Abstract
Scenarios of climate change, extensive land use, soil degradation, the loss of native forest cover due to monoculture expansion, and pasture scarcity pose new challenges to livestock farming worldwide. Associated crops emerge as an alternative to mitigate these factors; however, selecting compatible species that do not generate competition and optimize the attributes of the forage is a necessity. Therefore, this study evaluated the effect of a maize and bean association, and cutting time on the morphological variables, yield, and nutritional composition of forage. A randomized complete block design (RCBD) with a 3A × 3C factorial arrangement and three blocks was used. Factor A (associations) had three levels: INIA-604-Morocho maize monoculture (M), M+PER1003544 chaucha bean association (M+F1), and M+PER1003551 chaucha bean association (M+F2). Factor C (maize cutting stage) had three levels: R2 (blister grain), R3 (milky grain), and R4 (pasty grain). A total of 27 experimental units were established. No silage was made; the nutritional quality was evaluated as the raw material for silage. The treatments modulated key attributes for silage. In R4, the M+F2 association (INIA-604-Morocho + PER1003551) showed a higher percentage of dry matter in the system (32.36%) and better mixture quality due to a lower NDF and ADF (48.22% and 23.29%) and higher digestibility and protein values (62.10% and 9.53%). In addition, dry matter yields increased compared with R2 in M+F1 (134.16%), M+F2 (90.56%), and M (138.48%). Although R3 maximized green forage, R4 offered the best combination of quantity and quality for silage (as raw material), reducing the risk of deterioration and improving forage use efficiency. In general, combining maize with beans and adjusting the cut to R4 optimizes the production and quality of the raw material for silage, with the criterion that these findings pertain to pre-ensiled material and should be validated in future studies.
1. Introduction
Maize (Zea mays L.) is a grass of great global importance due to its versatility and strategic contribution to economic sectors such as agriculture, the food industry, livestock, and international trade. In 2023, green maize production exceeded 9.73 million tons; of that total, the Americas contributed 52.1%, with 4.6% of global production and 8.9% of the Americas’ total recorded in Peru [1]. Its relevance as fodder is associated with the expansion of the livestock sector, which grew 6.17% in the last decade and represents 11.36% of the gross value of national production [2,3]. In the Amazonas region, livestock is one of the main activities, especially in Chachapoyas, Rodríguez de Mendoza, and Luya [4], with a multifunctional role in small-scale agricultural and livestock systems [5]. From a productive and nutritional perspective, cattle generate daily income from the sale of milk, meat, and derivatives and contribute to food security by providing high-value proteins [6,7]; in addition, it constitutes a form of savings and an economic asset to manage financial risks and face situations of vulnerability [8,9].
However, the benefits of livestock production depend on the system’s capacity to adapt to new challenges, including climate change, which reduces forage availability during droughts associated with higher temperatures and lower precipitation, as well as growing market demands for meat and milk quality [10,11]. Land scarcity and limited labor have promoted a shift from extensive to stall-fed systems [12,13]. In this context, maize (Zea mays L.) has become a key forage in small-scale systems due to its high productivity, palatability, and energy content, especially when used as green forage or silage [14,15]. Nevertheless, as its reproductive maturity progresses, its digestibility declines, making it less suitable as green forage and usually destined for silage [16]. Therefore, the quality of the harvested material is critical, as it determines both the nutritional value of the fresh forage and its suitability as raw material for silage.
Most maize production in consecutive seasons is carried out in a monoculture system [17]. This practice facilitates crop management but is associated with multiple negative effects, such as soil degradation and erosion, fertility problems, increased carbon emissions, and reduced soil microbial diversity, destabilizing the biological balance of the soil and favoring the proliferation of pests and phytopathogens specific to the crop [18,19,20,21]. The expansion of this system is closely linked to the progressive loss of native forest cover, compromising ecological resilience and degrading ecosystem services, including nutrient cyclicity, the regulation of the hydrological cycle, and carbon sequestration capacity [22].
Faced with this problem, intercropping grasses with legumes is an effective strategy that offers multiple benefits, including improved forage quality, weed suppression, resistance to water stress, and reduced nitrate leaching [23,24,25]. In addition, by enhancing biological nitrogen fixation and transfer, these systems ensure nutrient cycling and long-term viability, allowing for a reduction in nitrogen fertilization of up to 31% [26,27].
However, the performance of these associations depends on selecting complementary varieties; otherwise, competition for light, water, and nutrients intensifies, decreasing productivity and favoring the dominance of one of the species [28,29]. In addition, the timing of cutting affects the nutritional composition of the forage, with late cutting compromising the nutritional value of legumes [30]. This limits the possibility of improving the combined nutritional composition associations compared with monoculture if management is not timely, restricting the expected benefits [31]. Therefore, clearly defining the phenological timing of cutting and associating compatible varieties is key to reducing competition and directing resources toward higher forage yield and quality.
Although it has been documented that intensive maize monoculture limits resource use efficiency and compromises production sustainability, there is still a gap in comprehensive studies on how association systems, particularly with native legumes such as beans, can improve the yield characteristics and nutritional quality of forage for silage under conditions in the Peruvian Amazon. In this context, the main objective was to evaluate, under agroecological conditions in the Peruvian Amazon, the effect of maize and native bean association on morphological variables, yield, and nutritional quality, compared with a maize monoculture as the raw material for silage. In addition, the optimal harvest time for mixed forage was determined, considering the R2, R3, and R4 cutting stages of maize. The hypothesis is that the combination of maize and native beans, compared with a monoculture, increases the combined yield and improves the nutritional composition of the forage intended for silage, and that it is possible to identify an optimal cut within stages R2, R3, and R4 in terms of yield and nutritional composition. This study presents a scientific basis for the performance of associations with native bean accessions from the Peruvian Amazon in terms of the yield and nutritional quality of forage as the raw material for silage, through different maize cutting times, providing real field data for future research on silo elaboration, since this study was limited to evaluating the raw material (pre-silage) and did not include the silage process. Nevertheless, this study provides viable agronomic alternatives that will contribute to optimizing the use of space and natural resources, improving the functional resilience of agricultural systems, strengthening the productive efficiency of livestock farming, and mitigating the soil degradation associated with monoculture in the Peruvian Amazon.
2. Materials and Methods
2.1. Description of the Research Area
This research was conducted at the Estación Experimental Agraria Amazonas del Instituto Nacional de Innovación Agraria (INIA), located in the San Juan district, Chachapoyas province, Amazonas region, Peru. The area is located at an altitude of 2445 m above sea level, at a latitude of 6°12′1.58″ S and a longitude of 77°52′9.37″ W. Meteorological data were recorded using a Vantage Pro2-Davis weather station during the experimental period from April to August 2024. As shown in Figure 1, the following parameters were monitored: temperature (maximum: 21.37 ± 1.56 °C; minimum: 9.67 ± 1.75 °C) (Figure 1a), relative humidity (76.59 ± 6.23%), and precipitation (0.68 ± 2.12 mm day−1) (Figure 1b). Prior to installation, soil sampling was carried out in accordance with soil study standards DS No. 013-2010-AG [32]. Sampling points were established in a zigzag pattern, the surface of each sampling point was cleaned, and a shovel was inserted to a depth of 30 cm to extract 1 kg of soil [33]. The samples were then coded and transferred to the Laboratorio de Suelos, Aguas y Foliares (LABSAF El Porvenir), accredited by the Instituto Nacional de Calidad, Ministerio de Producción, with registration no. LE-200. The physical parameters showed a sandy clay texture (sand 47%, silt 12%, and clay 41%), while the physical parameters were as follows: pH 7.80, electrical conductivity 12.60 mS m−1, organic matter 1.90%, total nitrogen 0.06%, available phosphorus 7.39 mg kg−1, and available potassium 208.4 mg kg−1. Exchangeable cations were also determined: Ca2+ 12.38, Mg2+ 0.88, Na+ 0.78, and K+ 0.15 cmol (+) kg−1; effective cation exchange capacity 14.19 cmol (+) kg−1.
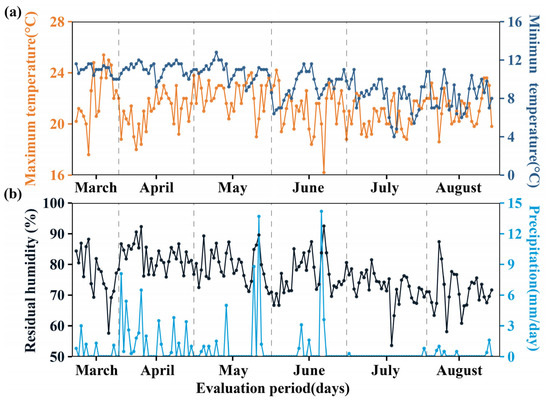
Figure 1.
Weather conditions during the experimental period. (a) Minimum and maximum temperature and (b) residual humidity (%) and precipitation.
2.2. Obtaining Plant Material
The seeds used in the experiment were provided by the Germplasm Bank of the Instituto Nacional de Innovación Agraria (INIA), located in the district of La Molina, Lima province, Peru. The INIA-604-Morocho maize variety was used, which is recognized for its high grain yield potential under experimental conditions (up to 8 t/ha), vigorous growth, lodging-resistant stalks, slightly conical ears, and semi-crystalline grain texture [34]. For the associated crop, two accessions of Andean green beans of chaucha type (PER1003544 [35] and PER1003551 [36]; also supplied by INIA) were selected for their higher yield as fodder, with registered provenance in the districts of Santo Tomás and La Jalca, department of Amazonas, and considered native genotypes.
2.3. Experimental Design
The experiment used a randomized complete block design (RCBD) with a 3A × 3C factorial arrangement, with 3 replicates. Factor A corresponded to maize and bean associations, while factor C represented cutting times of maize. The study included two associations: (i) INIA-604-Morocho maize associated with bean (type: chaucha) accession PER1003544 (M+F1); (ii) INIA-604-Morocho maize associated with bean (type: chaucha) accession PER1003551 (M+F2); (iii) a control (monoculture of INIA-604-Morocho). The cutting times were at the following phenological stages of maize: (i) grain in blister stage, 105 days (R2); (ii) milky grain, 125 days (R3); (iii) pasty grain, 145 days (R4). In total, nine treatments were carried out, as shown in Figure 2. However, for the nutritional analysis, only the monoculture with the R3 cutting phase was considered as a control.
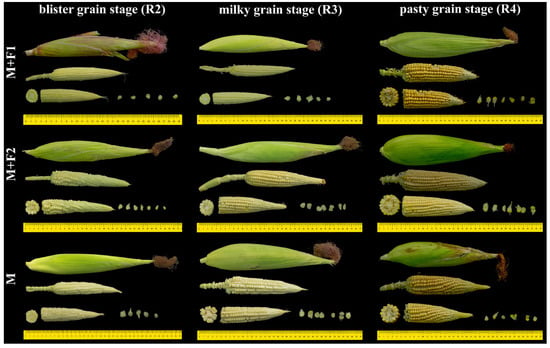
Figure 2.
Treatment design based on associations and cutting stages in maize. Associations: M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture).
2.4. Crop Installation
The soil was prepared using standard tillage operations and agricultural machinery (plowing and harrowing), followed by land layout, where it was divided into experimental units of 40 m2 (4 m × 10 m), with a distance of 1 m between plots and blocks, establishing six 10 m furrows in each plot. Sowing took place on 13 March 2024, and was carried out manually using a planting stick. For sowing, two maize seeds were used per hill (sowing point), with 0.40 m distancing between hills, which resulted in 50 plants per row and 300 per plot. Beans were established with one seed per maize hill, offset 15 cm from the hill. The control treatment consisted of maize only. Weed control was carried out manually after emergence, every 15 days, in order to prevent competition for nutrients. For irrigation, because sowing occurred during the rainy season, no supplemental irrigation was applied and crop water supply depended solely on precipitation. Fertilization was carried out using chicken manure, whose characteristics were as follows: pH (7.95), electrical conductivity (16.08 dS m−1), nitrogen (1.90%), phosphorus (152.52 ppm), carbon (24.51%), and organic matter (42.26%), with an application rate of 1 kg m−2. Harvesting was carried out at stages R2, R3, and R4 to evaluate yield in the associations and the control; however, for the nutritional composition of the control (monoculture), only evaluations at R3 were considered, excluding R2 and R4.
2.5. Evaluation of Indicators
2.5.1. Morphological Parameters
In each experimental unit, 10 hills were sampled for morphological measurements. Maize plant height was recorded from the base to the apex [37]; the length of the stem was measured from the base of the stem to the beginning of the spike [38]. For leaf dimensions, leaf length was recorded from the ligule to the apex and width at the midpoint of the length; five leaves per plant were measured using a flexometer, graduated ruler, and digital vernier caliper (Dasqua®, model 2000–2010, accuracy ±0.02 mm). Stem diameter was evaluated with a digital vernier caliper. To evaluate the number of leaves, only fully expanded and physiologically active leaves were considered, excluding those in senescence [39]. For beans, morphological evaluations were limited to plant height and leaf dimensions (leaf length and width).
2.5.2. Yield Parameters
Green forage yield was determined by harvesting 1 m2 per experimental unit by manual cutting at a height of 4 ± 1 cm. The green forage was weighed in situ with an electronic scale (accuracy ±5 g), and recorded in kg m−2 in the field notebook. A subsample of 1000 g of fresh forage was obtained from each sample and dried in a forced-air oven at 105 °C for 24 h. From these data, the percentage of dry matter was calculated by dividing the dry weight by the fresh weight of the subsample and multiplying the result by 100. The dry forage yield was calculated by multiplying the green forage extrapolated to t ha−1 by the percentage of dry matter.
2.5.3. Nutritional Composition
Nutritional composition analyses were performed by taking samples from a 1 m2 area of each experimental unit. The samples were taken in 3 repetitions per treatment. The samples were then transferred to the Laboratorio de Nutrición Animal y Bromatología (LABNUT) de la Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas. From each sample, 200 g of forage was weighed and dried in an oven at 60 °C for 48 h. The material was then ground in a fixed-speed mini mill (1730 rpm), followed by a series of protocols for protein, acid detergent fiber (ADF), neutral detergent fiber (NDF), and digestibility.
The crude protein content was estimated following the AOAC No. 928.08 method [40], subjecting the sample to digestion with sulfuric acid (H2SO4), converting the organic nitrogen to ammonia, then quantifying it by titration using hydrochloric acid (HCl). The total nitrogen content was calculated from the volume of HCl consumed and multiplied by the conversion factor 6.25 to estimate the protein content in the sample. To determine acid detergent fiber (ADF) and neutral detergent fiber (NDF), the ANKON A200 procedure [41] was followed. ADF was quantified with a neutral detergent solution, followed by filtration and oven drying. NDF was determined with a neutral detergent solution, alpha-amylase, and sodium sulfite, followed by rinsing with acetone and oven drying. The in vitro digestibility of dry matter was evaluated with the DAISY in vitro system. The procedure began with the pre-conditioning of the F57 filters with acetone and the recording of the initial weight. Then, 0.50 g of sample was added to each filter and a blank filter was included as a correction factor; the filters were sealed and placed in the digestion container of the DAISY II incubator. The buffer solution was prepared by mixing buffers A and B, supplemented with inoculum, for incubation at 39 °C. Finally, the post-incubation weight was recorded and digestibility was calculated as a percentage [42].
2.6. Statistical Analysis
The evaluations were carried out using a completely randomized block design (CRBD). For the variables evaluated, the Shapiro–Wilk normality test and Bartlett’s test of homogeneity of variances were applied, using functions from the base R package (stats) [43]. Data showing a normal distribution and homogeneity of variances were subjected to two-way ANOVA at 5% significance, followed by Tukey’s multiple comparison test. Likewise, data that did not meet normality were log-transformed. This process was performed using the agricolae package [44]. Graphical visualizations were performed with the ggplot2 package [45], and the entire process was carried out in R Studio (version 4.5.0) software.
3. Results
3.1. Morphological Parameters
3.1.1. Bean Evaluation
The morphological parameters of beans (Figure 3) showed significant effects of the association (A) and maize harvest time (C), except for plant height and leaf width, where the association was not significant (p > 0.05). Furthermore, for leaf width, the interaction (A × C) was not significant (Figure 3c). Bean height reached its highest averages for M+F1 and M+F2 when maize was cut at the blister grain stage (R2), with 148.33 and 150.00 cm, respectively, without differing from M+F2 with cutting at the pasty grain stage (R3) (153.33 cm) (Figure 3a). For leaf length, M+F2 with cutting at R2 had the highest average (19.00 cm), which was 29.51% higher than M+F1 at the same cutting time (14.66 cm) (Figure 3b). In general, the plant height and leaf length of the bean showed a decreasing trend as the cutting time of the maize progressed from the blister grain stage (R2) to the pasty grain stage (R4). Significant reductions of 16.81% and 31.11% in plant height and 23.83% and 42.11% in leaf length were observed for M+F1 and M+F2 (Figure 3a,b). These results suggest that, with association, beans reach their maximum height and leaf length when maize is at R2, with a progressive decrease from this vegetative stage onwards.
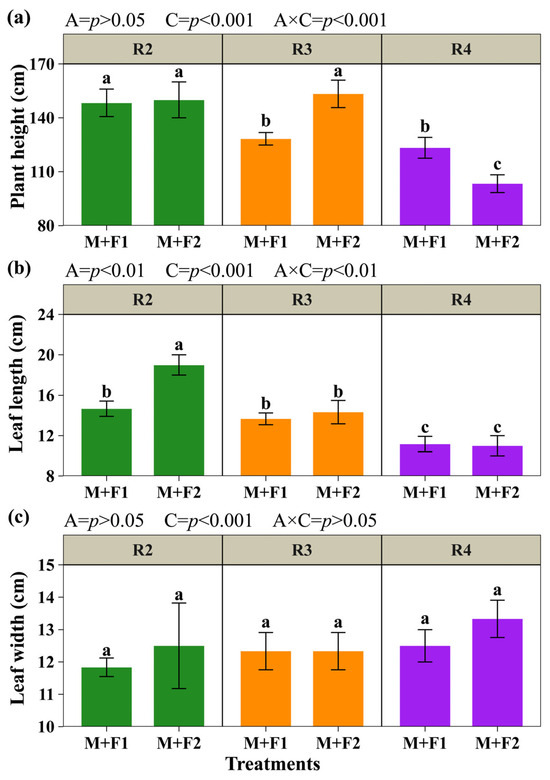
Figure 3.
Morphological evaluation of beans. Plant height (a), leaf length (b), and leaf width (c). Associations (A): M+F1 (maize + bean chaucha PER1003544) and M+F2 (maize + bean chaucha PER1003551). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns.
3.1.2. Maize Evaluation
The morphological parameters of maize (Figure 4) showed significant effects of the association (A) and maize cutting time (C). However, the association revealed effects only on stem height (p < 0.05). Similarly, for the number of leaves, the interaction (A × C) was not significant (Figure 4c). Plant height reached its highest values with cutting at the blister grain stage (R2) in M+F1, M+F2, and M, with 280, 310, and 296 cm, respectively, and showed a marked decrease at the milky grain stage (R3), decreasing by 22.66% and 16.33% in M+F2 and M (Figure 4a). For leaf length, the highest averages were observed with the cut in R2, with no differences between associations and monoculture (113.67, 117.33, and 111.33 cm for M+F1, M+F2, and M), and did not differ from the cut at R3 in M+F2 and M (109.67 and 111.67 cm) (Figure 4b). In addition, it was observed that, by delaying the cut from the blister grain stage (R2) to the pasty grain stage (R4), leaf length decreased by 10.27%, 19.31%, and 10.47% in M+F1, M+F2, and M. A similar pattern was observed in leaf width, with the highest values recorded in R2 regardless of the association, with reductions of 27.53%, 42.20%, and 23.71% when cutting the maize at R4 (Figure 4c,d). In R1, M+F2 and M did not differ in stem diameter, which was similar in R2. However, in M+F1, the value at R2 was 28.57% and 27.95% lower than those at R3 and R4; however, M+F2 showed no variation in stem diameter between stages, maintaining high averages in all cuts. By contrast, for stem height, the M+F2 association showed the highest values at R2 and R4 of 280.00 cm and 241.67 cm (Figure 4f). These patterns indicate that the cutting stage has a different impact depending on the type of system and association on the morphological parameters of maize.
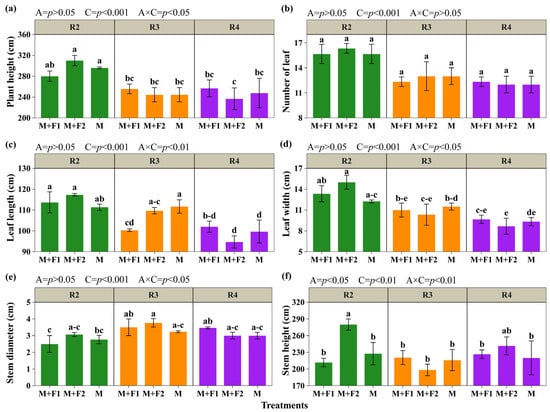
Figure 4.
Morphological evaluation of maize. Plant height (a), number of leaves (b), leaf length (c), leaf width (d), stem diameter (e), and stem height (f). Associations (A): M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns. Letter range (–).
3.2. Yield Parameters
3.2.1. Percentage of Dry Matter
The percentages of dry matter in the maize and beans, and the total for the system with the associations, are summarized in Figure 5 (Supplementary Materials, Table S1). It was determined that association (A) and maize cutting time (C) influenced the dry matter content. However, the association had no effect on maize dry matter; similarly, the interaction (A × C) was also not significant. The results indicated that all associations were statistically lower in dry matter content with cutting at the blister grain stage (R2) and the milky grain stage (R3) compared with pasty grain (R4) in the beans. The M+F1 association (56.69%) indicated a higher dry matter content in beans, being 40.32% higher than M+F2 (40.40%), with maize cutting at R4 (Figure 5a). On the other hand, the dry matter content did not vary in maize regardless of the time of cutting and the association (Figure 5b). Regarding the percentage of total dry matter in the system (maize + beans), the highest averages were observed with maize cut in R4, with M+F2 standing out with 32.36%, exceeding the monoculture (M) (27.76%) by 12.61%. In addition, it was observed that the combined dry matter percentages (maize + beans) were statistically lower in R2 and R3 compared with R4, indicating a higher moisture content during those cutting stages (Figure 5c).
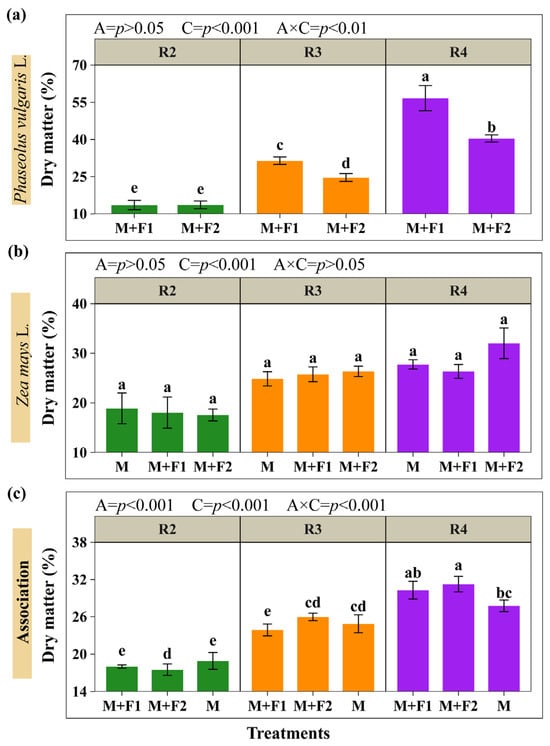
Figure 5.
Percentage of dry matter of beans (a), maize (b), and maize + beans system combined (c). Associations (A): M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns.
3.2.2. Green Forage and Dry Matter
The yields of green forage and the dry matter of the maize and beans, and the total yield of the system with the associations, are summarized in Figure 6 (Supplementary Materials, Table S1). It was determined that the association (A) and maize harvest time (C) influenced the yield parameters. However, the association had no effect on bean dry matter. Similarly, the interaction (A × C) was not significant for green and dry maize forage yield (Figure 6c,d). For beans, green forage was higher in M+F2 with maize cut at the blister grain stage (R2) and the milky grain stage (R3) (24.73 and 23.16 t ha−1). For dry matter, the maximum yield was observed with maize cutting in R3 in both the M+F1 and M+F2 associations with 6.19 and 5.69 t ha−1. For the pasty grain stage (R4), green forage yields in beans were the lowest; compared with R2, M+F2 decreased by 61.87% (from 24.73 to 9.43 t ha−1) and M+F1 decreased by 62.81% (from 15.46 to 5.75 t ha−1) (Figure 6a,b). According to the analysis, maize yields did not vary by association or cutting time, registering values ranging from 67.45 to 153.28 t ha−1 for green forage and 12.12 to 42.57 t ha−1 for dry matter. Based on the total system yield (maize + beans), green forage in R3 showed no significant differences between M+F1, M+F2, and M, and recorded the highest values (Figure 6e). In R4, the associations did not differ from each other, but were statistically surpassed by the monoculture (M). In terms of dry matter yield, R3 and R4 did not differ from each other regardless of the system (association or monoculture), and both surpassed R2 (Figure 6f). According to the analysis, maize yields did not vary by association or cutting time, registering values ranging from 67.45 to 153.28 t ha−1 for green forage and 12.12 to 42.57 t ha−1 for dry matter. Based on the total yield of the system (maize + beans), green forage (Figure 6e) in R3 showed no significant differences between M+F1, M+F2, and M; no differences were detected between systems in R3 and R4 in terms of dry matter (Figure 6f). The treatments showed significant increases in yield depending on the timing of the maize harvest; green forage was lower in R2. When harvested at R3, it increased by 76.73% in M+F1 (from 82.91 to 146.53 t ha−1), 28.33% in M+F2 (from 111.35 to 142.90 t ha−1), and 49.54% in M (from 96.43 to 144.20 t ha−1). In terms of dry matter, the harvest in R3 in-creased yield by 134.16% in M+F1 (from 14.93 to 34.96 t ha−1), 90.56% in M+F2 (from 19.50 to 37.16 t ha−1), and 96.56% in M (from 18.27 to 35.91 t ha−1), and in R4 134.16% in M+F1 (from 14.93 to 34.96 t ha−1), 90.56% in M+F2 (from 19.50 to 37.16 t ha−1), and 138.48% in M (from 18.27 to 43.57 t ha−1) compared with R2 (Figure 6e,f).
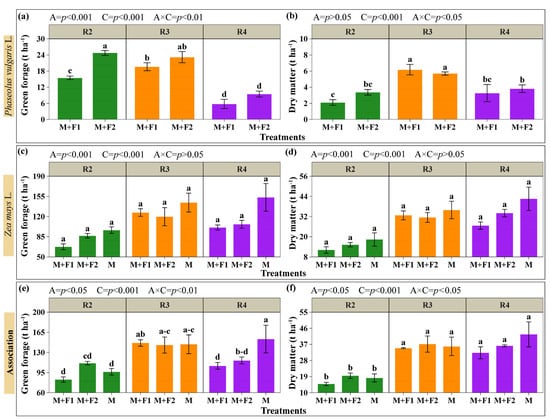
Figure 6.
Yield evaluation. Beans: green forage (a) and dry matter (b). Maize: green forage (c) and dry matter (d). Maize + beans system: combined yield in green forage (e) and dry matter (f). Associations (A): M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns. Letter range (–).
3.3. Nutritional Composition
3.3.1. Neutral Detergent Fiber and Acid Detergent Fiber
The neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents in the maize and beans, and total system content with associations, are shown in Figure 7 (Supplementary Materials, Table S2). The results indicated that the association (A) and maize harvest time (C) influenced the nutritional parameters of NDF and ADF. Likewise, the interaction (A × C) was significant for both variables. In general, beans had lower NDF relative contents than maize. Within beans, the maximum NDF was recorded in M+F2 (33.51%) with maize cut at the pasty grain stage (R4); the pattern was similar for ADF, with a value of 29.09% (Figure 7a,b). In maize, the highest NDF and ADF contents also corresponded to M+F2 (57.42% and 32.07%), but with cutting at the blister grain stage (R2). At the pasty grain stage (R3), M recorded an FDN and ADF of 53.19% and 27.83%, compared with 55.42% and 29.13% recorded in M+F2, which was 4.02% and 4.46% lower than M+F2 (Figure 7c,d). However, when considering the combined forage (maize + beans), the pattern was reversed, with the lowest NDF observed in M+F2 (44.64%) with maize cut at R3, while M (monoculture of maize) was higher (53.19%), with M+F2 being 19.07% lower. The same occurred in ADF, with relatively low averages recorded during maize cutting at R3 in M+F2 (26.47%), which was 2.07% and 4.89% below M+F1 (27.03%) and M (27.83%). In R4, in the combined evaluation (maize + beans), M+F2 presented the lowest values of NDF (48.22%) and ADF (23.29%), below M+F1 in the same stage (NDF 49.40%, ADF 25.24%). Likewise, these values were lower than those of the monoculture (M) measured in R3 (NDF 53.19%, ADF 27.83%) (Figure 7e,f).
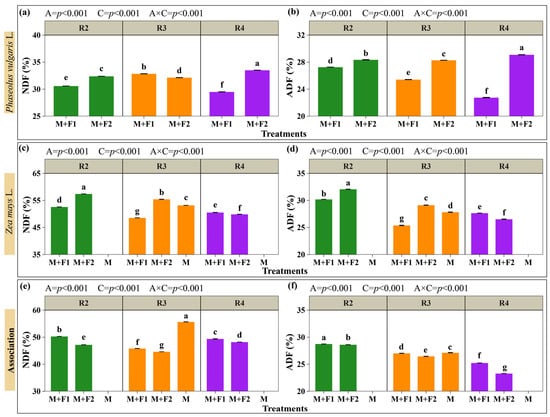
Figure 7.
Evaluation of neutral detergent fiber (NDF) and acid detergent fiber (ADF). Beans: NDF (a) and ADF (b). Maize: NDF (c) and ADF (d). Maize + beans system: combined NDF (e) and combined ADF (f). Associations (A): M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns.
3.3.2. Digestibility and Protein
The percentages of digestibility (DIV) and protein in the maize and beans, and the total for the system with the associations, are shown in Figure 8 (Supplementary Materials, Table S2). The results indicated that the association (A) and maize cutting time (C) influenced the nutritional parameters of digestibility and protein. Likewise, the interaction (A × C) was significant for both variables. In general terms, beans had higher relative values than maize in terms of digestibility and protein content. Cutting maize at the pasty grain stage (R4) benefited the digestibility and protein of beans, with a content of 81.66% and 16.7% in M+F1 (Figure 8a,b). The digestibility and protein content of maize was higher with blister grain stage (R2) cutting in M+F1 (63.09 y 8.26%); however, there was no difference in protein with M+F2 (7.81%). On the other hand, the monoculture (M) showed values of 56.28% and 6.43% in digestibility and protein content, being 8.27% and 23.65% higher than M+F2, which presented averages of 51.98% and 5.2% in both variables, with maize cut at the milky grain stage (R3) (Figure 8c,d). However, when maize and beans were combined, the analyses revealed that the highest digestibility and protein contents were recorded in M+F2 (70.74 and 10.12%), surpassing M (56.28 and 6.43%) by 25.69% in digestibility and 57.39% in protein (Figure 8e,f). These results support the idea that an optimal selection of associations can improve the nutritional composition of raw material intended for silage. In the R4 cutting stage, within the maize + bean system, the M+F2 combination was the most outstanding, with digestibility (62.1%) higher than M+F1; it also recorded the highest protein content (9.53%), standing out from the other treatments, except for M+F1 in R2, where there was no difference.
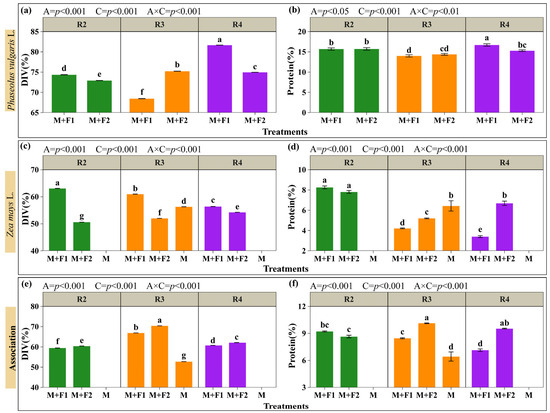
Figure 8.
Digestibility and protein evaluation. Beans: digestibility (a) and protein (b). Maize: digestibility (c) and protein (d). Maize + bean system: combined digestibility (e) and combined protein (f). Associations (A): M+F1 (maize + bean chaucha PER1003544), M+F2 (maize + bean chaucha PER1003551), and M (maize monoculture). Cutting times (C): grain in blister stage (R2), milky grain stage (R3), and pasty grain stage (R4). Significant differences are indicated by different letters in vertical columns.
4. Discussion
It was determined that cutting at the blister grain stage (R2) promoted a greater plant height in both species of the associated system. This pattern is consistent with the completion of stem elongation in maize around tasseling (VT) and stigma emission (R1), at which point resources are redirected toward reproductive development and grain filling, reducing height gain [46,47,48]. Overall, the synchrony between the end of vegetative growth and evaluation at R2 captured the maximum peak for the associated systems. In beans, on the other hand, the greater height under association appears to be related to the stem elongation induced by the shade of the maize canopy, which generates a morphological adjustment to improve light interception [49].
At the pasty grain stage (R4), the height of the bean in the M+F2 association was the lowest. Given that the trial depended on a rainy season without supplemental irrigation, it is plausible that competition for water contributed to this behavior, since maize increases its hydric demand during this grain stage [50], which can restrict availability for the associated crop, reflected in growth [51]. On the other hand, water retention may have been conditioned by the specific surface area and pore distribution of the clay fraction; a higher clay content tends to increase retention [52]. In our study, the range was intermediate, with sandy loam soils containing 47% clay. Among other factors that could have contributed to the reduced height is the reallocation of assimilates toward the formation of reproductive organs, prioritizing those structures with a higher probability of completing maturation [53,54].
In terms of maize plant height, no significant variations were observed between the associations and the monoculture when analyzed by cutting stage. Similarly, the number of leaves did not show significant differences between cutting stages or between association systems. This pattern is supported by several previous studies on association systems, where no differences in height and number of leaves were detected when compared with monoculture [55,56]. The maize stalk diameter remained stable in all associations when the trajectory of each was followed through the cutting stages, with the exception of M+F1, which increased significantly between R2 and R3. This behavior is in line with the literature, which reports more marked increases in diameter during the vegetative phase and a slowdown in thickening during the reproductive stage [57]. In this context, the specific increase in M+F1 between R2 and R3 suggests an atypical response within the early reproductive period, possibly modulated by the interaction of the association with accession PER1003544, which would have temporarily prolonged thickening before stabilizing.
Our results presented on maize and bean plant height differ from those reported by Aguiar et al. [58], who found that the early emergence of volunteer maize limited bean height, leaf area, and biomass. Although in our study plant height may have been influenced by elongation due to lack of light in beans, it did not influence the green and dry bean forage yield in the M+F2 treatment (maize + PER1003551 chaucha beans), with maize cut at the milky grain stage (R3). This result can be attributed to the fact that accession PER1003551 exhibits greater tolerance to the shading generated by maize, associated with a more efficient canopy architecture, higher photosynthetic rates, and greater stem resistance; together, these traits reduce yield loss under low-radiation conditions [59].
Based on the mixed yield of green forage (t ha−1) when cutting was performed in R2 and R3, the associations did not differ from monoculture, but the monoculture outperformed both associations in R4. These results differ from those reported by Ebel et al. [60] who indicated that associated cropping tends to produce 1.2 times the yield compared with monoculture. In addition, dry matter content (t ha−1) increased from the R3 cutting stage. This is consistent with the literature, which indicates the greater accumulation of dry matter as the plant matures [61].
The dry matter content is a determining factor for silage quality, and ranges of 30 to 35% are recommended [62]. In our study, the M+F2 combination with maize cut at R4 indicated 31.26% dry matter, surpassing the monoculture (M). In silage intended for prolonged storage, a low moisture content (680 g kg−1, equivalent to 32% dry matter) is associated with greater aerobic stability and the better preservation of fatty acids, reducing the risk of proliferation of yeasts such as Candida sp. [63], which is associated with fungal mastitis in cattle, characterized by an increased somatic cell count and udder inflammation [64,65]. The values observed in R2 and R3 ranged from 18.00 to 18.90% and 23.88 to 25.97%, indicating values significantly lower than those reported in the literature, with a higher moisture content. It is known that moisture affects the compaction capacity during the silage process [66]. In addition, values > 70% for moisture are associated with greater nutrient losses through effluents [67]. Regarding the percentage of dry matter in beans, the M+F1 association showed higher averages. Various studies have shown that dry matter tends to vary according to species and variety [68,69]. On the other hand, the R3 maize cut showed higher yields in the combined bean and maize system, with values ranging from 142.90 to 146.53 t ha−1 in green forage and 34.96 to 37.16 t ha−1 in dry forage. This finding may be related to higher photosynthetic rates and a longer duration of active photosynthesis [70], which together determine the net assimilation of carbon available for growth and dry matter accumulation [71].
In the associated mixed forage system, M+F2 cut at R4 had lower neutral detergent fiber (NDF) (48.22%) and acid detergent fiber (ADF) (23.29%) contents than M+F1 (R2 and R4) and the monoculture. Although it is known that a later harvest time promotes tissue senescence and lignification, and consequently NDF tends to increase [72], the association in intercropped M+F2 (maize + PER1003551 chaucha beans) seems to mitigate this impact and favors the nutritional quality of the forage. Furthermore, there is evidence that relatively low NDF and ADF contents can translate into higher digestibility [73]. This was reflected in the protein (9.53%) and digestibility (62.10%) being among the highest averages. However, it is known that when protein exceeds >17.5%, production benefits stagnate and nitrogen loss increases, aggravating the environmental burden [74,75]. However, these parameters may vary between the rainy and dry seasons [76].
It should be noted that this study was conducted in a single season; therefore, additional evaluations in different seasons and environments are required to corroborate the stability of the observed patterns. Furthermore, only the forage prior to ensiling was evaluated; the ensiling process was not carried out, so the fermentation and aerobic stability during storage remain undetermined. This limits the inferences about the efficiency of the raw material during ensiling; however, previous studies indicate that the inclusion of legumes can improve the quality of maize silage after about 45 days of fermentation [77]. This indicates that this study raises new questions when evaluating different parameters in the silage process.
5. Conclusions
Our study indicated that the combination of maize and beans, and the stage of cutting in maize, modulate key attributes for silage and system performance, including morphological parameters, yield, and nutritional composition. In the pasty grain stage (R4), the M+F2 combination (INIA-604-Morocho maize + PER1003551 chaucha bean accession) had a higher percentage of dry matter in the combined system (32.36%), along with a better mixture quality due to lower neutral detergent fiber (42.22%) and acid detergent fiber (23.29%) contents and higher digestibility (62.10%) and protein values (9.53%), conditions that favor silo stability and, therefore, better nutrient preservation. In addition, in R4, dry matter yields increased markedly compared with the blister grain stage (R2) in M+F1 (134.16%, from 14.93 to 34.96 t ha−1), M+F2 (90.56%, from 19.50 to 37.16 t ha−1), and monoculture (M) (138.48%, from 18.27 to 43.57 t ha−1), integrating favorable dry matter volume and concentration for silage. Although the milky grain stage (R3) maximized green forage, R4 offered a balanced combination of quantity and quality for silage, reducing the potential risks of the deterioration of the raw material in future uses for silage. For livestock systems, this implies greater feed self-sufficiency, less dependence on external inputs, and greater seasonal resilience, contributing to sustainability through diversified partnerships that stabilize supply and increase the nutritional value of silage. However, future studies are needed that include the ensiling process and evaluations in different seasons and environmental conditions in order to validate the efficiency of the raw material in silage and characterize its performance both during fermentation and in subsequent stability under different management schemes and climatic conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112445/s1, Table S1: Effect sizes (η2) of ANOVA for yield; Table S2: Effect sizes (η2) of ANOVA for nutritional composition.
Author Contributions
Conceptualization, H.V.V., L.V. and Y.P.; methodology, M.R., L.V.-V. and L.V.; software, J.L.M., M.A.A.-T., L.G.B. and J.Y.V.; validation, L.H.Z.L., J.M.I.P., M.A.A.-T. and J.Y.V.; formal analysis, J.L.M., M.A.A.-T., M.R. and J.Y.V.; investigation, L.V.-V., L.H.Z.L. and M.A.A.-T.; resources, M.R., L.H.Z.L. and Y.P.; data curation, L.G.B., J.M.I.P. and Y.P.; writing—original draft preparation, H.V.V., J.M.I.P. and L.G.B.; writing—review and editing, M.R., L.H.Z.L. and L.V.-V.; visualization, J.L.M., L.G.B. and Y.P.; supervision, M.A.A.-T., H.V.V. and L.V.; project administration, J.M.I.P., L.V.-V. and L.V.; funding acquisition, H.V.V. and J.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out and funded primarily by the Project CUI No. 2253484 “Creation of an Agrostology Laboratory Service at the Toribio Rodríguez de Mendoza National University” which was funded by the National Public Investment System (SNIP) of the Ministry of Economy and Finance (MEF) of Peru. In addition, we had the support of the Vice-Rectorate for Research of the Toribio Rodríguez de Mendoza National University of Amazonas—UNTRM.
Data Availability Statement
The original contributions presented in the study are included in this article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). FAOSTAT-Cultivos y Productos de Ganadería. Available online: https://www.fao.org/faostat/es/#data/QCL/visualize (accessed on 23 September 2025).
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). Perfil Competitivo de las Principales Especies y Productos Pecuarios. Available online: https://app.powerbi.com/view?r=eyJrIjoiYWM0MDIwYTktNTk3MS00OTc3LThiZTgtZjRmN2ZhMmZlNjVlIiwidCI6IjdmMDg0NjI3LTdmNDAtNDg3OS04OTE3LTk0Yjg2ZmQzNWYzZiJ9&pageName=ReportSection (accessed on 23 September 2025).
- Ferraretto, L.F.; Shaver, R.D.; Luck, B.D. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Oliva, M.; Collazos, R.; Vásquez, H.; Rubio, K.; Maicelo, J. Composición florística de especies herbáceas forrajeras en praderas naturales de las principales microcuencas ganaderas de la región Amazonas. Sci. Agropecu. 2019, 10, 109–117. [Google Scholar] [CrossRef]
- Hemingway, C.; Ruiz, L.; Vigne, M.; Aubron, C. The changing role of livestock in agrarian systems: A historical and multifunctional perspective from southern India. Agron. Sustain. Dev. 2025, 45, 7. [Google Scholar] [CrossRef]
- Postigo, J.C.; Young, K.R.; Crews, K.A. Change and continuity in a pastoralist community in the high Peruvian Andes. Hum. Ecol. 2008, 36, 535–551. [Google Scholar] [CrossRef]
- Radolf, M.; Wurzinger, M.; Gutiérrez, G. Livelihood and production strategies of livestock keepers and their perceptions on climate change in the Central Peruvian Andes. Small Rumin. Res. 2022, 215, 106763. [Google Scholar] [CrossRef]
- Alvarez-García, W.; Muñoz-Vílchez, Y.; Figueroa, D.; Estrada, R.; Quilcate, C. A review of sustainable cattle genetic improvement in the Peruvian Highlands. Vet. Anim. Sci. 2025, 27, 100427. [Google Scholar] [CrossRef]
- Gilardino, A.; Quispe, I.; Pacheco, M.; Bartl, K. Comparison of different methods for consideration of multifunctionality of Peruvian dairy cattle in life cycle assessment. Livest. Sci. 2020, 240, 104151. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W.B. Effects of nutritional factors on fat content, fatty acid composition, and sensorial properties of meat and milk from domesticated ruminants: An overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef]
- Lee, M.A.; Davis, A.P.; Chagunda, M.G.G.; Manning, P. Forage quality declines with rising temperatures, with implications for livestock production and methane emissions. Biogeosciences 2017, 14, 1403–1417. [Google Scholar] [CrossRef]
- Hadush, M. Exploring farmers’ seasonal and full year adoption of stall feeding of livestock in Tigrai region, Ethiopia. Econ. Agric. 2017, 64, 919–944. [Google Scholar] [CrossRef]
- Stür, W.; Khanh, T.T.; Duncan, A. Transformation of smallholder beef cattle production in Vietnam. Int. J. Agric. Sustain. 2013, 11, 363–381. [Google Scholar] [CrossRef]
- Gomes, V.C.; Meirelles, P.R.L.; Costa, C.; Barros, J.S.; Castilhos, A.M.; Souza, D.M.; Tardivo, R.; Pariz, C.M. Production and quality of corn silage with forage and pigeon peas in a crop-livestock system. Semin. Ciênc. Agrár. 2021, 42, 861–876. [Google Scholar] [CrossRef]
- Cañete, D.C.; Alvarez, T.S. Commercialization of green corn-based silage production for dairy in Cagayan Valley: Profitability and viability assessment. Univers. J. Agric. Res. 2021, 9, 79–90. [Google Scholar] [CrossRef]
- Ahmed, S.; Grecchi, I.; Ficuciello, V.; Bacciu, N.; Minuti, A.; Bani, P. Effects of hybrid and maturity stage on in vitro rumen digestibility of immature corn grain. Ital. J. Anim. Sci. 2014, 13, 3149. [Google Scholar] [CrossRef]
- Franco, S.; Pancino, B.; Martella, A.; De Gregorio, T. Assessing the presence of a monoculture: From definition to quantification. Agriculture 2022, 12, 1506. [Google Scholar] [CrossRef]
- Demirdogen, A.; Guldal, H.T.; Sanli, H. Monoculture, crop rotation policy, and fire. Ecol. Econ. 2023, 203, 107611. [Google Scholar] [CrossRef]
- Crews, T.E.; Carton, W.; Olsson, L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Glob. Sustain. 2018, 1, e11. [Google Scholar] [CrossRef]
- Figuerola, E.L.; Guerrero, L.D.; Türkowsky, D.; Wall, L.G.; Erijman, L. Crop monoculture rather than agriculture reduces the spatial turnover of soil bacterial communities at a regional scale. Environ. Microbiol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Dietrich, P.; Roeder, A.; Cesarz, S.; Eisenhauer, N.; Ebeling, A.; Schmid, B.; Schulze, E.-D.; Wagg, C.; Weigelt, A.; Roscher, C. Nematode communities, plant nutrient economy and life-cycle characteristics jointly determine plant monoculture performance over 12 years. Oikos 2020, 129, 466–479. [Google Scholar] [CrossRef]
- Suarez, A.; Gwozdz, W. On the relation between monocultures and ecosystem services in the Global South: A review. Biol. Conserv. 2023, 278, 109870. [Google Scholar] [CrossRef]
- Dhakal, D.; Islam, M.A. Grass–legume mixtures for improved soil health in cultivated agroecosystem. Sustainability 2018, 10, 2718. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Frossard, E.; Lüscher, A. Effects of legumes and fertilizer on nitrogen balance from intact leys and after tilling for subsequent crop. Agric. Ecosyst. Environ. 2024, 360, 108776. [Google Scholar] [CrossRef]
- Phelan, P.; Moloney, A.P.; McGeough, E.J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P. Forage legumes for grazing and conserving in ruminant production systems. Crit. Rev. Plant Sci. 2014, 34, 281–326. [Google Scholar] [CrossRef]
- Husse, S.; Lüscher, A.; Buchmann, N.; Hoekstra, N.J.; Huguenin-Elie, O. Effects of mixing forage species contrasting in vertical and temporal nutrient capture on nutrient yields and fertilizer recovery in productive grasslands. Plant Soil 2017, 420, 505–521. [Google Scholar] [CrossRef]
- Liu, Y.; Stomph, T.J.; Zhang, F.; Li, C.; van der Werf, W. Nitrogen Input Strategies Impact Fertilizer Nitrogen Saving by Intercropping: A Global Meta-Analysis. Field Crops Res. 2024, 318, 109607. [Google Scholar] [CrossRef]
- Tahir, M.; Li, C.; Zeng, T.; Xin, Y.; Chen, C.; Javed, H.H.; Yang, W.; Yan, Y. Mixture composition influenced the biomass yield and nutritional quality of legume–grass pastures. Agronomy 2022, 12, 1449. [Google Scholar] [CrossRef]
- Schmitt, M.B.; Berti, M.; Samarappuli, D.; Ransom, J.K. Factors affecting the establishment and growth of cover crops intersown into maize (Zea mays L.). Agronomy 2021, 11, 712. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Valqui-Valqui, L.; Bobadilla, L.G.; Reyna, M.; Maravi, C.; Pajares, N.; Altamirano-Tantalean, M.A. Influence of nitrogen fertilization and cutting dynamics on the yield and nutritional composition of white clover (Trifolium repens L.). Plants 2025, 14, 2765. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Ge, Q.; Yang, B.; Li, S. Effects of harvest period and mixed ratio on the characteristic and quality of mixed silage of alfalfa and maize. Anim. Feed. Sci. Technol. 2023, 306, 115796. [Google Scholar] [CrossRef]
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). Decreto Supremo N.º 013-2010-AG. Reglamento para la Ejecución de Levantamiento de Suelos. Available online: https://www.midagri.gob.pe/portal/download/pdf/marcolegal/normaslegales/decretossupremos/2010/ds13-2010-ag.pdf (accessed on 23 September 2025).
- Oliva-Cruz, M.; Cabañas-López, J.R.; Altamirano-Tantalean, M.A.; Juarez-Contreras, L.; Vigo, C.N. Agronomic behavior of peanut (Arachis hypogaea L.) cultivars under three planting densities in the northeast of Peru. Agronomy 2024, 14, 1905. [Google Scholar] [CrossRef]
- Estación Experimental Agraria Baños del Inca–Cajamarca. Maíz INIA 604—Morocho: Primera Variedad Mejorada de Maíz Morocho para la Sierra del Perú. Available online: https://repositorio.inia.gob.pe/handle/20.500.12955/698 (accessed on 23 September 2025).
- Instituto Nacional de Innovación Agraria (INIA). Detalle de la Accesión: PER1003544. Available online: https://genebankperu.inia.gob.pe/detalle?type=4&var=3889 (accessed on 23 September 2025).
- Instituto Nacional de Innovación Agraria (INIA). Detalle de la Accesión: PER1003551. Available online: https://genebankperu.inia.gob.pe/detalle?type=4&var=3896 (accessed on 23 September 2025).
- Yasin, S.; Zavala-García, F.; Niño-Medina, G.; Rodríguez-Salinas, P.A.; Gutiérrez-Diez, A.; Sinagawa-García, S.R.; Lugo-Cruz, E. Morphological and physiological response of maize (Zea mays L.) to drought stress during reproductive stage. Agronomy 2024, 14, 1718. [Google Scholar] [CrossRef]
- Marcos Solorio, B.; Martínez Campos, Á.R.; López Urquídez, G.A.; López Orona, C.A.; Arteaga Reyes, T.T. La biomasa de los sistemas productivos de maíz nativo (Zea mays) como alternativa a la captura de carbono. Rev. Int. Contam. Ambient. 2016, 32, 361–367. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Bobadilla, L.G.; Meseth, E.; Trigoso, M.J.; Zagaceta, L.H.; Valqui-Valqui, L.; Saravia-Navarro, D.; Barboza, E.; Maicelo, J.L. Agronomic and nutritional evaluation of INIA 910—Kumymarca ryegrass (Lolium multiflorum Lam.): An alternative for sustainable forage production in the Department of Amazonas (NW Peru). Agronomy 2025, 15, 100. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. (Eds.) Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; Available online: https://www.researchgate.net/publication/292783651_AOAC_2005 (accessed on 23 September 2025).
- ANKOM Technology. ANKOM A200 Fiber Analyzer. Available online: https://www.ankom.com/ (accessed on 16 June 2025).
- ANKOM Technology. Method 3: In Vitro True Digestibility Using the ANKOM DAISYII Incubator; ANKOM Technology: Macedon, NY, USA, 2005; Available online: https://www.ankom.com/sites/default/files/2024-08/Method_3_InVitro_D200_D200I.pdf (accessed on 16 June 2025).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 June 2025).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 16 June 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 16 June 2025).
- Lin, Y.; Watts, D.B.; Kloepper, J.W.; Torbert, H.A. Influence of plant growth-promoting rhizobacteria on corn growth under different fertility sources. Commun. Soil Sci. Plant Anal. 2018, 49, 1239–1255. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, J.; Wang, B.; de Leon, N.; Kaeppler, S.M.; Lima, D.C.; Zhang, Z. Estimation of maize yield and flowering time using multi-temporal UAV-based hyperspectral data. Remote Sens. 2022, 14, 3052. [Google Scholar] [CrossRef]
- Koca, Y.O.; Erekul, O. Changes of dry matter, biomass and relative growth rate with different phenological stages of corn. Agric. Agric. Sci. Procedia 2016, 10, 67–75. [Google Scholar] [CrossRef][Green Version]
- Raju, S.G.; Larkin, A. Optimizing French bean growth with organic manures in agroforestry and open systems. Int. J. Plant Soil Sci. 2025, 37, 85–90. [Google Scholar] [CrossRef]
- Sánchez, H.A.; Tadeo Robledo, M.; Espinosa Calderón, A.; Zaragoza Esparza, J.; López López, C. Productividad del agua y rendimiento de maíz bajo diferente disponibilidad de humedad. Rev. Mex. Cienc. Agrícolas 2020, 11, 1005–1016. [Google Scholar] [CrossRef]
- Lince Salazar, L.A.; Sadeghian-Khalajabadi, S.; Sarmiento Herrera, N.G. Evaluación de parámetros relacionados con el crecimiento de plantas de café (Coffea arabica L.) en respuesta al déficit hídrico del suelo. Rev. Investig. Agrar. Ambient. 2024, 15, 11–34. [Google Scholar] [CrossRef]
- Tuller, M.; Or, D. Water films and scaling of soil characteristic curves at low water contents. Water Resour. Res. 2005, 41, W09403. [Google Scholar] [CrossRef]
- Nakamura, R.R. Maternal investment and fruit abortion in Phaseolus vulgaris. Am. J. Bot. 1986, 73, 1049–1057. [Google Scholar] [CrossRef]
- Manson, J.B.; Denton, M.D.; Lake, L.; Brand, J.; Sadras, V.O. Linking pod-set and seed yield of faba bean across organ, phytomer, plant, and population scales. J. Exp. Bot. 2025, 76, 4472–4489. [Google Scholar] [CrossRef]
- Ma’Ruf, M.A.; Numba, S.; Suriyanti, S.; Tjoneng, A. Pertumbuhan dan produksi tumpang sari tanaman jagung (Zea mays L.) dan tanaman kedelai (Glycine max L.). AGrotekMAS J. Indones. J. Ilmu Pertan. 2024, 5, 349–355. [Google Scholar] [CrossRef]
- Davis, J.H.C.; Garcia, S. The effects of plant arrangement and density on intercropped beans (Phaseolus vulgaris L.) and maize I. Traits related to dry matter and seed productivity. Field Crops Res. 1987, 16, 105–115. [Google Scholar] [CrossRef]
- Shahniza, S.S.; Mohd Firdaus, I.; Roslan, I. Effect of time of application and concentrations of plant growth regulators on growth and yield of sweet corn (Zea mays L.). Res. Crops 2020, 21, 46–53. [Google Scholar] [CrossRef]
- Aguiar, A.C.M.; Basso, C.J.; Silva, D.R.O.; Gheller, D.P.; Novello, B.D.; Rieder, E. Relative competitiveness of common bean cultivars in coexistence with volunteer corn. Rev. Bras. Herb. 2019, 37, 97. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, L.; Liu, R.; Wang, W.; Yu, R.; Zhou, T.; Ahmad, I.; Raza, A.; Jiang, S.; Xu, M.; et al. Shade-tolerant soybean reduces yield loss by regulating its canopy structure and stem characteristics in the maize–soybean strip intercropping system. Front. Plant Sci. 2022, 13, 848893. [Google Scholar] [CrossRef]
- Ebel, R.; Pozas Cárdenas, J.G.; Soria Miranda, F.; Cruz González, J. Organic milpa: Yields of maize, beans, and squash in mono- and polycropping systems. Rev. Terra Latinoam. 2017, 35, 149–160. [Google Scholar] [CrossRef]
- Bantihun, A.; Asmare, B.; Mekuriaw, Y. Comparative Evaluation of Selected Grass Species for Agronomic Performance, Forage Yield, and Chemical Composition in the Highlands of Ethiopia. Adv. Agric. 2022, 2022, 6974681. [Google Scholar] [CrossRef]
- Adesogan, A.; Newman, Y. Silage harvesting, storing, and feeding. EDIS 2010, 2010, AG180. [Google Scholar] [CrossRef]
- Bai, C.; Wang, C.; Sun, L.; Xu, H.; Jiang, Y.; Na, N.; Yin, G.; Liu, S.; Xue, Y. Dynamics of bacterial and fungal communities and metabolites during aerobic exposure in whole-plant corn silages with two different moisture levels. Front. Microbiol. 2021, 12, 663895. [Google Scholar] [CrossRef]
- Wataradee, S.; Boonserm, T.; Samngamnim, S.; Ajariyakhajorn, K. Characterization of virulence factors and antimicrobial susceptibility of Streptococcus agalactiae associated with bovine mastitis cases in Thailand. Animals 2024, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Luo, H.; Wang, Y.; Liu, X.; Zhou, X. Epidemiological investigation of non-albicans Candida species recovered from mycotic mastitis of cows in Yinchuan, Ningxia of China. BMC Vet. Res. 2018, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Cordukes, W.E.; Shearer, D.A.; Cooper, D.J. The effect of initial compaction and moisture content on ensiling losses of forage crops. Can. J. Plant Sci. 1959, 39, 127–134. [Google Scholar] [CrossRef]
- Lajús, C.R.; Sebben, C.; Pasqualotto, D.L.; Spode, M.R.; Sabadini, P.B.; Dalcanton, F.; da Luz, G.L.; Onofre, S.B.; Cericato, A.; Topolski Pavan Batiston, T.F. Production and nutritive value of silage corn in different reproductive stages. Int. J. Adv. Eng. Res. Sci. 2020, 7, 130–136. [Google Scholar] [CrossRef]
- Meehan, E.J.; Gilliland, T.J. Differences in dry matter content between forage varieties of Lolium perenne L. Biol. Environ. Proc. R. Ir. Acad. 2019, 119B, 123–137. [Google Scholar] [CrossRef]
- Kebede, G.; Feyissa, F.; Faji, M.; Mohammed, K.; Dejene, M.; Mengistu, G.; Geleti, D.; Assefa, G.; Alemayehu, M.; Mengistu, S.; et al. Dry matter accumulation dynamics, morphological characteristics and nutritive value of desho (Pennisetum glaucifolium) grass varieties in the central Highlands of Ethiopia. J. Agric. Environ. Sci. 2023, 8, 111–124. [Google Scholar] [CrossRef]
- Lewis-Beck, C.; Walker, V.A.; Niemi, J.; Caragea, P.; Hornbuckle, B.K. Extracting agronomic information from SMOS vegetation optical depth in the US Corn Belt using a nonlinear hierarchical model. Remote Sens. 2020, 12, 827. [Google Scholar] [CrossRef]
- Inamoto, K.; Nagasuga, K.; Yano, T. Effect of CO2 enrichment on the photosynthesis and dry matter accumulation in the oriental hybrid lily ‘Siberia’. Hortic. J. 2022, 91, 541–550. [Google Scholar] [CrossRef]
- Geren, H.; Kavut, Y.T.; Unlu, H.B. Effect of different cutting intervals on the forage yield and some silage quality characteristics of giant king grass (Pennisetum hybridum) under Mediterranean climatic conditions. Turk. J. Field Crops 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Nguyen, H.T.D.; Schonewille, J.T.; Pellikaan, W.F.; Nguyen, T.X.; Hendriks, W.H. In vitro gas production of common Southeast Asian grasses in response to variable regrowth periods in Vietnam. Fermentation 2024, 10, 280. [Google Scholar] [CrossRef]
- Grøseth, M.; Karlsson, L.; Steinshamn, H.; Johansen, M.; Kidane, A.; Prestløkken, E. Effects of dry matter concentration in grass silage on milk production of dairy cows fed concentrates high or low in metabolizable protein concentration. Livest. Sci. 2025, 291, 105611. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Wilkinson, R.G.; Sinclair, L.A. Reducing dietary protein and supplementation with starch or rumen-protected methionine and its effect on performance and nitrogen efficiency in dairy cows fed a red clover and grass silage–based diet. J. Dairy Sci. 2024, 107, 3543–3557. [Google Scholar] [CrossRef] [PubMed]
- Valqui, L.; Saucedo-Uriarte, J.A.; Altamirano-Tantalean, M.A.; Bobadilla, L.G.; Portocarrero Villegas, S.M.; Bardales, W.; Frias, H.; Zagaceta Llanca, L.H.; Valqui-Valqui, L.; Puerta-Chavez, L.J.; et al. Influence of tree species on soil physicochemical composition, macrofauna, and forage production. J. Agric. Food Res. 2025, 23, 102220. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Shao, T. Effect of ensiling corn stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the Tibetan Plateau. Grassl. Sci. 2017, 63, 236–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).