Chlormequat Chloride and Uniconazole Regulate Lodging Resistance and Yield Formation of Wheat Through Different Strategies
Abstract
1. Introduction
2. Materials and Methods
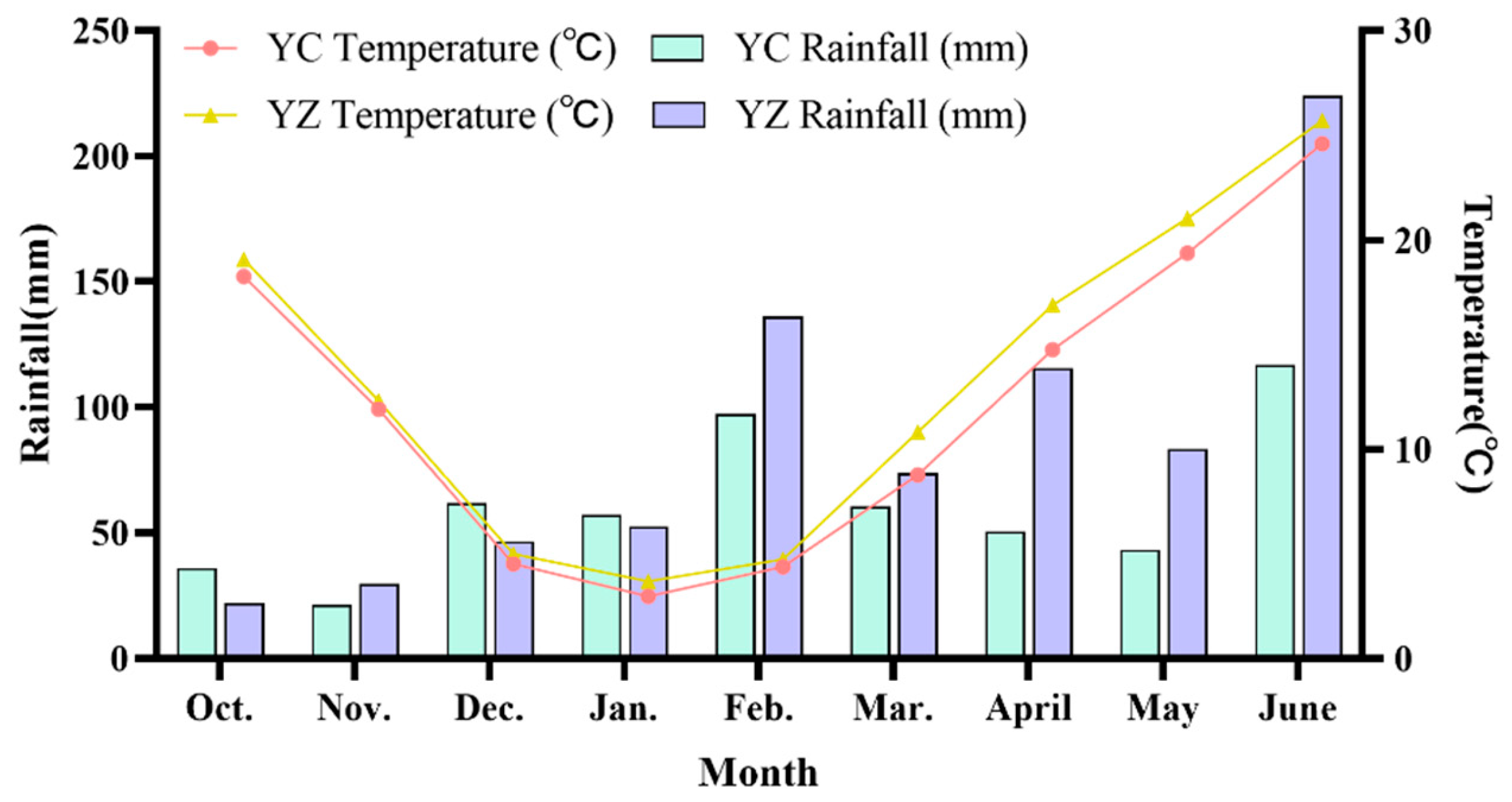
2.1. Experimental Location
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Grain Yield and Yield Components
2.3.2. Plant Type Characteristics
2.3.3. Breaking Strength, Culm Lodging Index, and Lodging Rate
2.3.4. Dry Matter Accumulation
2.3.5. Leaf Area Index and Effective Leaf Area
2.3.6. SPAD Value
2.3.7. Endogenous Hormone Content
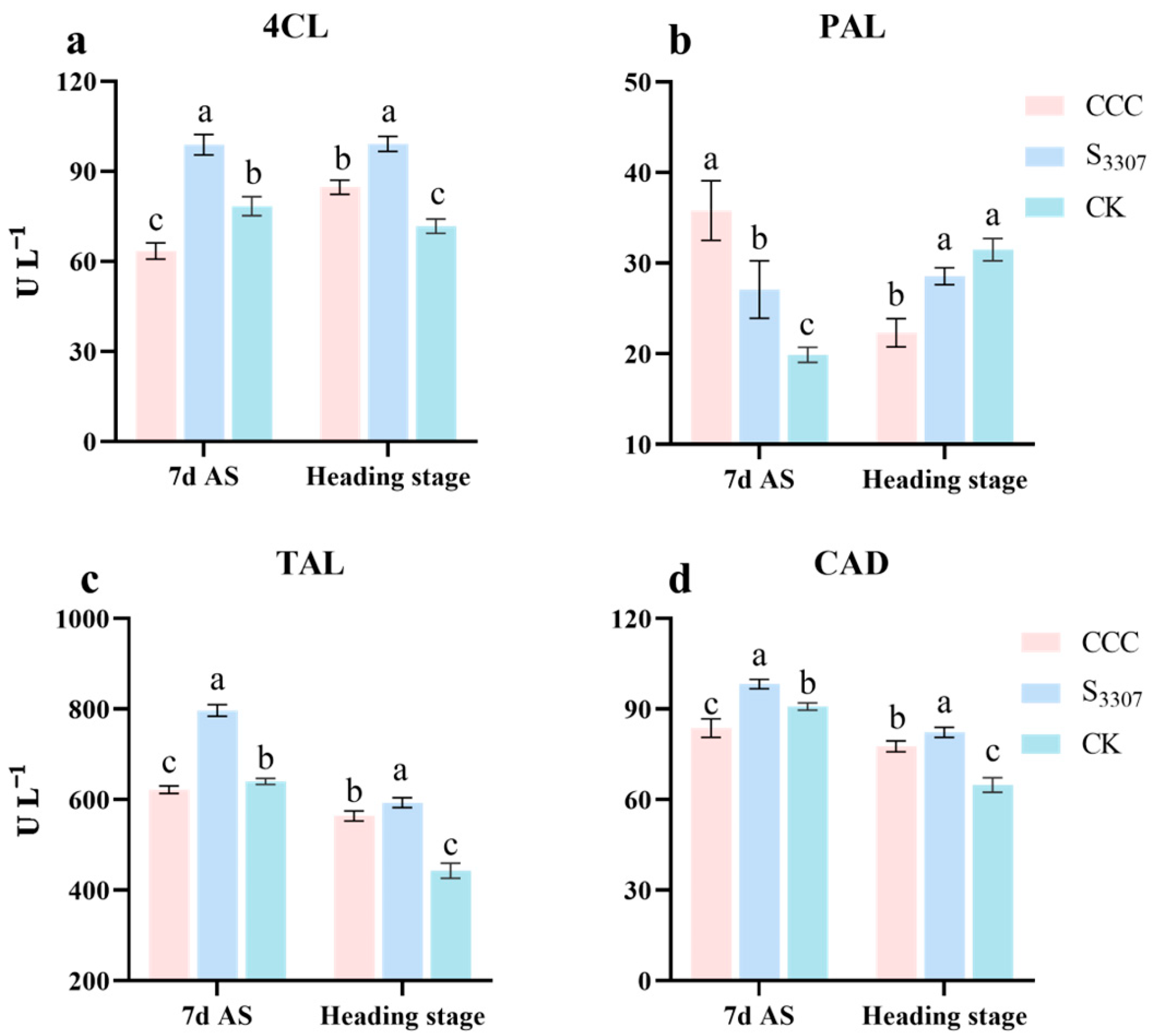
2.3.8. The Activity of Lignin-Related Enzymes
2.3.9. The Activity of Carbon Metabolism-Related Enzymes
2.4. Statistical Analysis
3. Results
3.1. Stem Agronomic Traits and Lodging Resistance
3.2. Yield
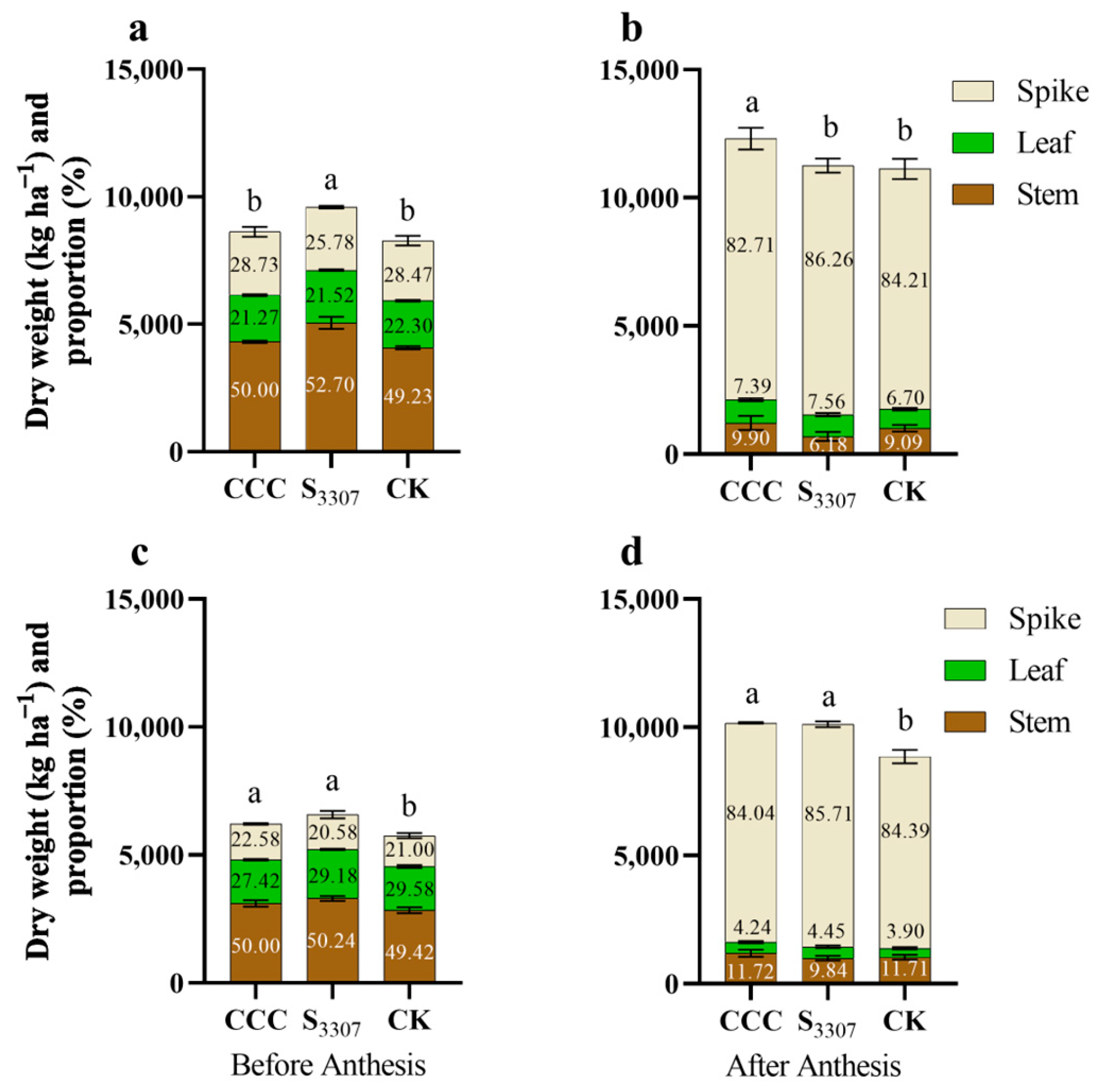
3.3. Dry Matter Accumulation Pre-Anthesis and Post-Anthesis
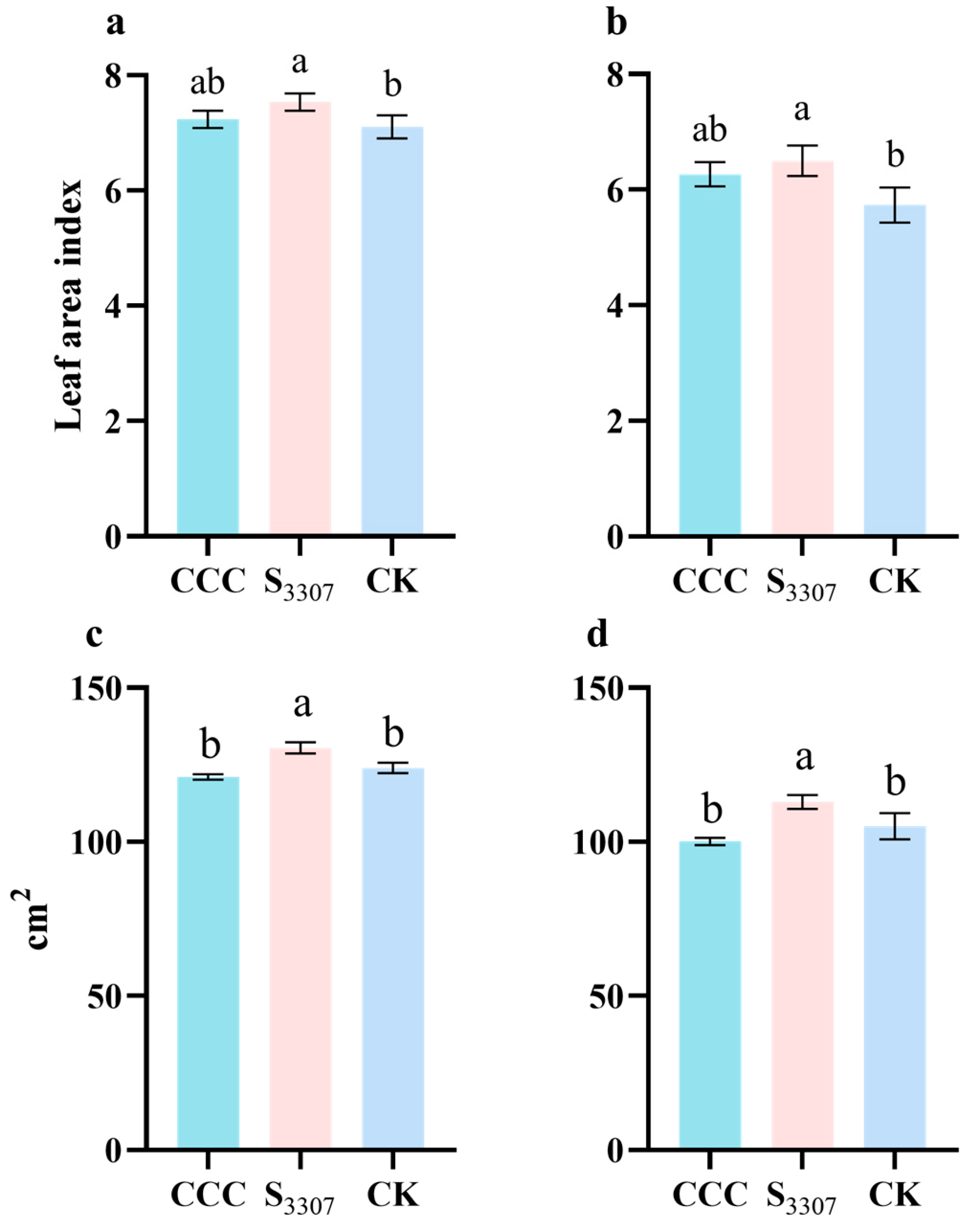
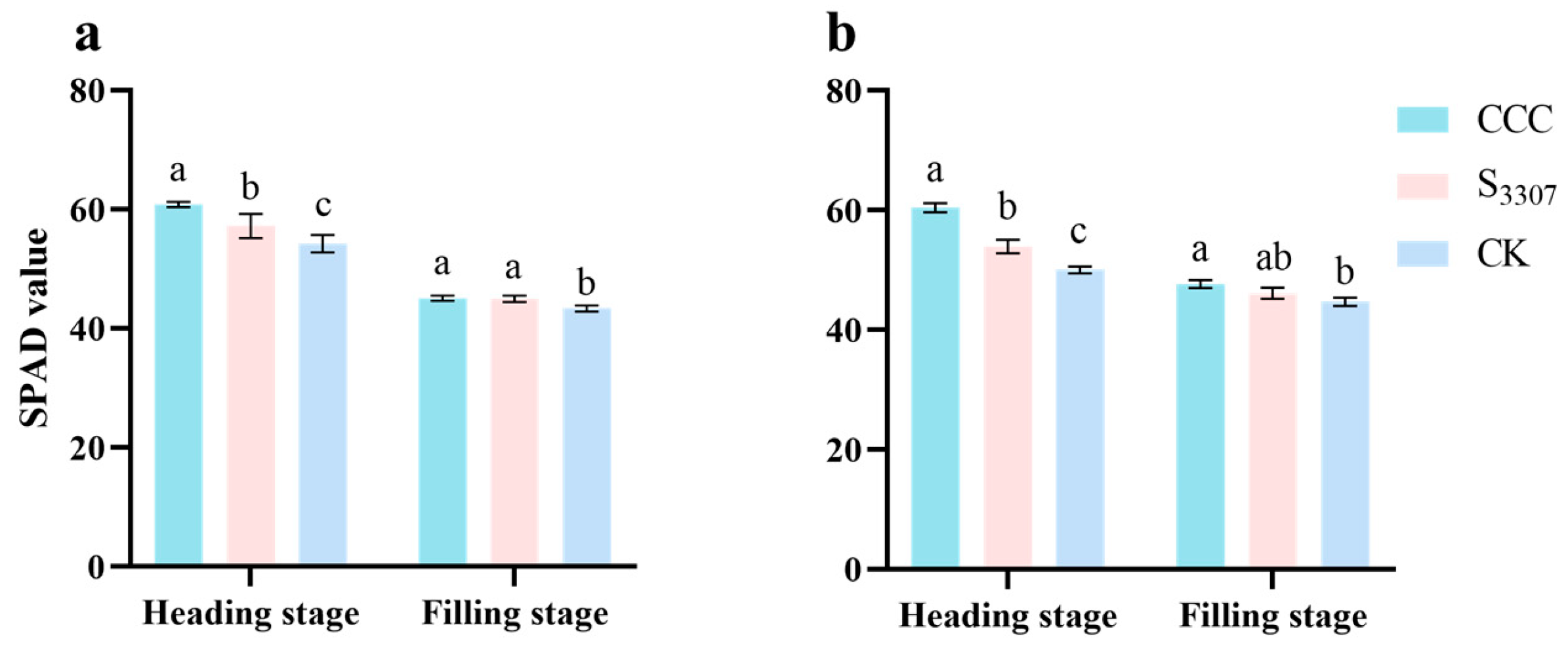
3.4. LAI, Effective Leaf Area and SPAD
3.5. Stem Plant Hormones
3.6. Stem Lignin-Related Enzymes
3.7. Leaf Carbon Metabolism-Related Enzymes
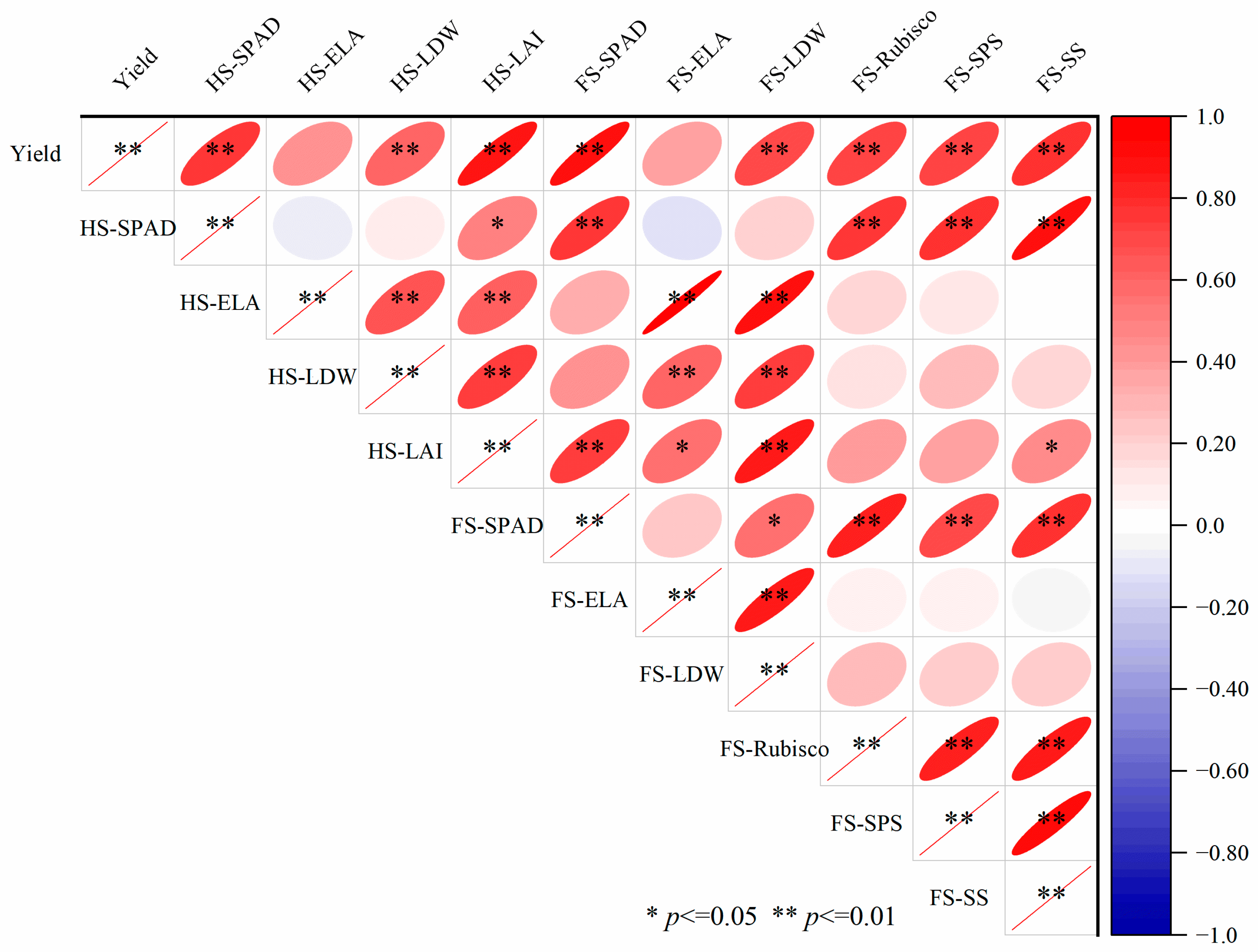
3.8. Correlation Between Lodging Resistance Indexes and Correlation Between Yield and Material Production
4. Discussion
4.1. Effects and Differences in CCC and S3307 on the Stem Morphology and Lodging Resistance
4.2. Effects of CCC and S3307 on Wheat Yield and the Differences in Yield-Increase Approaches
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Yang, X.; Guan, Z.; Zhang, Q.; Li, X.; Gul, C.; Xia, X. The impacts of temperature averages, variabilities and extremes on China’s winter wheat yield and its changing rate. Environ. Res. Commun. 2023, 5, 071002. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Available online: https://www.stats.gov.cn/sj/ndsj/ (accessed on 11 March 2025).
- Zhang, G.P.; Chen, J.X.; Bull, D.A. The effects of timing of N application and plant growth regulators on morphogenesis and yield formation in wheat. Plant Growth Regul. 2001, 35, 239–245. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Lodging yield penalties as affected by breeding in Mediterranean wheats. Field Crops Res. 2011, 122, 40–48. [Google Scholar] [CrossRef]
- Berry, P.M.; Spink, J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 2012, 137, 19–26. [Google Scholar] [CrossRef]
- Jin, Z.; Mu, Y.; Li, Y.; Nie, L. Effect of different rice planting methods on the water, energy and carbon footprints of subsequent wheat. Front. Sustain. Food Syst. 2023, 7, 1173916. [Google Scholar] [CrossRef]
- Berry, P.M. Lodging Resistance in Cereals. In Crop Science; Encyclopedia of sustainability science and technology series; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Mangin, A.; Brule-Babel, A.; Flaten, D.; Wiersma, J.; Lawley, Y. Canopy management: The balance between lodging risk and nitrogen use for spring wheat production in the Canadian Prairies. Can. J. Plant Sci. 2022, 102, 984–1000. [Google Scholar] [CrossRef]
- Li, H.M.; Cui, G.G.; Li, G.Y.; Lu, H.; Wei, H.Y.; Zhang, H.P.; Zhang, H.C. Assessing the efficacy and residual impact of plant growth retardants on crop lodging and overgrowth: A review. Eur. J. Agron. 2024, 159, 127276. [Google Scholar] [CrossRef]
- Zheng, R.; Yan, W.; Xia, Y. Highly water-dispersible hydroxyl functionalized covalent organic frameworks as matrix for enhanced MALDI-TOF MS identification and quantification of quaternary ammonium salts in water and fruits. Anal. Chim. Acta 2022, 1227, 340269. [Google Scholar] [CrossRef]
- Khan, H.; Parkash, O.; Mamrutha, H.M.; Bairwa, R.K.; Mishra, C.N.; Kumar, R.; Jasrotia, P.; Kumar, S.; Krishnappa, G.; Ahlawat, O.P.; et al. Foliar application of chlormequat chloride improves lodging resistance and grain yield in bread wheat. Plant Physiol. Rep. 2025, 30, 199–205. [Google Scholar] [CrossRef]
- Gebre, E.; Schluter, U.; Hedden, P.; Kunert, K. Gibberellin biosynthesis inhibitors help control plant height for improving lodging resistance in E. tef (Eragrostis tef). J. Crop Improv. 2012, 26, 375–388. [Google Scholar] [CrossRef]
- Miranzadeh, H.; Emam, Y.; Seyyed, H.; Zare, S. Productivity and radiation use efficiency of four dryland wheat cultivars under different levels of nitrogen and chlormequat chloride. J. Agric. Sci. Technol. 2011, 13, 339–351. [Google Scholar]
- Yang, M.; Chen, S.; Chao, K.; Ji, C.; Shi, Y. Effects of nano silicon fertilizer on the lodging resistance characteristics of wheat basal second stem node. BMC Plant Biol. 2024, 24, 54. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Kamran, M.; Guo, Z.; Meng, X.; Ali, S.; Zhang, P.; Liu, T.; Cai, T.; Han, Q. Effects of uniconazole or ethephon foliar application on culm mechanical strength and lignin metabolism, and their relationship with lodging resistance in winter wheat. Crop Pasture Sci. 2020, 71, 12–22. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Mathews, P.R.; Caldicott, J.J.B. The effects of chlormequat chloride formulated with choline chloride on the height and yield of winter wheat. Ann. Appl. Biol. 1981, 97, 227–236. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef]
- Fischer, R.A. Wheat physiology: A review of recent developments. Crop Pasture Sci. 2011, 62, 95–114. [Google Scholar] [CrossRef]
- Tian, Z.; Jing, Q.; Dai, T.; Jiang, D.; Cao, W. Effects of genetic improvements on grain yield and agronomic traits of winter wheat in the Yangtze River Basin of China. Field Crops Res. 2011, 124, 417–425. [Google Scholar] [CrossRef]
- Matysiak, K. Influence of trinexapac-ethyl on growth and development of winter wheat. J. Plant Prot. Res. 2006, 46, 133–143. [Google Scholar]
- Espindula, M.C.; Rocha, V.S.; Rezende Fontes, P.C.; Correa Da Silva, R.C.; De Souza, L.T. Effect of nitrogen and trinexapac-Ethyl rates on the SPAD index of wheat Leaves. J. Plant Nutr. 2009, 32, 1956–1964. [Google Scholar] [CrossRef]
- Shekoofa, A.; Emam, Y. Effects of nitrogen fertilization and plant growth regulators (PGRs) on yield of wheat (Triticum aestivum L.) cv. Shiraz. J. Agric. Sci. Technol. 2008, 10, 101–108. [Google Scholar]
- Chhokar, R.S.; Kumar, N.; Gill, S.C.; Tripathi, S.C.; Singh, G. Enhancing wheat productivity through genotypes and growth regulators application under higher fertility conditions in sub-humid climate. Int. J. Plant Prod. 2024, 18, 85–95. [Google Scholar] [CrossRef]
- Schluttenhofer, C.M.; Massa, G.D.; Mitchell, C.A. Use of uniconazole to control plant height for an industrial/pharmaceutical maize platform. Ind. Crops Prod. 2011, 33, 720–726. [Google Scholar] [CrossRef]
- Gutierrez, J.; Lopez Nunez-Flores, M.J.; Gomez-Ros, L.V.; Novo Uzal, E.; Esteban Carrasco, A.; Diaz, J.; Sottomayor, M.; Cuello, J.; Ros Barcelo, A. Hormonal regulation of the basic peroxidase isoenzyme from Zinnia elegans. Planta 2009, 230, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xi, K.; Liu, X.; Han, S.; Han, X.; Li, G.; Yang, L.; Ma, D.; Fang, Z.; Gong, S.; et al. Silica nanoparticles promote wheat growth by mediating hormones and sugar metabolism. J. Nanobiotechnol. 2023, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J. Plant Physiol. 2003, 160, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Ahmad, I.; Meng, X.; Kamran, M.; Ali, S.; Ahmad, S.; Liu, T.; Cai, T.; Han, Q.F. Effects of uniconazole with or without micronutrient on the lignin biosynthesis, lodging resistance, and winter wheat production in semiarid regions. J. Integr. Agric. 2020, 19, 62–77. [Google Scholar] [CrossRef]
- Jesus, W.C., Jr.; Vale, F.X.R.; Coelho, R.R.; Paul, P.A.; Hau, B.; Bergamin, A. Relationships between angular leaf spot, healthy leaf area, effective leaf area and yield of Phaseolus vulgaris. Eur. J. Plant Pathol. 2003, 109, 625–632. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Kamínek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Liu, J.F.; Ding, J.; Yuan, B.F.; Feng, Y.Q. Magnetic solid phase extraction coupled with in situ derivatization for the highly sensitive determination of acidic phytohormones in rice leaves by UPLC-MS/MS. Analyst 2014, 139, 5605–5613. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.H.; Hahlbrock, K. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur. J. Biochem. 1975, 52, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.a.J.; Andralojc, P.J.; Parmar, S.; Keys, A.J.; Habash, D.; Paul, M.J.; Alred, R.; Quick, W.P.; Servaites, J.C. Regulation of Rubisco by inhibitors in the light. Plant Cell Environ. 1997, 20, 528–534. [Google Scholar] [CrossRef]
- Pavlinova, O.A.; Balakhontsev, E.N.; Prasolova, M.F.; Turkina, M.V. Sucrose-phosphate synthase, sucrose synthase, and invertase in sugar beet leaves. Russ. J. Plant Physiol. 2002, 49, 68–73. [Google Scholar] [CrossRef]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Ghuman, L.; Ram, H. Enhancing wheat (Triticum aestivum L.) grain yield and quality by managing lodging with growth regulators under different nutrition levels. J. Plant Nutr. 2021, 44, 1916–1929. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Shi, Y.; Li, Y.; Yin, Y.; Yang, D.; Luo, Y.; Pang, D.; Xu, X.; Li, W.; et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2017, 7, 41805. [Google Scholar] [CrossRef]
- Stubbs, C.J.; Kunduru, B.; Bokros, N.; Verges, V.; Porter, J.; Cook, D.D.; Debolt, S.; Mcmahan, C.; Sekhon, R.S.; Robertson, D.J. Moving toward short stature maize: The effect of plant height on maize stalk lodging resistance. Field Crops Res. 2023, 300, 109008. [Google Scholar] [CrossRef]
- Ma, Q.H.; Xu, Y.; Lin, Z.B.; He, P. Cloning of cDNA encoding COMT from wheat which is differentially expressed in lodging-sensitive and -resistant cultivars. J. Exp. Bot. 2002, 53, 2281–2282. [Google Scholar] [CrossRef]
- Berry, P.M.; Spink, J.H.; Gay, A.P.; Craigon, J. A comparison of root and stem lodging risks among winter wheat cultivars. J. Agric. Sci. 2003, 141, 191–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, Z.; Yao, Y.; Nie, X.; Sun, Q. Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.). BMC Genet. 2007, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.K.; Hu, J.; Rotman, A.; Zheng, C.; Halaly, T.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Lichter, A.; Sun, T.P.; et al. Functional characterization and developmental expression profiling of gibberellin signalling components in Vitis vinifera. J. Exp. Bot. 2015, 66, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Ordonio, R.L.; Ito, Y.; Hatakeyama, A.; Ohmae-Shinohara, K.; Kasuga, S.; Tokunaga, T.; Mizuno, H.; Kitano, H.; Matsuoka, M.; Sazuka, T. Gibberellin deficiency pleiotropically induces culm bending in sorghum: An insight into sorghum semi-dwarf breeding. Sci. Rep. 2014, 4, 5287. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Orozco-Melendez, L.R.; Hernandez-Rodriguez, O.A.; Cruz-Alvarez, O.; Robles-Hernandez, L.; Avila-Quezada, G.D.; Chavez, E.S.; Porras-Flores, D.A.; Ojeda-Barrios, D.L. Paclobutrazol and its use in fruit production: A review. Phyton-Int. J. Exp. Bot. 2022, 91, 1–12. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Clouse, S.D. Brassinosteroids. Arab. Book. 2011, 9, e0151. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.; Wu, X.; Xu, J.; Liu, T.; Ding, R.; Han, Q. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- Kamran, M.; Cui, W.; Ahmad, I.; Meng, X.; Zhang, X.; Su, W.; Chen, J.; Ahmad, S.; Fahad, S.; Han, Q.; et al. Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul. 2018, 84, 317–332. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Li, H.; Chiang, V.L.; Fu, Y. Cooperative regulation of flavonoid and lignin biosynthesis in plants. Crit. Rev. Plant Sci. 2021, 40, 109–126. [Google Scholar] [CrossRef]
- Han, H.; Yang, W. Influence of uniconazole and plant density on nitrogen content and grain quality in winter wheat in South China. Plant Soil Environ. 2009, 55, 159–166. [Google Scholar] [CrossRef]
- Ramburan, S.; Greenfield, P.L. The effects of chlormequat chloride and ethephon on agronomic and quality characteristics of South African irrigated wheat. S. Afr. J. Plant Soil. 2007, 24, 106–113. [Google Scholar] [CrossRef]
- Stahli, D.; Perrissin-Fabert, D.; Blouet, A.; Guckert, A. Contribution of the wheat (Triticum aestivum L.) flag leaf to grain yield in response to plant growth regulators. Plant Growth Regul. 1995, 16, 293–297. [Google Scholar] [CrossRef]
- Monostori, I.; Arendas, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vagujfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Bode, J.; Wild, A. The influence of (2-Chloroethyl)trimethylammoniumchloride (CCC) on growth and photosynthetic metabolism of young wheat plants (Triticum aestivum L.). J. Plant Physiol. 1984, 116, 435–446. [Google Scholar] [CrossRef]





| Experimental Site | pH | Organic Matter (g kg−1) | Total N (g kg−1) | Available P (g kg−1) | Available K (g kg−1) |
|---|---|---|---|---|---|
| YC | 7.78 | 23.4 | 1.3 | 47.5 | 192.17 |
| YZ | 7.40 | 22.6 | 1.2 | 33.2 | 125.6 |
| Experimental Site | Treatment | PH (cm) | SD (mm) | 1st IL (cm) | 2nd IL (cm) | 3rd IL (cm) | 4th IL (cm) | 5th IL (cm) |
|---|---|---|---|---|---|---|---|---|
| YC | CCC | 71.7 c | 5.5 a | 6.8 a | 12.3 b | 19.1 b | 14.6 b | 10.5 b |
| S3307 | 75.4 b | 5.3 a | 7.0 a | 13.5 a | 20.0 a | 14.6 b | 10.9 b | |
| CK | 78.8 a | 5.1 b | 7.0 a | 143 a | 20.7 a | 15.7 a | 12.4 a | |
| YZ | CCC | 71.4 c | 5.1 a | 6.4 b | 13.5 b | 19.2 b | 15.4 b | 8.7 a |
| S3307 | 73.5 b | 5.0 a | 5.9 c | 13.1 c | 20.3 a | 16.5 a | 9.2 a | |
| CK | 76.2 a | 4.6 b | 7.3 a | 15.0 a | 19.8 ab | 16.0 ab | 9.9 a |
| Experiment Site | Treatment | Lodging Stage | Lodging Level | Lodging Rate (%) |
|---|---|---|---|---|
| YC | CCC | - | 0 b | 0 b |
| S3307 | - | 0 b | 0 b | |
| CK | Filling stage | 3 a | 7.1 a | |
| YZ | CCC | - | 0 b | 0 b |
| S3307 | - | 0 b | 0 b | |
| CK | Anthesis stage | 2 a | 15.6 a |
| Experimental Site | Treatments | Spikes per hectare (×104) | Grain Number Per Spike | 1000-Grain Weight (g) | Crop Yield (kg ha−1) |
| YC | CCC | 453.3 b | 41.5 b | 50.6 a | 7693.2 a |
| S3307 | 474.3 a | 43.3 a | 46.5 b | 7682.8 a | |
| CK | 428.3 c | 43.4 a | 47.5 b | 7258.4 b | |
| YZ | CCC | 353.1 b | 38.5 b | 50.4 a | 7299.4 a |
| S3307 | 379.2 a | 39.3 ab | 46.0 b | 7247.5 a | |
| CK | 340.6 b | 41.7 a | 46.4 b | 6824.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, T.; Weng, W.; Cui, G.; Zhang, H.; Xing, Z.; Fu, L.; Liu, B.; Wei, H.; Zhang, H.; et al. Chlormequat Chloride and Uniconazole Regulate Lodging Resistance and Yield Formation of Wheat Through Different Strategies. Agronomy 2025, 15, 2475. https://doi.org/10.3390/agronomy15112475
Li H, Li T, Weng W, Cui G, Zhang H, Xing Z, Fu L, Liu B, Wei H, Zhang H, et al. Chlormequat Chloride and Uniconazole Regulate Lodging Resistance and Yield Formation of Wheat Through Different Strategies. Agronomy. 2025; 15(11):2475. https://doi.org/10.3390/agronomy15112475
Chicago/Turabian StyleLi, Huimin, Tao Li, Wenan Weng, Gege Cui, Haipeng Zhang, Zhipeng Xing, Luping Fu, Bingliang Liu, Haiyan Wei, Hongcheng Zhang, and et al. 2025. "Chlormequat Chloride and Uniconazole Regulate Lodging Resistance and Yield Formation of Wheat Through Different Strategies" Agronomy 15, no. 11: 2475. https://doi.org/10.3390/agronomy15112475
APA StyleLi, H., Li, T., Weng, W., Cui, G., Zhang, H., Xing, Z., Fu, L., Liu, B., Wei, H., Zhang, H., & Li, G. (2025). Chlormequat Chloride and Uniconazole Regulate Lodging Resistance and Yield Formation of Wheat Through Different Strategies. Agronomy, 15(11), 2475. https://doi.org/10.3390/agronomy15112475







