Coupling Effects of Organic Fertilizer Substituting Chemical Fertilizer on Potato Yield, Quality and Soil Nitrogen Content in the Erhai Lake Basin of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Measurements and Calculations
2.3.1. Soil Sampling
2.3.2. Sampling of Plants
2.3.3. Soil Nutrients and Enzymes
2.3.4. Leaf Area Index (LAI) and Relative Chlorophyll Content (SPAD)
2.3.5. Plant N, P, K Accumulation Amounts
2.3.6. Activities of Nitrogen Metabolism Enzymes in Potato Leaves
2.3.7. Soluble Protein Content in Potato Leaves
2.3.8. Potato Yield and Nutritional Quality
2.4. Comprehensive Evaluation
2.4.1. Membership Function Method
2.4.2. Principal Component Analysis
2.4.3. Weighted TOPSIS Method
2.4.4. Gray Correlation Degree Analysis Method
2.4.5. Evaluation Model Using Aggregate Differences
2.5. Data Analysis
3. Results
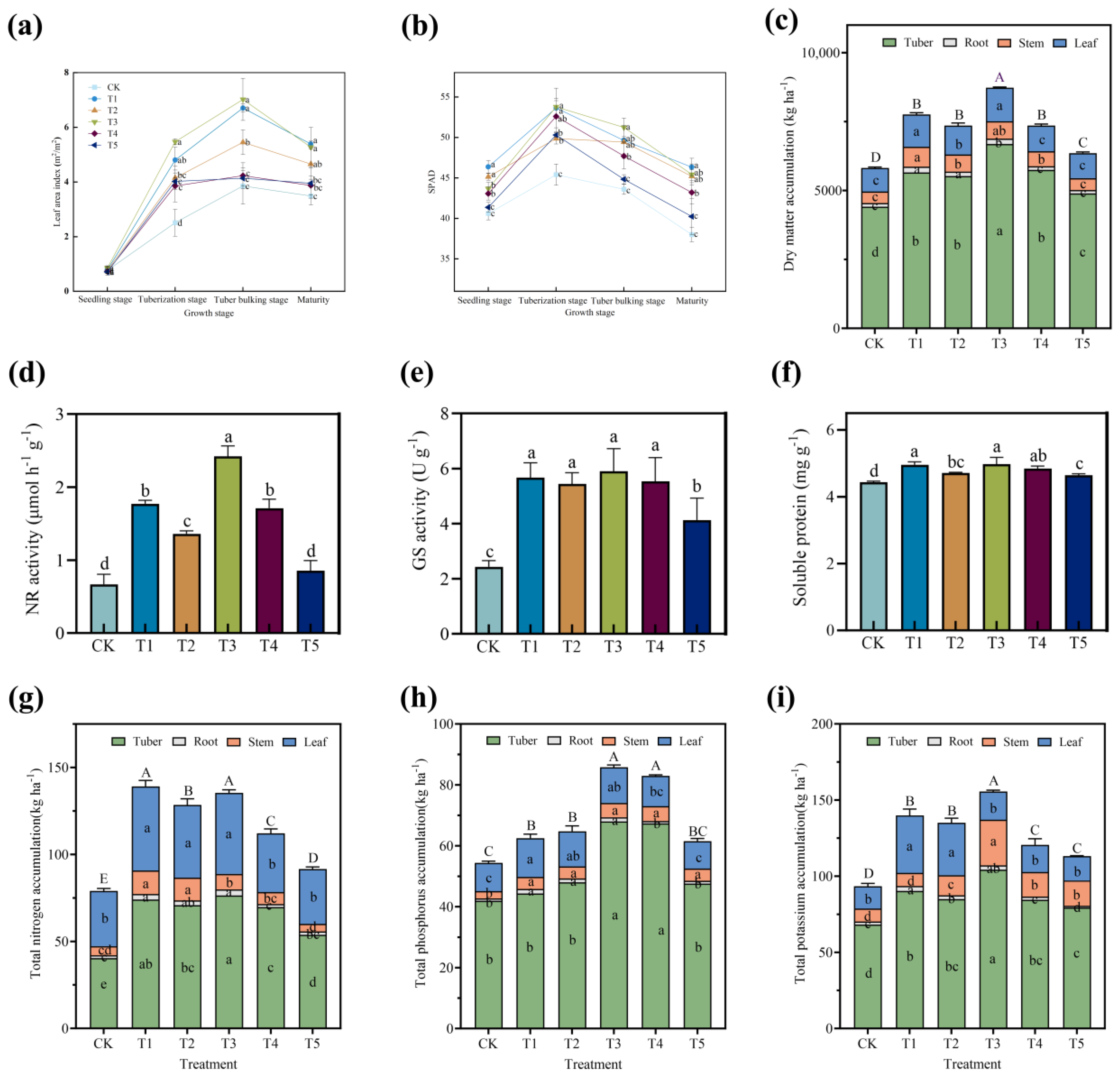
3.1. Effects of Different Fertilization Treatments on Growth and Metabolic Absorption of Potato
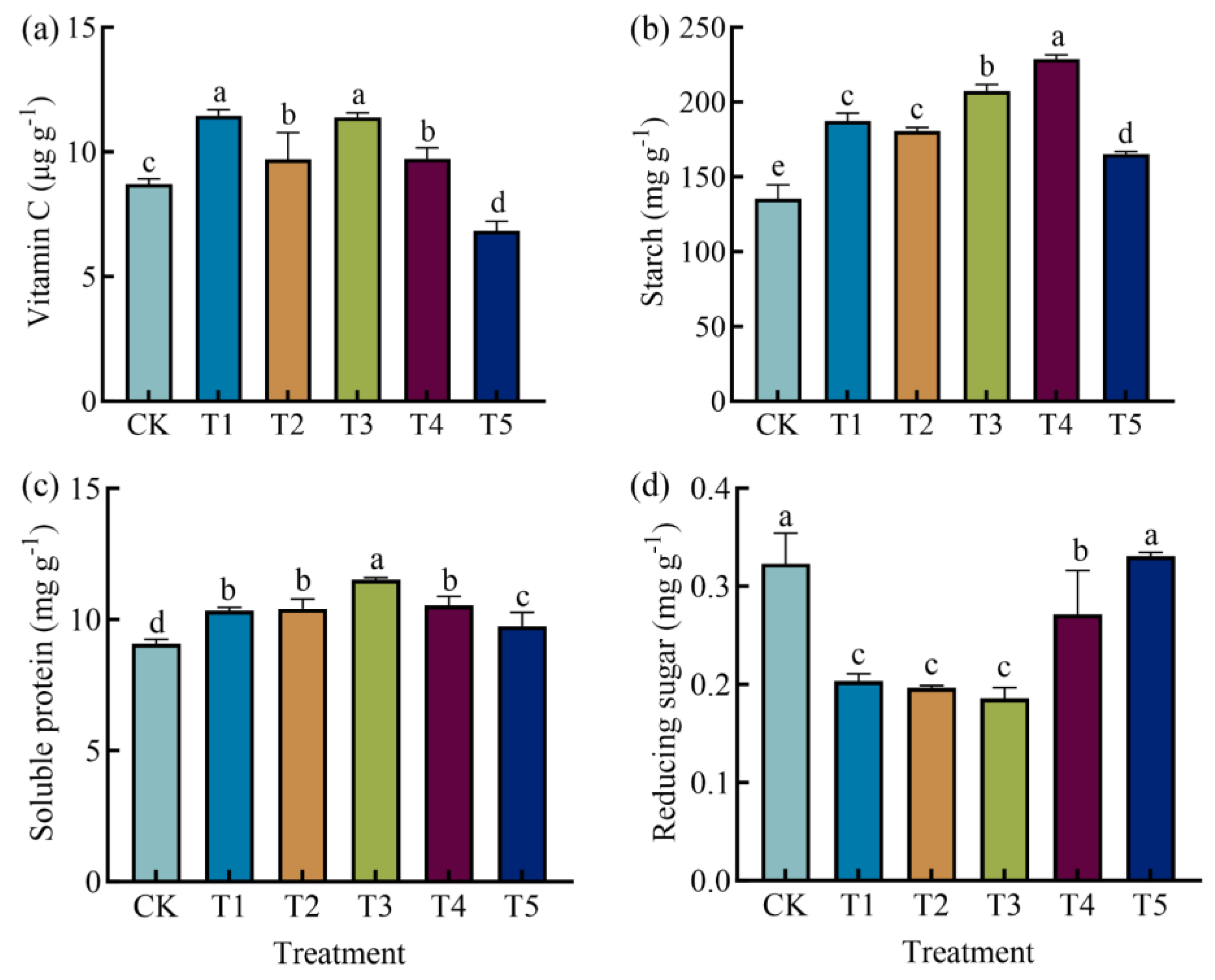
3.2. Effects of Different Fertilization Treatments on Yield and Quality of Potato
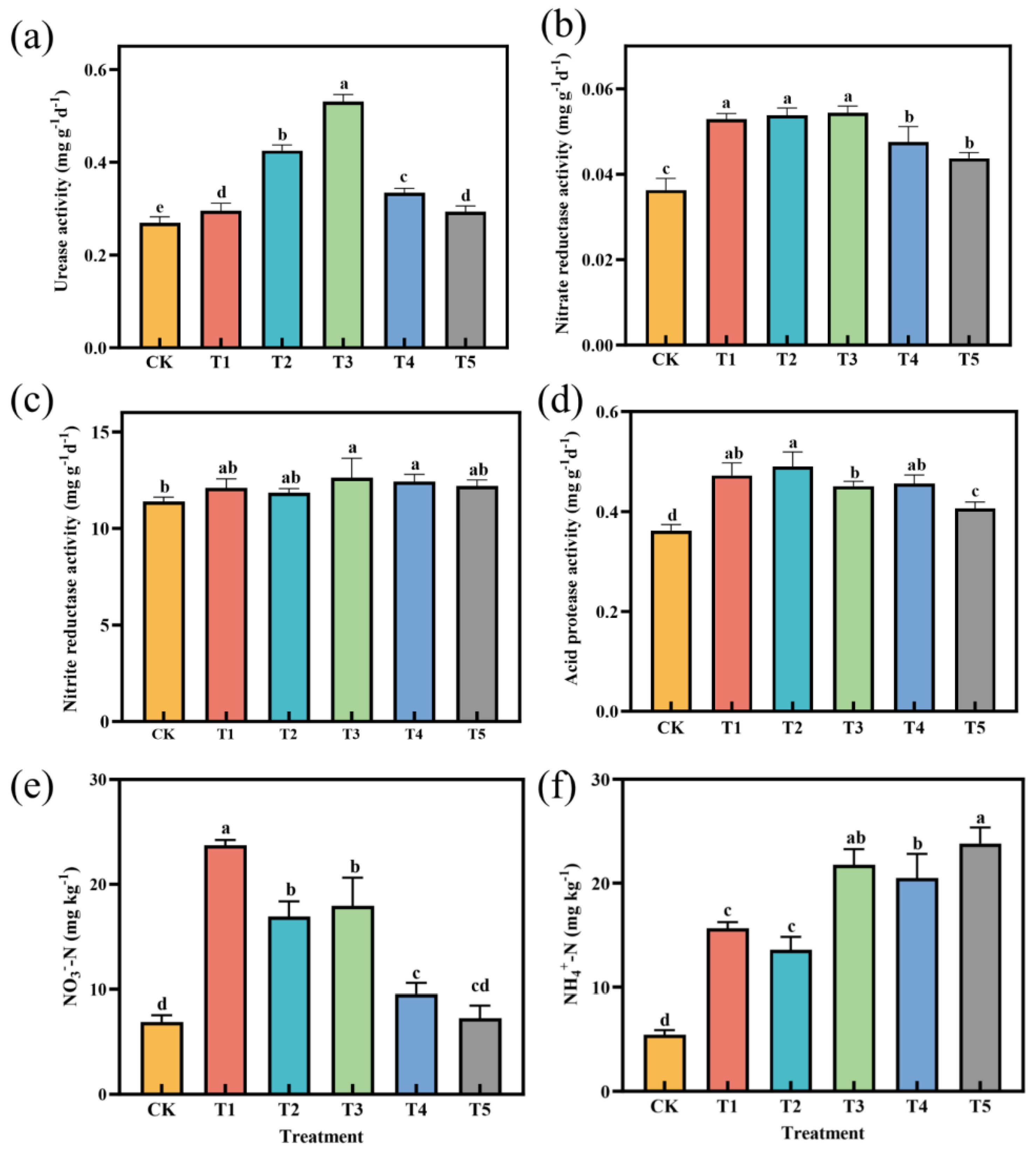
3.3. Effects of Different Fertilization Treatments on Soil Enzyme and Soil Nitrogen Content
3.4. Effects of Different Fertilization Treatments on Economic Benefits
3.5. Coupling and Decision Optimization of Multi-Objective Evaluation Models Based on Minimization of Overall Differences
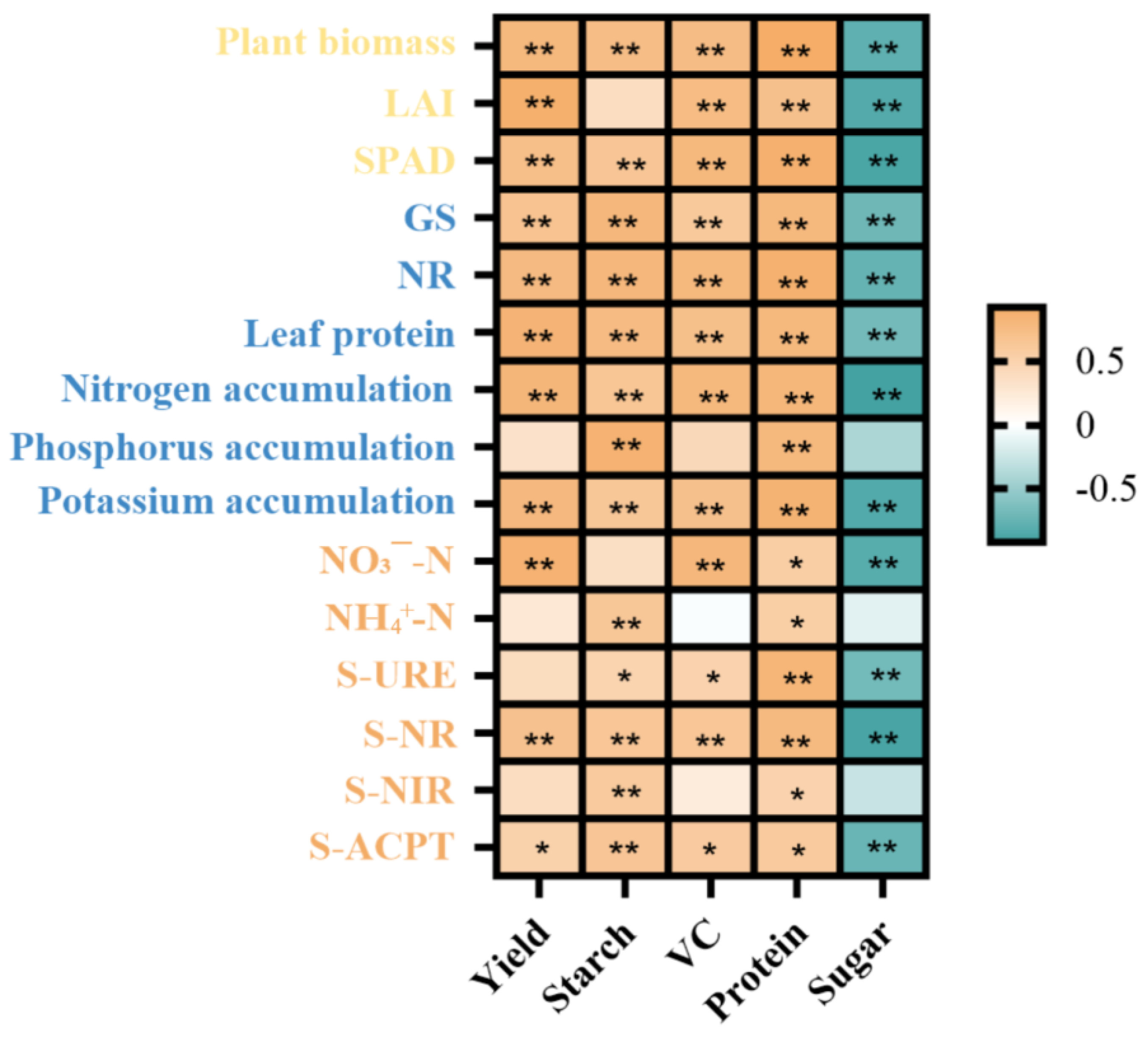
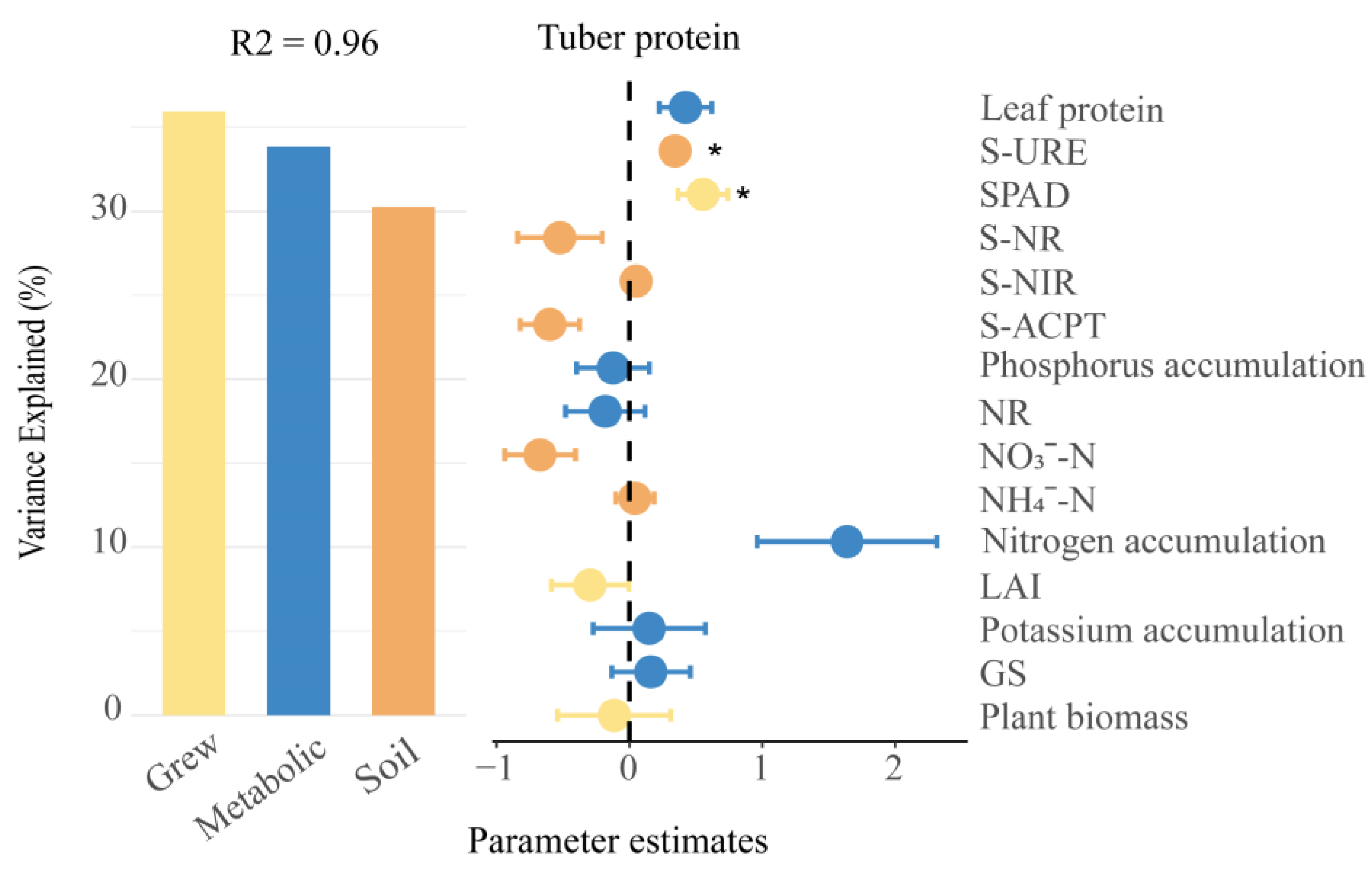
3.6. Factors Influencing Soil Fertility and Potato Metabolism and Growth to Promote Tuber Protein Content
4. Discussion
4.1. Effects of Equal Replacement of Chemical Fertilizer Nitrogen with Organic Fertilizer Nitrogen in the Rhizosphere Soil of Potatoes by Increasing Soil Enzyme Activity
4.2. Effects of Equal Replacement of Chemical Fertilizer Nitrogen with Organic Fertilizer Nitrogen on Nutrient Uptake and Yield of Potato
4.3. Effects of Equal Replacement of Chemical Fertilizer Nitrogen with Organic Fertilizer Nitrogen on Nitrogen Metabolism and Potato Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, G.; Cambouris, A.N.; Bertrand, A.; Ziadi, N.; Li, H.; Khelifi, M. Nitrogen Fertilization Effects on the Leaf Chemical Concentrations in Russet Burbank Potato. Field Crop. Res. 2019, 232, 40–48. [Google Scholar] [CrossRef]
- Xie, C.; Hao, D.; Duan, X.; Tao, C.; Yang, J.; Yang, X. Green and Efficient Cultivation Techniques of Potato around the Erhai Lake Basin. Chin. Potato J. 2023, 37, 436–439. [Google Scholar] [CrossRef]
- Chen, X.; Qian, X.; Li, X.; Wei, Z.; Hu, S. Long-term Trend of Eutrophication State of Lake Erhai in 1988–2013 and Analyses of its Socio-Economic Drivers. J. Lake Sci. 2018, 30, 70–78. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.-R.; Bermejo, A.; Legaz, F.; Quiñones, A. Liquid Organic Fertilizers for Sustainable Agriculture: Nutrient Uptake of Organic Versus Mineral Fertilizers in Citrus Trees. PLoS ONE 2016, 11, e0161619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Sun, S.; Liu, W.; Zhu, L.; Yan, X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Abd–Elrahman, S.H.; Saudy, H.S.; El–Fattah, D.A.A.; Hashem, F.A.-E. Effect of Irrigation Water and Organic Fertilizer on Reducing Nitrate Accumulation and Boosting Lettuce Productivity. J. Soil Sci. Plant Nutr. 2022, 22, 2144–2155. [Google Scholar] [CrossRef]
- Xing, Y.; Niu, X.; Wang, N.; Jiang, W.; Gao, Y.; Wang, X. The Correlation Between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR. Sustainability 2020, 12, 1588. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; De Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Morishita, A.; Kinoshita, R.; Tani, M.; Sugawara, M.; Ikeda, S. Effects of Phosphate Fertilization Rates on Physicochemical Properties of Potato Starch. Starch-Stärke 2023, 76, 2300190. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Xu, W.; Wang, J.; Jiang, Y.; Ali, W.; Liu, W. Substitution of Inorganic Fertilizer with Organic Fertilizer Influences Soil Carbon and Nitrogen Content and Enzyme Activity under Rubber Plantation. Forests 2024, 15, 756. [Google Scholar] [CrossRef]
- Yan, J.; Weng, H.; He, T. Effects of Replacing Chemical Fertilizers Nitrogen with Organic Fertilizers Nitrogen on Growth Characteristics, Leaf Carbon and Nitrogen Balance and Nitrogen Metabolism Enzyme Activities of Hulless barleyon. J. Triticeae Crops. 2025, 45, 121–129. [Google Scholar] [CrossRef]
- Yang, J.; Mattoo, A.K.; Liu, Y.; Zvomuya, F.; He, H. Trade-Offs of Organic and Organic-Inorganic Fertilizer Combinations in Tomato Quality and Yield: A Global Meta-Analysis (1992–2021). Eur. J. Agron. 2023, 151, 126985. [Google Scholar] [CrossRef]
- Liang, B.; Yang, X.; Murphy, D.V.; He, X.; Zhou, J. Fate of 15 N-Labeled Fertilizer in Soils Under Dryland Agriculture after 19 Years of Different Fertilizations. Biol. Fertil. Soils 2013, 49, 977–986. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.K.; Singh, R.P. Application of Plant Growth Promoting Microbes to Enrich Zinc in Potato for Nutritional Security and Sustainable Agriculture. Rhizosphere 2023, 25, 100665. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, M.; Zhang, S.; Fan, J.; Feng, H.; Zhang, F.; Wang, X.; Sun, L.; Xiang, Y. Optimization of Irrigation Amount and Fertilization Rate of Drip-Fertigated Potato Based on Analytic Hierarchy Process and Fuzzy Comprehensive Evaluation Methods. Agric. Water Manag. 2021, 256, 107130. [Google Scholar] [CrossRef]
- Zhou, L.; Mu, T.; Ma, M.; Zhang, R.; Sun, Q.; Xu, Y. Nutritional Evaluation of Different Cultivars of Potatoes (Solanum tuberosuml.) from China by Grey Relational Analysis (GRA) and its Application in Potato Steamed Bread Making. J. Integr. Agric. 2019, 18, 231–245. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Yang, Q.; Wang, X.; Cui, N. Determining Optimal Deficit Irrigation and Fertilization to Increase Mango Yield, Quality, and WUE in a Dry Hot Environment Based on TOPSIS. Agric. Water Manag. 2021, 245, 106650. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, B.; Zhang, H.; Shen, Y.; Zhang, B.; Chen, Y.; Yu, B.; Li, Y.; Cai, Y. Responses of Zoysia Matrella Growth, Soil Mineral Nitrogen, and Nitrogen Balance to Different Levels of Irrigation and Mowing. Pratac. Sci. 2023, 40, 2787–2799. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Y.; Zhang, X.; Shen, B.; Qin, S.; Liu, Z.; Wang, L.; Li, C.; Zhang, J. Effects of Deficit Irrigation on Growth, Yield and Water Use of Potato Plants. Trans. Chin. Soc. Agric. Eng. 2019, 35, 114–123. [Google Scholar] [CrossRef]
- Cang, K.; Du, Y.; Mu, L.; Yang, C.; Chen, Y.; Zhang, Y. Principal Component Analysis and Comprehensive Evaluation of Main Agronomic Traits of New Varieties of Tartary Buckwheat in Southern Mountainous Areas of Ningxia. Heilongjiang Agric. Sci. 2024, 12, 21–28. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.; Fu, J.; Zhang, X.; Li, Y.; Shao, Y.; Xing, Y.; Wang, X. Coupling Effects of Irrigation Amount and Fertilization Rate on Yield, Quality, Water and Fertilizer Use Efficiency of Different Potato Varieties in Northwest China. Agric. Water Manag. 2023, 287, 108446. [Google Scholar] [CrossRef]
- Qiu, S.J.; Ju, X.T.; Ingwersen, J.; Qin, Z.C.; Li, L.; Streck, T.; Christie, P.; Zhang, F.S. Changes in Soil Carbon and Nitrogen Pools after Shifting from Conventional Cereal to Greenhouse Vegetable Production. Soil Tillage Res. 2010, 107, 80–87. [Google Scholar] [CrossRef]
- Zhai, Z.; Wang Li Li, H.; Qiu, J.; Yang, J.; Dong, X. Nitrous Oxide Emissions and Net Greenhouse Effect from Spring-Maize Field as Influenced by Combined Application of Manure and Inorganic Fertilizer. J. Agro Environ. Sci. 2013, 32, 2502–2510. [Google Scholar] [CrossRef]
- Zilio, M.; Motta, S.; Tambone, F.; Scaglia, B.; Boccasile, G.; Squartini, A.; Adani, F. The Distribution of Functional N-Cycle Related Genes and Ammonia and Nitrate Nitrogen in Soil Profiles Fertilized with Mineral and Organic N Fertilizer. PLoS ONE 2020, 15, e0228364. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Liu, X.; Zhang, C.; Zeng, Z.; Lan, T. Effects of Biochar and Bionitrification Inhibitors on Nitrogen Transformation in Calcareous Purple Soil. Soils 2025, 57, 558–567. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, W.; He, Y.; Yu, C.; Wu, C.; Li, J. Effects of Understory Taxus chinensis var. mairei Planting on Soil Fertility of Chinese Fir Plantations. Chin. J. Appl. Environ. Biol. 2024, 30, 344–352. [Google Scholar] [CrossRef]
- Li, C.; Ma, S.; Shao, Y.; Ma, S.; Zhang, L. Effects of Long-Term Organic Fertilization on Soil Microbiologic Characteristics, Yield and Sustainable Production of Winter Wheat. J. Integr. Agric. 2018, 17, 210–219. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, H.J.; Cha, S.J.; Kwon, S.J.; Park, J.H. Effect of Pyroligneous Acid on Soil Urease, Amidase, and Nitrogen Use Efficiency by Chinese Cabbage (Brassica campestris var. pekinensis). Environ. Pollut. 2021, 291, 118132. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Luo, J.; Lindsey, S.; Wang, L.; Zhang, L.; Shi, Y. Potential of Chamomilla Recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils. Atmosphere 2021, 12, 1223. [Google Scholar] [CrossRef]
- Liu, N.; Liao, P.; Zhang, J.; Zhou, Y.; Luo, L.; Huang, H.; Zhang, L. Characteristics of Denitrification Genes and Relevant Enzyme Activities in Heavy-Metal Polluted Soils Remediated by Biochar and Compost. Sci. Total Environ. 2020, 739, 139987. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Shu, A.; Liu, J.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Effects of Long-Term Fertilization with Different Substitution Ratios of Organic Fertilizer on Paddy Soil. Pedosphere 2022, 32, 637–648. [Google Scholar] [CrossRef]
- Gao, Y.; Long, X.; Liao, Y.; Lin, Y.; He, Z.; Kong, Q.; Kong, X.; He, X. Influence of Arbuscular Mycorrhizal Fungi on Nitrogen Dynamics during Cinnamomum Camphora Litter Decomposition. Microorganisms 2025, 13, 151. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, K.; Guo, W.; Huang, H.; Gou, Z.; Yang, L.; Chen, Y.; Oh, K.; Fang, C.; Luo, L. The Effects of Partial Substitution of Fertilizer Using Different Organic Materials on Soil Nutrient Condition, Aggregate Stability and Enzyme Activity in a Tea Plantation. Plants 2023, 12, 3791. [Google Scholar] [CrossRef]
- Yokoyama, D.; Imai, N.; Kitayama, K. Effects of Nitrogen and Phosphorus Fertilization on Activities of Four Different Classes of Fine-Root and Soil Phosphatases in Bornean Tropical Rain Forests. Plant Soil 2017, 416, 463–476. [Google Scholar] [CrossRef]
- Chang, X.; He, H.; Cheng, L.; Yang, X.; Li, S.; Yu, M.; Zhang, J.; Li, J. Combined Application of Chemical and Organic Fertilizers: Effects on Yield and Soil Nutrients in Spring Wheat under Drip Irrigation. Agronomy 2024, 14, 655. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, P.; Liu, X.; Guo, T. Effects of Organic Fertilizers Partially Replacing Chemical Fertilizers on Potato Yield, Water and Nitrogen Utilization in Semi-Arid Areas. Chin. J. Soil Fertil. Sci. 2023, 10, 143–149. [Google Scholar] [CrossRef]
- Liu, J.; Si, Z.; Wu, L.; Li, S.; Sun, Y.; Wang, N.; Gao, Y.; Duan, A. Effects of Water and Nitrogen Coupling on Winter Wheat Yield and Water and Nitrogen Use Efficiency under High-Low Seedbed Cultivation Pattern. Trans. Chin. Soc. Agric. Eng. 2023, 39, 144–154. [Google Scholar] [CrossRef]
- Chen, M.; Xing, Y.; Xie, Y.; Liu, X.; Shao, Y.; Li, S.; Zhao, T.; Lu, J.; Wang, X. Effects of Organic Manure and Other Nitrogen Substitutes on Spring Maize Growth, Yield, and Water and Fertilizer Utilization Efficiency. J. Soil Water Cons. 2024, 38, 369–381. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different Organic Manure Sources and NPK Fertilizer on Soil Chemical Properties, Growth, Yield and Quality of Okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Yuan, M.; Wu, G.; Wang, J.; Sun, Y. Study on the Input and Demand of Crop Nutrients and Potential of Fertilizer Reduction in Anhui Province. Chin. J. Eco Agric. 2020, 28, 221–235. [Google Scholar] [CrossRef]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated Use of Bio-organic Fertilizers for Enhancing Soil Fertility–Plant Nutrition, Germination Status and Initial Growth of Corn (Zea mays L.). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Wan, M.; Man, B.; Liu, W.; Liu, H.; Ma, F.; Ma, X.; Li, T.; Liu, J.; Wu, N. Effects of Intercropping with Legumes on the Activities of Key Enzymes of Carbon and Nitrogen Metabolism in Potato Leaves and Crop Yield. Chin. J. Eco Agric. 2024, 32, 2070–2080. [Google Scholar] [CrossRef]
- Xudong, G.; Fengju, Z.; Teng, W.; Xiaowei, X.; Xiaohui, J.; Xing, X. Effects of Nitrogen and Phosphorus Addition on Growth and Leaf Nitrogen Metabolism of Alfalfa in Alkaline Soil in Yinchuan Plain of Hetao Basin. PeerJ 2022, 10, e13261. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, C.; Guo, S.; Lu, K.; Wu, N. Effect of Nitrogen form on Growth and Nitrogen Metaolism of Atropa Belladonna. Pratac. Sci. 2017, 34, 1669–1676. [Google Scholar] [CrossRef]
- Dai, T.; Cao, W.; Sun, C.; Jiang, D.; Jing, Q. Effect of Enhanced Ammonium Nutrition on Photosynthesis and Nitrate Reductase and Glutamine Synthetase Activities of Winter Wheat. Appl. Ecol. 2003, 14, 1529–1532. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Yao, Y.; Dang, P.; Farooq, T.H.; Wu, X.; Wang, J.; Yan, W. Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes. Plants 2023, 12, 4188. [Google Scholar] [CrossRef]
- Zheng, J.; Su, Z.; Liu, J.; Wang, Y. Suitable Model and Evaluation of Nitrogen Fertilizer Replacement by Greenhouse Tomato Digestate Based on Nutrient Balance Principle. J. China Agric. Univ. 2024, 29, 77–90. [Google Scholar] [CrossRef]
- Shu, X.; Jin, M.; Wang, S.; Xu, X.; Deng, L.; Zhang, Z.; Zhao, X.; Yu, J.; Zhu, Y.; Lu, G. The Effect of Nitrogen and Potassium Interaction on the Leaf Physiological Characteristics, Yield, and Quality of Sweet Potato. Agronomy 2024, 14, 2319. [Google Scholar] [CrossRef]
- Khan, M.Z.; Akhtar, M.E.; Mahmood-ul-Hassan, M.; Mahmood, M.M.; Safdar, M.N. Potato Tuber Yield and Quality as Affected by Rates and Sources of Potassium Fertilizer. J. Plant Nutr. 2012, 35, 664–677. [Google Scholar] [CrossRef]
- Tetlow, I. Starch Biosynthesis in Crop Plants. Agronomy 2018, 8, 81. [Google Scholar] [CrossRef]
- Wu, M.; Shi, M.; Sun, D.; Liu, J.; Zhang, W.; Kang, Y.; Liu, Y.; Qin, S. Effect of Nitrogen Reduction with Organic and Bacterial Fertilizers on Physiological Characteristics, Yield, and Storage Quality of Potato. J. Gansu Agric. Univ. 2025, 60, 106–113. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Ma, Z.; Zhang, M.; Wei, H.; Zhang, H.; Liu, G.; Hu, Q.; Li, G.; Xu, F. Effects of Light Intensity and Nitrogen Fertilizer Interaction on Carbon and Nitrogen Metabolism at Grain-Filling Stage and its Relationship with Yield and Quality of Southern Soft Japonica Rice. Acta Agron. Sin. 2023, 49, 3042–3062. [Google Scholar] [CrossRef]
- Ren, M.; Mao, G.; Liu, S.; Wang, W.; Zheng, H.; Tang, Q. Research Progress on the Effects of Light Quality on Plant Growth and Development, Photosynthesis, and Carbon and Nitrogen Metabolism. Plant Physiol. J. 2023, 59, 1211–1228. [Google Scholar] [CrossRef]
- Wang, X.; Guo, T.; Wang, Y.; Xing, Y.; Wang, Y.; He, X. Exploring the Optimization of Water and Fertilizer Management Practices for Potato Production in the Sandy Loam Soils of Northwest China Based on PCA. Agric. Water Manag. 2020, 237, 106180. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Bi, L.; Wang, Y.; Fan, J.; Zhang, F.; Hou, X.; Cheng, M.; Hu, W.; Wu, L.; et al. Multi-Objective Optimization of Water and Fertilizer Management for Potato Production in Sandy Areas of Northern China Based on TOPSIS. Field Crops Res. 2019, 240, 55–68. [Google Scholar] [CrossRef]
| Treatments | Potato Root System Morphology | |||
|---|---|---|---|---|
| Total Root Length (cm) | Total Root Surface Area (cm2) | Mean Diameter of Root System (mm) | Total Root Volume (cm3) | |
| CK | 655.88 ± 18.39 b | 94.77 ± 4.72 d | 0.68 ± 0.05 d | 1.03 ± 0.01 c |
| T1 | 805.19 ± 24.41 a | 143.79 ± 13.14 a | 1.1 ± 0.02 ab | 2.32 ± 0.1 a |
| T2 | 758.02 ± 25 ab | 126.13 ± 1.75 b | 1.13 ± 0.03 a | 2.23 ± 0.04 a |
| T3 | 800.07 ± 19.02 a | 136.69 ± 2.31 ab | 1.17 ± 0.03 a | 2.22 ± 0.13 a |
| T4 | 758.51 ± 13.45 ab | 113.86 ± 1.86 c | 1.04 ± 0.06 b | 1.47 ± 0.03 b |
| T5 | 726.2 ± 27.11 ab | 105.44 ± 2.6 c | 0.83 ± 0.05 c | 1.07 ± 0.05 c |
| Treatments | Tuber Yield (kg·ha−1) | Commodity Tubers (kg·ha−1) |
|---|---|---|
| CK | 33,384.64 ± 1036.33 c | 30,596.18 ± 919.39 c |
| T1 | 51,039.26 ± 2775.88 a | 48,366.31 ± 2961.37 a |
| T2 | 37,653.33 ± 555.59 b | 34,584.78 ± 729.9 b |
| T3 | 48,041.31 ± 2084.52 a | 45,585.07 ± 2139.6 a |
| T4 | 37,678 ± 322.66 b | 34,850.97 ± 318.23 b |
| T5 | 35,259.9 ± 2853.83 bc | 31,635.21 ± 2211.19 bc |
| Treatment | Input (CNY·ha−2) | Gross Income | Net Income | Income/Input | ||
|---|---|---|---|---|---|---|
| Fertilizer Input | Other Inputs | Total Input | ||||
| CK | 0 | 15,000.00 | 15,000.00 | 33,384.64 | 18,384.64 | 2.23 |
| T1 | 4591.968 | 15,000.00 | 19,591.97 | 51,039.26 | 31,447.29 | 2.61 |
| T2 | 4498.84 | 15,000.00 | 19,498.84 | 37,653.33 | 18,154.49 | 1.93 |
| T3 | 5568.523 | 15,000.00 | 20,568.52 | 48,041.31 | 27,472.78 | 2.34 |
| T4 | 6638.203 | 15,000.00 | 21,638.2 | 37,678 | 16,039.8 | 1.74 |
| T5 | 7707.883 | 15,000.00 | 22,707.88 | 35,259.9 | 12,552.02 | 1.55 |
| Treatment | Membership Function | Principal Component Analysis | Weighted TOPSIS | Gray Relation Analysis | Combined Evaluation Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | |
| CK | 0.110 | 6 | −3.620 | 6 | 0.149 | 6 | 0.576 | 6 | 0.142 | 6 |
| T1 | 0.662 | 2 | 1.393 | 2 | 0.561 | 2 | 0.793 | 2 | 0.808 | 2 |
| T2 | 0.542 | 3 | 0.277 | 4 | 0.492 | 3 | 0.723 | 4 | 0.710 | 4 |
| T3 | 0.866 | 1 | 3.297 | 1 | 0.841 | 1 | 0.900 | 1 | 0.976 | 1 |
| T4 | 0.550 | 4 | 0.567 | 3 | 0.484 | 4 | 0.734 | 3 | 0.722 | 3 |
| T5 | 0.294 | 5 | −1.913 | 5 | 0.324 | 5 | 0.642 | 5 | 0.492 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, W.; Wang, T.; Li, W.; Li, Y.; Yan, B.; Zhang, M.; Zhao, J.; Fan, M. Coupling Effects of Organic Fertilizer Substituting Chemical Fertilizer on Potato Yield, Quality and Soil Nitrogen Content in the Erhai Lake Basin of China. Agronomy 2025, 15, 2470. https://doi.org/10.3390/agronomy15112470
Sun X, Zhang W, Wang T, Li W, Li Y, Yan B, Zhang M, Zhao J, Fan M. Coupling Effects of Organic Fertilizer Substituting Chemical Fertilizer on Potato Yield, Quality and Soil Nitrogen Content in the Erhai Lake Basin of China. Agronomy. 2025; 15(11):2470. https://doi.org/10.3390/agronomy15112470
Chicago/Turabian StyleSun, Xuemei, Wenmei Zhang, Ting Wang, Wanting Li, Yongmei Li, Benshuai Yan, Mengge Zhang, Jixia Zhao, and Maopan Fan. 2025. "Coupling Effects of Organic Fertilizer Substituting Chemical Fertilizer on Potato Yield, Quality and Soil Nitrogen Content in the Erhai Lake Basin of China" Agronomy 15, no. 11: 2470. https://doi.org/10.3390/agronomy15112470
APA StyleSun, X., Zhang, W., Wang, T., Li, W., Li, Y., Yan, B., Zhang, M., Zhao, J., & Fan, M. (2025). Coupling Effects of Organic Fertilizer Substituting Chemical Fertilizer on Potato Yield, Quality and Soil Nitrogen Content in the Erhai Lake Basin of China. Agronomy, 15(11), 2470. https://doi.org/10.3390/agronomy15112470




