DL | 644 | - | Yes | |
| [85] | PCN | Microscope | Detection | CNN | 3376 | - | Prec., Rec. | No |
| [82] | EPN | Microscope | Detection | DL | 1135 | 2 | Prec., Rec., mAP | No |
| [81] | RKN and FLN | Microscope | Detection | DL | 4606 | 2 | Prec, Rec., F-score, mAP | No |
| [86] | PPN | Microscope | Detection | DL | 3503 | - | Prec., Rec. mAP | No |
| [76] | PWN | Drone | Detection | DL | 4862 | - | Prec., Rec., Acc. | No |
| [87] | PWN | Drone | Detection | DL | 1872 | - | Prec., Rec. | No |
| [88] | PWN | Drone | Detection | DL | 2478 | - | Prec., Rec, F-score, mAP | No |
| [45] | RKN | Microscope | Detection | DL | 4742 | - | Acc. | Yes |
| [89] | PPN | Microscope | Detection | DL | 525 | - | mAP | No |
| [90] | PWN | Drone | Detection | DL | 894 | - | mAP, Prec., Rec. | No |
References
- Mukherjee, S.; Ray, S. Nematodes-Ecology, Adaptation and Parasitism: Ecology, Adaptation and Parasitism; BoD–Books on Demand: Norderstedt, Germany, 2024. [Google Scholar]
- Kantor, C.; Eisenback, J.D.; Kantor, M. Biosecurity risks to human food supply associated with plant-parasitic nematodes. Front. Plant Sci. 2024, 15, 1404335. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Haris, M.; Hussain, T.; Khan, A.A.; Laasli, S.E.; Lahlali, R.; Mokrini, F. Counter-attack of biocontrol agents: Environmentally benign Approaches against Root-knot nematodes (Meloidogyne spp.) on Agricultural crops. Heliyon 2023, 9, e21653. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Vázquez, I.; Montoya-Martínez, A.C.; De los Santos-Villalobos, S.; Ek-Ramos, M.J.; Montesinos-Matías, R.; Martínez-Anaya, C. Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. World J. Microbiol. Biotechnol. 2022, 38, 26. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Carreira, D.N.; Braga, F.R.; Soares, F.E.d.F. First report of the nematicidal activity of Flammulina velutipes, its spent mushroom compost and metabolites. 3 Biotech 2019, 9, 410. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Nicol, J.; Turner, S.; Coyne, D.L.; Nijs, L.D.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Vieira, P.; Gleason, C. Plant-parasitic nematode effectors—Insights into their diversity and new tools for their identification. Curr. Opin. Plant Biol. 2019, 50, 37–43. [Google Scholar] [CrossRef]
- Kantor, C.; Teixeira, M.; Kantor, M.; Gleason, C. Tiny Invaders, Big Trouble: Emerging Nematode Threats in the United States. Phytopathology 2025, 115, 587–595. [Google Scholar] [CrossRef]
- Mani, J.; Nagachandrabose, S.; Somasundaram, P.; Deenan, S. Artificial Intelligence integrated Nano biosensor Technology: A breakthrough in early detection and sustainable management of phytonematodes. Physiol. Mol. Plant Pathol. 2025, 139, 102756. [Google Scholar] [CrossRef]
- Seesao, Y.; Gay, M.; Merlin, S.; Viscogliosi, E.; Aliouat-Denis, C.; Audebert, C. A review of methods for nematode identification. J. Microbiol. Methods 2017, 138, 37–49. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, P.; Peng, D.; Huang, W.; Kong, L.A.; Li, C.; Liu, E.; Peng, H. Current advances in the identification of plant nematode diseases: From lab assays to in-field diagnostics. Front. Plant Sci. 2023, 14, 1106784. [Google Scholar] [CrossRef] [PubMed]
- Porazinska, D.L.; Morgan, M.J.; Gaspar, J.M.; Court, L.N.; Hardy, C.M.; Hodda, M. Discrimination of plant-parasitic nematodes from complex soil communities using ecometagenetics. Phytopathology 2014, 104, 749–761. [Google Scholar] [CrossRef]
- Been, T.; Meijer, E.M.; Benier, A.; Knol, J. Using image analysis for counting larvae of potato cyst nematodes (Globodera spp.). Fundam. Appl. Nematol. 1996, 19, 297–304. [Google Scholar]
- Hallmann, J.; Subbotin, S.A. Methods for extraction, processing and detection of plant and soil nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CAB International: Wallingford, UK, 2018; pp. 87–119. [Google Scholar]
- Cesarz, S.; Schulz, A.E.; Beugnon, R.; Eisenhauer, N. Testing soil nematode extraction efficiency using different variations of the Baermann-funnel method. Soil Org. 2019, 91, 61. [Google Scholar]
- Tintori, S.C.; Sloat, S.A.; Rockman, M.V. Rapid isolation of wild nematodes by Baermann funnel. J. Vis. Exp. 2022, 179, e63287. [Google Scholar]
- Arjoune, Y.; Sugunaraj, N.; Peri, S.; Nair, S.V.; Skurdal, A.; Ranganathan, P.; Johnson, B. Soybean cyst nematode detection and management: A review. Plant Methods 2022, 18, 110. [Google Scholar] [CrossRef]
- Syifa, M.; Park, S.J.; Lee, C.W. Detection of the pine wilt disease tree candidates for drone remote sensing using artificial intelligence techniques. Engineering 2020, 6, 919–926. [Google Scholar] [CrossRef]
- Bhat, K.A.; Mir, R.A.; Farooq, A.; Manzoor, M.; Hami, A.; Allie, K.A.; Wani, S.M.; Khan, M.; Sayyed, R.; Poczai, P.; et al. Advances in nematode identification: A journey from fundamentals to evolutionary aspects. Diversity 2022, 14, 536. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, S.; Dutta, S.; Mondal, S. Advancements in Nematode Management: Exploring Machine Learning in Precision Agriculture. Indian J. Nematol. 2024, 53, 106–113. [Google Scholar]
- Ma, P.; Li, C.; Rahaman, M.M.; Yao, Y.; Zhang, J.; Zou, S.; Zhao, X.; Grzegorzek, M. A state-of-the-art survey of object detection techniques in microorganism image analysis: From classical methods to deep learning approaches. Artif. Intell. Rev. 2023, 56, 1627–1698. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, L.; Chen, J.; Fang, Q.; Ablameyko, S.; Yin, Z.; Xu, Y. A survey on applications of deep learning in microscopy image analysis. Comput. Biol. Med. 2021, 134, 104523. [Google Scholar] [CrossRef] [PubMed]
- Pun, T.B.; Thapa Magar, R.; Koech, R.; Owen, K.J.; Adorada, D.L. Emerging Trends and Technologies Used for the Identification, Detection, and Characterisation of Plant-Parasitic Nematode Infestation in Crops. Plants 2024, 13, 3041. [Google Scholar] [CrossRef]
- Pun, T.B.; Neupane, A.; Koech, R. A deep learning-based decision support tool for plant-parasitic nematode management. J. Imaging 2023, 9, 240. [Google Scholar] [CrossRef]
- Qing, X.; Wang, Y.; Lu, X.; Li, H.; Wang, X.; Li, H.; Xie, X. NemaRec: A deep learning-based web application for nematode image identification and ecological indices calculation. Eur. J. Soil Biol. 2022, 110, 103408. [Google Scholar] [CrossRef]
- Qazi, F.; Khalid, A.; Poddar, A.; Tetienne, J.P.; Nadarajah, A.; Aburto-Medina, A.; Shahsavari, E.; Shukla, R.; Prawer, S.; Ball, A.S.; et al. Real-time detection and identification of nematode eggs genus and species through optical imaging. Sci. Rep. 2020, 10, 7219. [Google Scholar] [CrossRef]
- Sommer, C. Digital image analysis and identification of eggs from bovine parasitic nematodes. J. Helminthol. 1996, 70, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, M.; Prasad, Y.; Rao, M.N. Remote sensing of biotic stress in crop plants and its applications for pest management. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 517–545. [Google Scholar]
- Carneiro, R.G.; Mazzafera, P.; Ferraz, L.C.C.; Muraoka, T.; Trivelin, P.C.O. Uptake and translocation of nitrogen, phosphorus and calcium in soybean infected with Meloidogyne incognita and M. javanica. Fitopatol. Bras. 2002, 27, 141–150. [Google Scholar] [CrossRef]
- Santos, L.B.; Bastos, L.M.; de Oliveira, M.F.; Soares, P.L.M.; Ciampitti, I.A.; da Silva, R.P. Identifying nematode damage on soybean through remote sensing and machine learning techniques. Agronomy 2022, 12, 2404. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Ren, L. Detection of pine wood nematode infestation using hyperspectral drone images. Ecol. Indic. 2024, 162, 112034. [Google Scholar] [CrossRef]
- Sun, Z.; Ibrayim, M.; Hamdulla, A. Detection of pine wilt nematode from drone images using UAV. Sensors 2022, 22, 4704. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Shaikh, T.A.; Rasool, T.; Lone, F.R. Towards leveraging the role of machine learning and artificial intelligence in precision agriculture and smart farming. Comput. Electron. Agric. 2022, 198, 107119. [Google Scholar] [CrossRef]
- Saleem, R.M.; Bashir, R.N.; Faheem, M.; Haq, M.A.; Alhussen, A.; Alzamil, Z.S.; Khan, S. Internet of things based weekly crop pest prediction by using deep neural network. IEEE Access 2023, 11, 85900–85913. [Google Scholar] [CrossRef]
- Saranya, T.; Subbiah, S.; Dhanaseelan, V. AI Based Identification of Plant Health Issues. In Proceedings of the 2024 9th International Conference on Communication and Electronics Systems (ICCES), Tamil Nadu, India, 16–18 December 2024; IEEE: New York, NY, USA, 2024; pp. 1513–1517. [Google Scholar]
- Martins, G.D.; Galo, M.d.L.B.T.; Vieira, B.S. Detecting and mapping root-knot nematode infection in coffee crop using remote sensing measurements. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 5395–5403. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, R.; Shi, W.; Yu, Q.; Li, X.; Chen, X. Automatic detection and classification of dead nematode-infested pine wood in stages based on YOLO v4 and GoogLeNet. Forests 2023, 14, 601. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Samiappan, S.; Wubben, M.J.; Brooks, J.P.; Shrestha, A.; Panda, R.M.; Reddy, K.R.; Bheemanahalli, R. Hyperspectral reflectance and machine learning approaches for the detection of drought and root–knot nematode infestation in cotton. Remote Sens. 2022, 14, 4021. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Fei-Fei, L. Imagenet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; IEEE: New York, NY, USA, 2009; pp. 248–255. [Google Scholar]
- Yosinski, J.; Clune, J.; Bengio, Y.; Lipson, H. How transferable are features in deep neural networks? In Advances in Neural Information Processing Systems, Proceedings of the Conference and Workshop on Neural Information Processing Systems 2014, Montreal, BC, Canada, 12–13 December 2014; NeurIPS Foundation: La Jolla, CA, USA, 2014; Volume 27. [Google Scholar]
- Shahi, T.B.; Sitaula, C.; Bhandari, K.P.; Poudel, S.; Bhandari, R.; Mishra, R.; Sharma, B.K.; Mishra, B. Deep Learning-Based Method for Irrigation Status Detection in Tomato Using Plant Leaves. IEEE Trans. Artif. Intell. 2025, 6, 1849–1858. [Google Scholar] [CrossRef]
- Saikai, K.K.; Bresilla, T.; Kool, J.; de Ruijter, N.C.; Van Schaik, C.; Teklu, M.G. Counting nematodes made easy: Leveraging AI-powered automation for enhanced efficiency and precision. Front. Plant Sci. 2024, 15, 1349209. [Google Scholar] [CrossRef]
- Singh, N.; Singh, A.K.; Dhruw, L.; Kaur, S.; Hazarika, S.; Mishra, K.; Mishra, V.; Kant, L. Development of a Deep Learning-Assisted Mobile Application for the Identification of Nematodes Through Microscopic Images. Mod. Agric. 2024, 2, e70000. [Google Scholar] [CrossRef]
- Tzutalin. LabelImg. Free Software: MIT License. 2015. Available online: https://github.com/tzutalin/labelImg (accessed on 15 June 2025).
- Chatterjee, S.; Dey, D.; Munshi, S. Integration of morphological preprocessing and fractal based feature extraction with recursive feature elimination for skin lesion types classification. Comput. Methods Programs Biomed. 2019, 178, 201–218. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shahi, T.B.; Xu, C.Y.; Neupane, A.; Guo, W. Recent advances in crop disease detection using UAV and deep learning techniques. Remote Sens. 2023, 15, 2450. [Google Scholar] [CrossRef]
- de la Blanca, N.P.; Fdez-Valdivia, J.; Castillo, P.; Gomez-Barcina, A. Detecting nematode features from digital images. J. Nematol. 1992, 24, 289. [Google Scholar]
- Pun, T.B.; Neupane, A.; Koech, R.; Owen, K.J. Detection and quantification of root-knot nematode (Meloidogyne spp.) eggs from tomato plants using image analysis. IEEE Access 2022, 10, 123190–123204. [Google Scholar] [CrossRef]
- Pun, T.B.; Neupane, A.; Koech, R. Quantification of root-knot nematode infestation in tomato using digital image analysis. Agronomy 2021, 11, 2372. [Google Scholar] [CrossRef]
- Moore, B.T.; Jordan, J.M.; Baugh, L.R. WormSizer: High-throughput analysis of nematode size and shape. PLoS ONE 2013, 8, e57142. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.G. A new method for measuring the size of nematodes using image processing. Biol. Methods Protoc. 2019, 4, bpz020. [Google Scholar] [CrossRef] [PubMed]
- Fraher, S.P.; Watson, M.; Nguyen, H.; Moore, S.; Lewis, R.S.; Kudenov, M.; Yencho, G.C.; Gorny, A.M. A comparison of three automated root-knot nematode egg counting approaches using machine learning, image analysis, and a hybrid model. Plant Dis. 2024, 108, 2625–2629. [Google Scholar] [CrossRef]
- Holladay, B.H.; Willett, D.S.; Stelinski, L.L. High throughput nematode counting with automated image processing. BioControl 2016, 61, 177–183. [Google Scholar] [CrossRef]
- Kranse, O.P.; Ko, I.; Healey, R.; Sonawala, U.; Wei, S.; Senatori, B.; De Batté, F.; Zhou, J.; Eves-van den Akker, S. A low-cost and open-source solution to automate imaging and analysis of cyst nematode infection assays for Arabidopsis thaliana. Plant Methods 2022, 18, 134. [Google Scholar] [CrossRef]
- Jjagwe, P.; Chandel, A.K.; Langston, D.B. Impact Assessment of Nematode Infestation on Soybean Crop Production Using Aerial Multispectral Imagery and Machine Learning. Appl. Sci. 2024, 14, 5482. [Google Scholar] [CrossRef]
- Kurtulmuş, F.; Ulu, T.C. Detection of dead entomopathogenic nematodes in microscope images using computer vision. Biosyst. Eng. 2014, 118, 29–38. [Google Scholar] [CrossRef]
- Silva, C.; Magalhaes, K.; Neto, A.D. An intelligent system for detection of nematodes in digital images. In Proceedings of the International Joint Conference on Neural Networks, Portland, OR, USA, 20–24 July 2003; IEEE: New York, NY, USA, 2003; Volume 1, pp. 612–615. [Google Scholar]
- de Jesus, S.B.; Vieira, D.; Gheller, P.; Cunha, B.P.; Gallucci, F.; Fonseca, G. Machine learning algorithms accurately identify free-living marine nematode species. PeerJ 2023, 11, e16216. [Google Scholar] [CrossRef]
- Chen, L.; Strauch, M.; Daub, M.; Jansen, M.; Luigs, H.G.; Merhof, D. Instance segmentation of nematode cysts in microscopic images of soil samples. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: New York, NY, USA, 2019; pp. 5932–5936. [Google Scholar]
- Ropelewska, E.; Skwiercz, A.; Sobczak, M. Distinguishing cyst nematode species using image textures and artificial neural networks. Agronomy 2023, 13, 2277. [Google Scholar] [CrossRef]
- Radvansky, M.; Kadlecova, A.; Kudelka, M. Utilizing Image Recognition Methods for the Identification and Counting of Live Nematodes in Petri Dishes. In Proceedings of the 2024 25th International Carpathian Control Conference (ICCC), Krynica Zdrój, Poland, 22–24 May 2024; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar]
- Filgueiras, C.C.; Kim, Y.; Wickings, K.G.; El Borai, F.; Duncan, L.W.; Willett, D.S. The Smart Soil Organism Detector: An instrument and machine learning pipeline for soil species identification. Biosens. Bioelectron. 2023, 221, 114417. [Google Scholar] [CrossRef]
- Niu, H.; Zhao, T.; Westphal, A.; Chen, Y. A low-cost proximate sensing method for early detection of nematodes in walnut using Walabot and scikit-learn classification algorithms. In Proceedings of the Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping V, Online, 27 April–8 May 2020; SPIE: Paris, France, 2020; Volume 11414, pp. 119–125. [Google Scholar]
- Shabrina, N.H.; Lika, R.A.; Indarti, S. Deep learning models for automatic identification of plant-parasitic nematode. Artif. Intell. Agric. 2023, 7, 1–12. [Google Scholar] [CrossRef]
- Uhlemann, J.; Cawley, O.; Kakouli-Duarte, T. Nematode Identification using Artificial Neural Networks. In Proceedings of the 1st International Conference on Deep Learning Theory and Applications, Virtual, 8–10 July 2020; pp. 13–22. [Google Scholar]
- Abade, A.; Porto, L.F.; Ferreira, P.A.; de Barros Vidal, F. NemaNet: A convolutional neural network model for identification of soybean nematodes. Biosyst. Eng. 2022, 213, 39–62. [Google Scholar] [CrossRef]
- Lu, S.; Fung, S.; Wang, Y.; Lu, X.; Ouyang, W.; Qing, X.; Li, H. I-nema: A large-scale microscopic image dataset for nematode recognition. Neural Comput. Appl. 2025, 37, 2763–2773. [Google Scholar] [CrossRef]
- Thevenoux, R.; Van Linh, L.; Villessèche, H.; Buisson, A.; Beurton-Aimar, M.; Grenier, E.; Folcher, L.; Parisey, N. Image based species identification of Globodera quarantine nematodes using computer vision and deep learning. Comput. Electron. Agric. 2021, 186, 106058. [Google Scholar] [CrossRef]
- Fudickar, S.; Nustede, E.J.; Dreyer, E.; Bornhorst, J. Mask R-CNN based C. elegans detection with a DIY microscope. Biosensors 2021, 11, 257. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, J.; Xiao, J.; Song, K.; Lv, L.; Lao, S. An algorithm based on attention mask for fine-grained object detection. In Proceedings of the 2021 3rd International Conference on Advances in Computer Technology, Information Science and Communication (CTISC), Shanghai, China, 23–25 April 2021; IEEE: New York, NY, USA, 2021; pp. 324–327. [Google Scholar]
- Kalwa, U.; Legner, C.; Wlezien, E.; Tylka, G.; Pandey, S. New methods of removing debris and high-throughput counting of cyst nematode eggs extracted from field soil. PLoS ONE 2019, 14, e0223386. [Google Scholar] [CrossRef]
- Qin, J.; Wang, B.; Wu, Y.; Lu, Q.; Zhu, H. Identifying pine wood nematode disease using UAV images and deep learning algorithms. Remote Sens. 2021, 13, 162. [Google Scholar] [CrossRef]
- Shen, J.; Xu, Q.; Gao, M.; Ning, J.; Jiang, X.; Gao, M. Aerial Image Segmentation of Nematode-Affected Pine Trees with U-Net Convolutional Neural Network. Appl. Sci. 2024, 14, 5087. [Google Scholar] [CrossRef]
- Oliveira, A.J.; Assis, G.A.; Faria, E.R.; Souza, J.R.; Vivaldini, K.C.; Guizilini, V.; Ramos, F.; Mendes, C.C.; Wolf, D.F. Analysis of nematodes in coffee crops at different altitudes using aerial images. In Proceedings of the 2019 27th European Signal Processing Conference (EUSIPCO), A Coruña, Spain, 2–6 September 2019; IEEE: New York, NY, USA, 2019; pp. 1–5. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 39, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.Y.; Berg, A.C. Ssd: Single shot multibox detector. In Proceedings of the Computer Vision–ECCV 2016: 14th European Conference, Amsterdam, The Netherlands, 11–14 October 2016; Proceedings, Part I 14. Springer: Berlin/Heidelberg, Germany, 2016; pp. 21–37. [Google Scholar]
- Pun, T.B.; Neupane, A.; Koech, R.; Walsh, K. Detection and counting of root-knot nematodes using YOLO models with mosaic augmentation. Biosens. Bioelectron. X 2023, 15, 100407. [Google Scholar] [CrossRef]
- Phuyued, U.; Kangkachit, T.; Jitkongchuen, D. Detection of Infective Juvenile Stage of Entomopathogenic Nematodes Using Deep Learning. In Proceedings of the 2024 5th International Conference on Big Data Analytics and Practices (IBDAP), Bangkok, Thailand, 23–25 August 2024; IEEE: New York, NY, USA, 2024; pp. 50–55. [Google Scholar]
- Akintayo, A.; Lee, N.; Chawla, V.; Mullaney, M.; Marett, C.; Singh, A.; Singh, A.; Tylka, G.; Ganapathysubramaniam, B.; Sarkar, S. An end-to-end convolutional selective autoencoder approach to Soybean Cyst Nematode eggs detection. arXiv 2016, arXiv:1603.07834. [Google Scholar]
- Akintayo, A.; Tylka, G.L.; Singh, A.K.; Ganapathysubramanian, B.; Singh, A.; Sarkar, S. A deep learning framework to discern and count microscopic nematode eggs. Sci. Rep. 2018, 8, 9145. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Strauch, M.; Daub, M.; Jiang, X.; Jansen, M.; Luigs, H.G.; Schultz-Kuhlmann, S.; Krüssel, S.; Merhof, D. A CNN framework based on line annotations for detecting nematodes in microscopic images. In Proceedings of the 2020 IEEE 17th international symposium on biomedical imaging (ISBI), Iowa City, IA, USA, 3–7 April 2020; IEEE: New York, NY, USA, 2020; pp. 508–512. [Google Scholar]
- Agarwal, S.; Curran, Z.C.; Yu, G.; Mishra, S.; Baniya, A.; Bogale, M.; Hughes, K.; Salichs, O.; Zare, A.; Jiang, Z.; et al. Plant parasitic nematode identification in complex samples with deep learning. J. Nematol. 2023, 55, 20230045. [Google Scholar] [CrossRef]
- Lim, W.; Choi, K.; Cho, W.; Chang, B.; Ko, D.W. Efficient dead pine tree detecting method in the Forest damaged by pine wood nematode (Bursaphelenchus xylophilus) through utilizing unmanned aerial vehicles and deep learning-based object detection techniques. For. Sci. Technol. 2022, 18, 36–43. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Ji, Y.; Xu, Z.; Gu, J.; Jing, W. A spatio-temporal multi-scale fusion algorithm for pine wood nematode disease tree detection. J. For. Res. 2024, 35, 109. [Google Scholar] [CrossRef]
- Yuan, Z.; Musa, N.; Dybal, K.; Back, M.; Leybourne, D.; Yang, P. Quantifying Nematodes through Images: Datasets, Models, and Baselines of Deep Learning. In Proceedings of the 2023 IEEE 22nd International Conference on Trust, Security and Privacy in Computing and Communications (TrustCom), Exeter, UK, 31 October–3 November 2023; IEEE: New York, NY, USA, 2023; pp. 2448–2455. [Google Scholar]
- Su, J.; Qin, B.; Sun, F.; Lan, P.; Liu, G. Identification of Pine Wilt-Diseased Trees Using UAV Remote Sensing Imagery and Improved PWD-YOLOv8n Algorithm. Drones 2024, 8, 404. [Google Scholar] [CrossRef]
- Kirillov, A.; Mintun, E.; Ravi, N.; Mao, H.; Rolland, C.; Gustafson, L.; Xiao, T.; Whitehead, S.; Berg, A.C.; Lo, W.Y.; et al. Segment anything. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Paris, France, 2–6 October 2023; pp. 4015–4026. [Google Scholar]
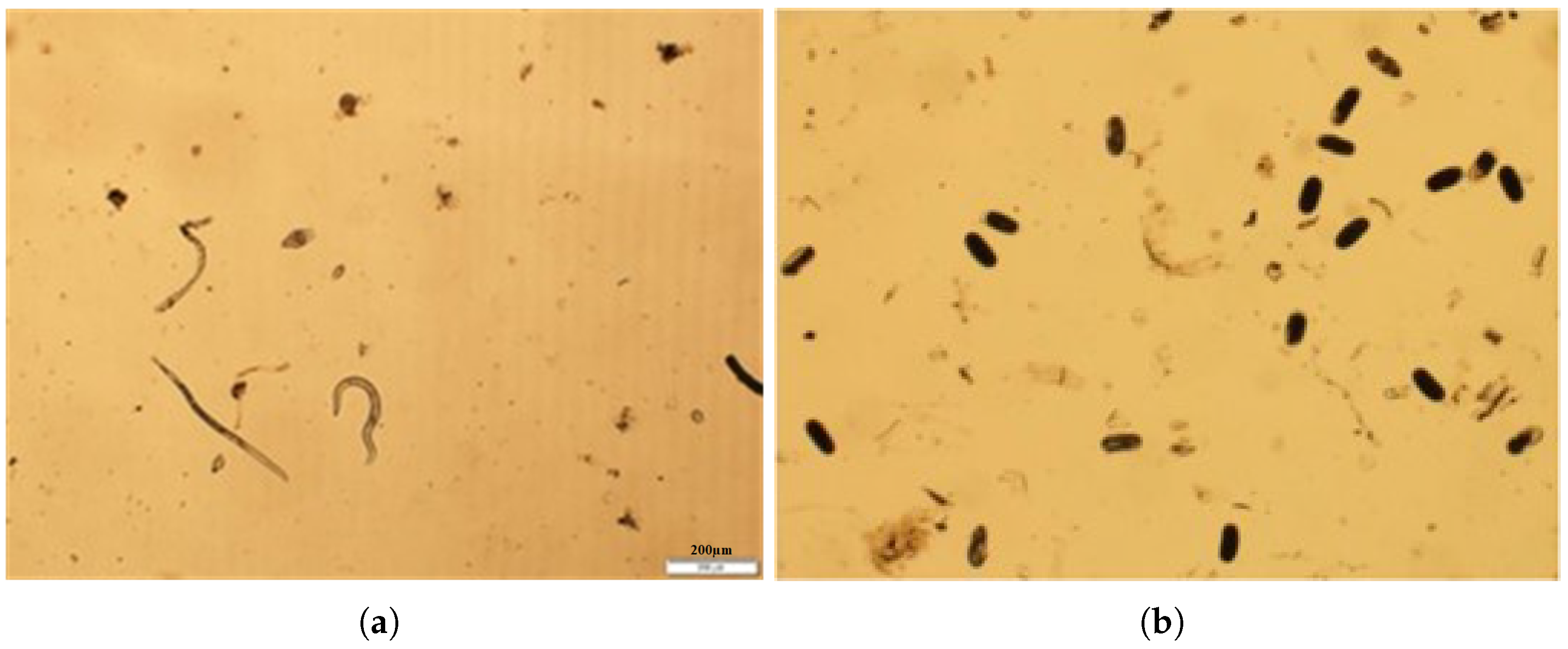

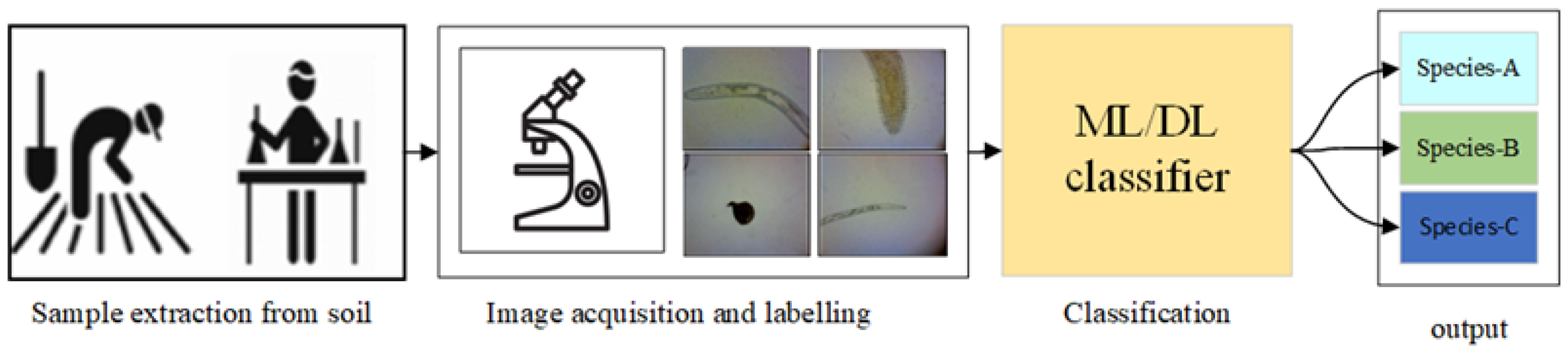

| Ref | Description | Field | Datasets | Limitation and Gaps |
|---|---|---|---|---|
| [18] | soybean cyst nematode (SCN) detection and management | Nematode detection | Microscopy, remote sensing and hyperspectral imaging |
|
| [22] | Transition from traditional image processing and ML to DL methods. | Image analysis | Microorganism images |
|
| [23] | Microscopy image analysis with DL. | General image analysis | Microscopy images |
|
| [24] | Review of emerging techniques for PPN. | PPN identification | Microscopy, remote sensing, and hyperspectral imaging |
|
| [20] | Nematode identification methods. | Nematode identification | Diverse array of datasets |
|
| Database | Search Strategy | Remarks |
|---|---|---|
| MDPI | (Search within = ‘All fields’, Article Types = ‘Article’ and Years = ‘2012–2025’) | Article types and Years are used as search filters. |
| Scopus | (Search within = ‘Article title, abstract, keywords’, Years = ‘2012–2025’, Document Types = ‘Article’, language = ‘English’) | Document type, Years, and Language are used as search filters |
| PubMed | (Search within = ‘All fields’, Text Availability = ‘full text’, Years = ‘2012–2025’, Language = ‘English’) | We utilise text availability as an extra search filter in this case. |
| Google Scholar | (Search within = ‘title’, Years = ‘2012–2025’) | Advanced setting such as with all of the words = ‘Nematodes’, with at least one of the words = (‘machine learning’, ‘deep learning’) were used as search filters. |
| Ref | Input Images | Methods | Remarks |
|---|---|---|---|
| [52] | Nematode egg | Image analysis techniques such as CA, SS, and EP | The semi-automated counting methods for RKN eggs achieved with overall of 0.90. |
| [56] | RKN Eggs | Contour-based method | The counting of three types of RKN (M. enterolobii, M. incognita, M. javanica) was highly correlated with human raters with , , , respectively. |
| [57] | EPN image | Standard curve method | The nematode area was estimated using image processing, and the results showed that the nematode pixel area from image analysis was well correlated with the total number of nematodes in the sample (). |
| [53] | RKN image | Image analysis techniques such as CA, thin structure and skeleton graph | When these methods were tested on 517 microscopy images, the result of automated counting was highly correlated with manual counting of the nematode, with the highest using the CA method. |
| [58] | Cyst nematodes | Image analysis techniques such as thresholding colour, removing outliers, and watershed, etc. | A low-cost and open-source imaging method for nematode counting was developed. |
| [59] | SCN | Statistical models | Nematode infestation on soybean using machine learning and high resolution multispectral aerial imagery. |
| [60] | EPN images | Image analysis techniques such as skeleton and two-path analysis | Detected and counted the dead nematodes in microscopy images. |
| [61] | Nematode image | Image analysis techniques, such as auto-contrast technique and segmentation, are used. | An automatic and intelligent technique for nematode identification was developed using a neural network. |
| Ref. | Dataset | ML Methods | P | R | F | Acc. | Remarks |
|---|---|---|---|---|---|---|---|
| [31] | RS images | RF, CIT, LR | - | - | - | 71.00 | Multispectral imagery acquired with drone. |
| [62] | Microscopy image | RF, SGBoost, SVM, KNN | - | - | - | 93.00 | Two nematode dataset Acantholaimus (D1), Sabatieria (D2) are used. |
| [63] | Microscopy image | SVM | 68.05 | 74.94 | - | - | Sugar beet nematodes were analysed. |
| [64] | Microscopy image | ANN | 89.80 | - | 89.70 | 86.30 | Cyst nematodes were analysed. |
| [65] | Flatbed scanner image | SVM | 96.73 | 98.15 | 97.13 | 96.21 | Nematode (Caenorhabditis elegans) in Petri dish scanned photo were analysed. |
| [41] | HS spectra | MLC | - | - | - | 93.00 | |
| [66] | Microscopy image | KNN, SVM, XGBoost | - | 91.60 | - | 95.50 | EPN were classified. |
| [67] | RS data | SVM, RF, KNN DT | - | - | - | 72.00 | Root lesion nematodes by proximal sensor were analysed. |
| Ref. | Dataset | DL Methods | P | R | F | Acc. | Remarks |
|---|---|---|---|---|---|---|---|
| [68] | Microscopy image (PPN) | ResNet101v2, CoAtNet-0, EfficientNetV2B0, EfficientNetV2M | 98.26 | 97.26 | 97.99 | 98.66 | 957 image of PPN from Indonesia, representing 11 classes/species |
| [69] | Microscopy image (Juveniles and Adult) | Xception | - | - | - | 88.28 | Juveniles and adults nematode image by light microscope |
| [70] | Microscopy image | NemaNet and other DL models | 98.96 | 98.87 | 98.91 | 98.80 | Microscopy images of different nematode species |
| [26] | Microscopy image (NemaRec) | ResNet101 | - | - | - | 54.7 | Microscopy image consisting of 19 nematode species (2769 images) collected in China |
| [71] | Microscopy image (I-Nema) | Xception, ResNet50, ViT, and so on | - | - | - | 86.78 | This includes the 40 nematode species (9215 microscopy images) |
| [72] | Microscopy image (PPN) | EB-Net | - | - | - | 71.00 | It includes the PPN image of 14 species from Peru, Mexico and Europe |
| [46] | Microscopy image (EPN) | Custom CNN | 95.66 | 95.56 | 95.56 | 98.52 | A custom CNN was developed to classify EPN species using microscopy images |
| Ref. | Dataset | DL Methods | P | R | F | Acc. | Remarks |
|---|---|---|---|---|---|---|---|
| [76] | RS images | SCANet, CANet, SNet, DeepLabV3+, HRNet | 86.00 | 91.00 | - | 79.00 | PWN image acquired by drone |
| [77] | RS images | VGG with UNet, ResNet50 with DeepLabV3+ | - | - | 88.50 | 99.13 | Pine Wood Nematode (PWN) disease identified based on drone imagery |
| [74] | Microscopy images | UNet and Attention-UNet | - | - | - | 85.00 | Microscopy images of nematodes |
| [78] | RS image | UNet | 66.00 | 74.66 | 69.00 | - | Nematode pest detection in coffee crops using drone imagery |
| [73] | Microscopy image | Mask R-CNN * | 96.00 | 95.66 | 95.8 | - | Microscopy image fo C. elegans |
| Ref | Dataset | Methods | P | R | F | Acc. | Remarks |
|---|---|---|---|---|---|---|---|
| [84] | Microscopy image | CSAE | 93.73 | - | 94.40 | 95.05 | Detection and counting of SCN in a microscopic image. |
| [85] | Microscopy image | CNN | 84.20 | 85.63 | - | - | CNN-based on Line Annotations was implemented to detect PCN in Microscopy images. |
| [82] | Microscopy image | YOLO-v5s | 78.10 | 78.30 | - | - | Detection of the infective juvenile stage of EPN using a bounding box. |
| [81] | Microscopy image | YOLOv5 | 100.00 | 99.80 | 99.90 | - | YOLO models with mosaic data augmentation were implemented to detect RKN. |
| [86] | Microcopy images | YOLOv5 | 85.10 | 75.30 | - | - | Plant parasite nematode were detected in complex microscopy samples. |
| [76] | RS images | YOLOv5 | 98.70 | 98.10 | 97.30 | - | Infestation of nematode on Pine wood was estimated using multi-spectral drone images. |
| [87] | RS images | YOLOv3 | 84.38 | 99.09 | - | - | Dead pine tree detection due to pine wood nematode (PWN) using drone imagery. |
| [88] | RS images | Improved-YOLOv8 | 85.20 | 64.30 | - | - | PWN disease tree detection using drone imagery. |
| [45] | Microscopy image | YOLOv8x | - | - | - | 94.00 | Detection and counting fo Nematode eggs. |
| [89] | Microscopy image | YOLOv6 | 96.53 * | - | - | - | AgriNema dataset. |
| [90] | RS image | YOLOV8 | 87.90 | 87.00 | - | - | PWN infected trees were detected using drone images. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, A.; Shahi, T.B.; Koech, R.; Walsh, K.; Langat, P.K. Nematode Detection and Classification Using Machine Learning Techniques: A Review. Agronomy 2025, 15, 2481. https://doi.org/10.3390/agronomy15112481
Neupane A, Shahi TB, Koech R, Walsh K, Langat PK. Nematode Detection and Classification Using Machine Learning Techniques: A Review. Agronomy. 2025; 15(11):2481. https://doi.org/10.3390/agronomy15112481
Chicago/Turabian StyleNeupane, Arjun, Tej Bahadur Shahi, Richard Koech, Kerry Walsh, and Philip Kibet Langat. 2025. "Nematode Detection and Classification Using Machine Learning Techniques: A Review" Agronomy 15, no. 11: 2481. https://doi.org/10.3390/agronomy15112481
APA StyleNeupane, A., Shahi, T. B., Koech, R., Walsh, K., & Langat, P. K. (2025). Nematode Detection and Classification Using Machine Learning Techniques: A Review. Agronomy, 15(11), 2481. https://doi.org/10.3390/agronomy15112481









