Enhancing Almond Seed Germination and Growth Through Microbial Priming: A Biostimulation Strategy for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Seeds Collecting
2.2. Treatments Preparation
2.3. Seeds Preparation for Germination Test
- First day of germination (FDG)
- Germination percentage (GP) (%)
- Mean germination time (MGT)
- Germination rate index (GRI)
2.4. Growth Chamber Experiment
2.5. Mycorrhization Assessment
2.5.1. Spore Extraction and Identification
2.5.2. AMF Colonization
2.6. Soil Physicochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification and Density of AMF SPORES
3.2. Germination Parameters
3.2.1. Germination Percentage (GP)
3.2.2. Germination Indicators
3.3. Seedling Growth Assessment
3.4. Assessment of Physicochemical Properties of Soils
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome Engineering and Plant Biostimulants for Sustainable Crop Improvement and Mitigation of Biotic and Abiotic Stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Andrade-Linares, D.R.; Lehmann, A.; Rillig, M.C. Microbial Stress Priming: A Meta-analysis. Environ. Microbiol. 2016, 18, 1277–1288. [Google Scholar] [CrossRef]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring Plant-Associated Microbial Inoculants in Agriculture: A Roadmap for Successful Application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef]
- Azaroual, S.E.; Kasmi, Y.; Aasfar, A.; El Arroussi, H.; Zeroual, Y.; El Kadiri, Y.; Zrhidri, A.; Elfahime, E.; Sefiani, A.; Meftah Kadmiri, I. Investigation of Bacterial Diversity Using 16S rRNA Sequencing and Prediction of Its Functionalities in Moroccan Phosphate Mine Ecosystem. Sci. Rep. 2022, 12, 3741. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between Arbuscular Mycorrhizal Fungi and Bacteria and Their Potential for Stimulating Plant Growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Bessis, A.; Pozo, M.J. Microbial Priming of Plant and Animal Immunity: Symbionts as Developmental Signals. Trends Microbiol. 2014, 22, 607–613. [Google Scholar] [CrossRef]
- Woodmansee, A.; Aakairi, M.; Gérard, B.; Hassani, O.S.; Ouarghidi, A.; Power, A.G.; Rossiter, D.G.; McDonald, A. Characterizing Rural Livelihoods in a Changing Environment: A Case Study in the High Atlas Mountains of Morocco. Discov. Sustain. 2025, 6, 31. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, Economic Importance, Genomics. In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and Classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Zeinalabedini, M.; Khayam-Nekoui, M.; Grigorian, V.; Gradziel, T.M.; Martínez-Gómez, P. The Origin and Dissemination of the Cultivated Almond as Determined by Nuclear and Chloroplast SSR Marker Analysis. Sci. Hortic. 2010, 125, 593–601. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/statistics/en (accessed on 18 October 2024).
- Lionello, P.; Abrantes, F.; Gacic, M.; Planton, S.; Trigo, R.; Ulbrich, U. The Climate of the Mediterranean Region: Research Progress and Climate Change Impacts. Reg. Environ. Change 2014, 14, 1679–1684. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate Change and Interconnected Risks to Sustainable Development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Fernandes De Oliveira, A.; Mameli, M.G.; De Pau, L.; Satta, D. Almond Tree Adaptation to Water Stress: Differences in Physiological Performance and Yield Responses among Four Cultivar Grown in Mediterranean Environment. Plants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Savoia, M.A.; Del Faro, L.; Venerito, P.; Gaeta, L.; Palasciano, M.; Montemurro, C.; Sabetta, W. The Relevance of Discovering and Recovering the Biodiversity of Apulian Almond Germplasm by Means of Molecular and Phenotypic Markers. Plants 2022, 11, 574. [Google Scholar] [CrossRef]
- Lipan, L.; Romero, A.; Echeverria, G.; Maldonado, M.; Company, T.; Escalona, J.M.; Ruiz, J.; Miarnau, X. Native versus Modern Almond Cultivars of Mallorca Island: From Biodiversity to Industrial Aptitude and Fruit Quality. Agronomy 2022, 12, 1933. [Google Scholar] [CrossRef]
- Vasilikiotis, C.; Li, M.; Schmidt, J.E.; Azimi, A.; Garcia, J.; Volder, A.; Lampinen, B.; Gaudin, A.C. Orchard Management Practices Affect Arbuscular Mycorrhizal Fungal Root Colonisation of Almond. Biol. Agric. Hortic. 2020, 36, 230–248. [Google Scholar] [CrossRef]
- Roldan-Fajardo, B.E.; Barea, J.M.; Ocampo, J.A.; Azcon-Aguilar, C. The Effect of Season on VA Mycorrhiza of the Almond Tree and of Phosphate Fertilization and Species of Endophyte on Its Mycorrhizal Dependency. Plant Soil 1982, 68, 361–367. [Google Scholar] [CrossRef]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology® 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Aguilar, C.; Palenzuela, J.; Roldán, A.; Bautista, S.; Vallejo, R.; Barea, J.M. Analysis of the Mycorrhizal Potential in the Rhizosphere of Representative Plant Species from Desertification-Threatened Mediterranean Shrublands. Appl. Soil Ecol. 2003, 22, 29–37. [Google Scholar] [CrossRef]
- Tamburini, E.; Mandaresu, M.; Lussu, R.; Sergi, S.; Vitali, F.; Carucci, A.; Cappai, G. Metal Phytostabilization by Mastic Shrub (Pistacia lentiscus L.) and Its Root-Associated Bacteria in Different Habitats of Sardinian Abandoned Mining Areas (Italy). Environ. Sci. Pollut. Res. 2023, 30, 122107–122120. [Google Scholar] [CrossRef]
- Szili-Kovács, T.; Takács, T. Advanced Research of Rhizosphere Microbial Activity. Agriculture 2023, 13, 911. [Google Scholar] [CrossRef]
- Applebaum, I.; Jeyaraman, M.; Sherman, C.; Doniger, T.; Steinberger, Y. Structure and Function of the Soil Rhizosphere Fungal Communities in Medicinal Plants—A Preliminary Study. Agriculture 2022, 12, 152. [Google Scholar] [CrossRef]
- Steinberger, Y.; Doniger, T.; Sherman, C.; Jeyaraman, M.; Applebaum, I. Soil Bacterial Community of Medicinal Plant Rhizosphere in a Mediterranean System. Agriculture 2024, 14, 664. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Torres, M.P.; Torrecillas, E.; Díaz, G.; Roldán, A. Plant Type Differently Promote the Arbuscular Mycorrhizal Fungi Biodiversity in the Rhizosphere after Revegetation of a Degraded, Semiarid Land. Soil Biol. Biochem. 2011, 43, 167–173. [Google Scholar] [CrossRef]
- Manaut, N.; El Bzar, Z.; El-Bachri, C.; Bouabidi, Z.; Hafidi, M.; Douma, M. Exploring the potentialities of Chamaerops humilis l. Soil to improve growth and quality of saffron. Appl. Ecol. Environ. Res. 2023, 21, 5977–5988. [Google Scholar] [CrossRef]
- Howard, M.M.; Bell, T.H.; Kao-Kniffin, J. Soil Microbiome Transfer Method Affects Microbiome Composition, Including Dominant Microorganisms, in a Novel Environment. FEMS Microbiol. Lett. 2017, 364, fnx092. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.; Luera, J.; Lavallee, K.; Soti, P. Influence of Microbial Priming and Seeding Depth on Germination and Growth of Native Wildflowers. Ecol. Process 2021, 10, 19. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Cornea-Cipcigan, M.; Pamfil, D.; Sisea, C.R.; Mărgăoan, R. Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days. Agronomy 2020, 10, 516. [Google Scholar] [CrossRef]
- Coffey, K.L.; Kirkman, L.K. Seed Germination Strategies of Species with Restoration Potential in a Fire-Maintained Pine Savanna. Nat. Areas J. 2006, 26, 289–299. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Towards a Rational Basis for Testing Seed Quality. In Seed Production; Butterworths: London, UK, 1980; pp. 605–635. [Google Scholar]
- Esechie, H.A. Interaction of Salinity and Temperature on the Germination of Sorghum. J. Agron. CropSci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Relationship Between Decarboxylation of Glutamic Acid and Vigor in Soybean Seed 1. Crop. Sci. 1973, 13, 227–232. [Google Scholar] [CrossRef]
- The International Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM). Available online: https://invam.ku.edu/species-descriptions (accessed on 15 February 2025).
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trovelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure Du Taux de Mycorrhization VA d’un Systeme Radiculaire. Recherche Des Methodes d’estimation Ayant Une Signification Fonctionnelle. In The Mycorrhizae: Physiology and Genetic; INRA Press: Paris, Freance, 1986; pp. 217–221. [Google Scholar]
- Aubert, G. Methodes d’analyses Des Sols: Documents de Travail Tous Droits Reserves. In Centre Régional de Documentation Pédagogique; Centre Régional de Documentation Pédagogique: Marseille, France, 1978. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Agronomy Monographs; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; pp. 539–579. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius Z. F. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Zhu, J.; Kang, H.; Tan, H.; Xu, M. Effects of Drought Stresses Induced by Polyethylene Glycol on Germination of Pinus Sylvestris Var. Mongolica Seeds from Natural and Plantation Forests on Sandy Land. J. For. Res. 2006, 11, 319–328. [Google Scholar] [CrossRef]
- Duponnois, R.; Ramanankierana, H.; Hafidi, M.; Baohanta, R.; Baudoin, É.; Thioulouse, J.; Sanguin, H.; Bâ, A.; Galiana, A.; Bally, R.; et al. Native Plant Resources to Optimize the Performances of Forest Rehabilitation in Mediterranean and Tropical Environment: Some Examples of Nursing Plant Species That Improve the Soil Mycorrhizal Potential. Comptes Rendus. Biol. 2013, 336, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.G.; Rubio, R.; Rouanet, J.L.; Borie, F. Early Effects of Tillage and Crop Rotation on Arbuscular Mycorrhizal Fungal Propagules in an Ultisol. Biol. Fertil. Soils 2006, 43, 83–92. [Google Scholar] [CrossRef]
- Oruru, M.B.; Njeru, E.M. Upscaling Arbuscular Mycorrhizal Symbiosis and Related Agroecosystems Services in Smallholder Farming Systems. BioMed Res. Int. 2016, 2016, 4376240. [Google Scholar] [CrossRef]
- Rocha, I.; Duarte, I.; Ma, Y.; Souza-Alonso, P.; Látr, A.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy 2019, 9, 471. [Google Scholar] [CrossRef]
- Barea, J.-M.; Azcón, R.; Azcón-Aguilar, C. Mycorrhizosphere Interactions to Improve Plant Fitness and Soil Quality. Antonie Van Leeuwenhoek 2002, 81, 343–351. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Awan, S.; Footitt, S.; Finch-Savage, W.E. Interaction of Maternal Environment and Allelic Differences in Seed Vigour Genes Determines Seed Performance in Brassica oleracea. Plant J. 2018, 94, 1098–1108. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, W.; Yin, H.; Luo, X.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the Mother Plant during Seed Development Promotes Subsequent Seed Germination in Soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial Seed Endophyte Shapes Disease Resistance in Rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus Subtilis Biofilm Formation and Social Interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Zhao, S.; Yang, C.; Meng, Y.; Shuai, H.; Luo, X.; Dai, Y.; Yin, H.; Du, J.; et al. DA-6 Promotes Germination and Seedling Establishment from Aged Soybean Seeds by Mediating Fatty Acid Metabolism and Glycometabolism. J. Exp. Bot. 2019, 70, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Toju, H.; Kurokawa, H.; Kenta, T. Factors Influencing Leaf- and Root-Associated Communities of Bacteria and Fungi Across 33 Plant Orders in a Grassland. Front. Microbiol. 2019, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Van Overbeek, L.S.; Franke, A.C.; Nijhuis, E.H.M.; Groeneveld, R.M.W.; Da Rocha, U.N.; Lotz, L.A.P. Bacterial Communities Associated with Chenopodium Album and Stellaria Media Seeds from Arable Soils. Microb. Ecol. 2011, 62, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacques, M.-A. Emergence Shapes the Structure of the Seed Microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Zulueta-Rodríguez, R.; Hernández-Montiel, L.; Murillo-Amador, B.; Rueda-Puente, E.; Capistrán, L.; Troyo-Diéguez, E.; Córdoba-Matson, M. Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction. Forests 2015, 6, 3109–3122. [Google Scholar] [CrossRef]
- Yaqoob, H.S.; Shoaib, A.; Anwar, A.; Perveen, S.; Javed, S.; Mehnaz, S. Seed Biopriming with Ochrobactrum Ciceri Mediated Defense Responses in Zea mays (L.) against Fusarium Rot. Physiol. Mol. Biol. Plants 2024, 30, 49–66. [Google Scholar] [CrossRef]
- Homet-Gutiérrez, P.; Schupp, E.W.; Gómez, J.M. Naturalization of almond trees (Prunus dulcis) in semi-arid regions of the Western Mediterranean. J. Arid Environ. 2015, 113, 108–113. [Google Scholar] [CrossRef]
- Badalamenti, E.; Bueno, R.S.; Sala, G.; Cusimano, D.; La Mantia, T.; Ilardi, V. The naturalization of the almond Prunus dulcis in different ecological contexts in the Mediterranean: An underestimated process? Flora 2022, 294, 152117. [Google Scholar] [CrossRef]

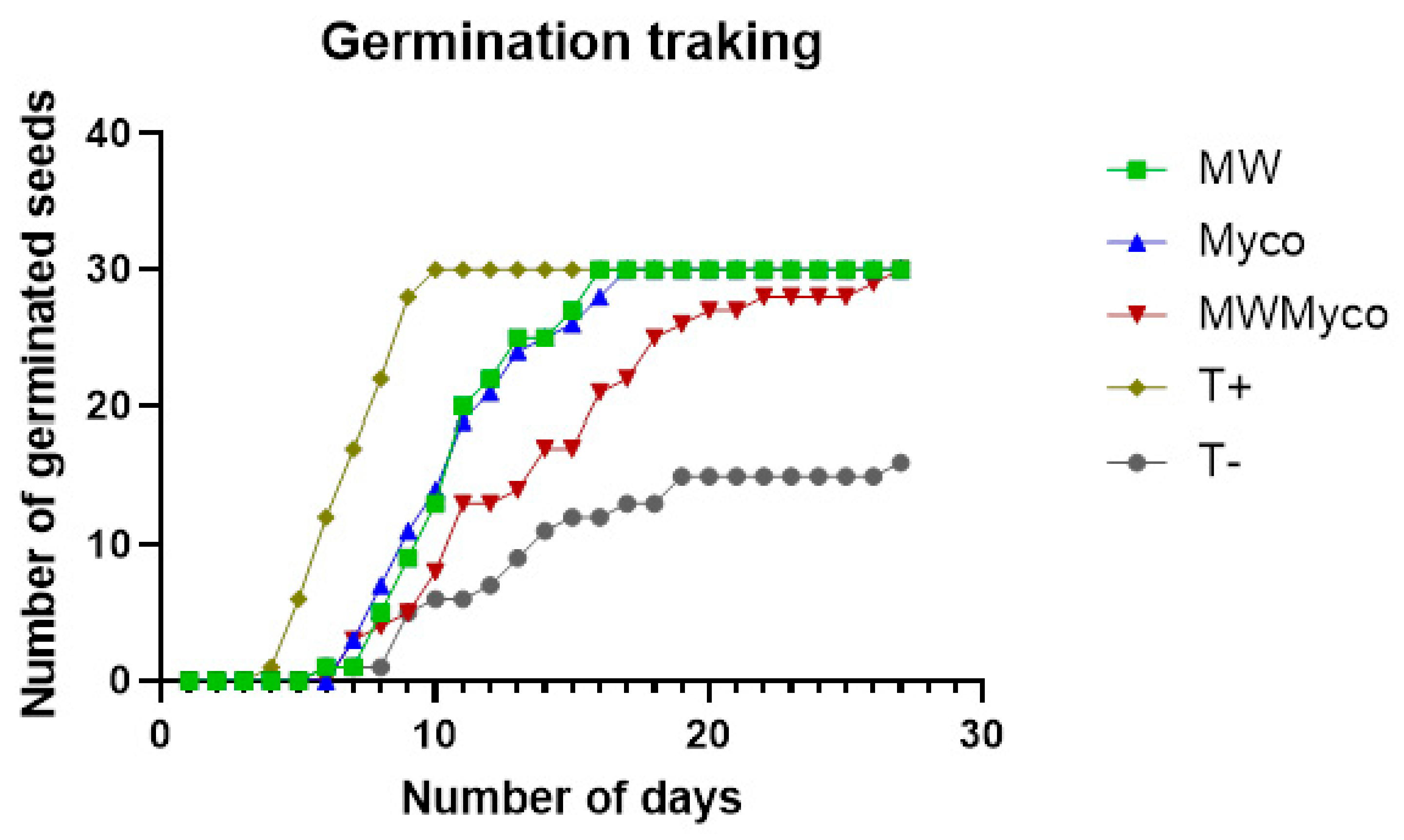
| Treatments | Number of AMF Spores/100 g of Soil | Intensity of Mycorrhization (M%) | Frequency (F%) |
|---|---|---|---|
| PNS | 935 ± 1 | 94.17 ± 1.44 | 100% ± 0.0 |
| MW | 00 ± 0.0 a | 3.37% ± 0.23 a | 20 ± 0.0 a |
| Myco | 2099 ± 4.5 b | 86.17 ± 1.25 c | 100% ± 0.0 a |
| MW + Myco | 1087 ± 3 a | 52.67 ± 2.3 b | 100% ± 0.0 a |
| T+ | 00 ± 0 a | 0.73% ± 0.23 a | 20.00 ± 0.0 a |
| T− | 00 ± 0 c | 0.23 ± 0.23 a | 10 ± 0.0 a |
| Treatment | Day 10 | Day 20 | Day 27 |
|---|---|---|---|
| MW | 43.33 ± 0.33 c | 100 ± 0.0 c | 100 ± 0.0 b |
| Myco | 46.67 ± 0.68 d | 100 ± 0.0 c | 100 ± 0.0 b |
| MW + Myco | 26.66 ± 0.66 b | 90 ± 0.45 b | 100 ± 0.0 b |
| T+ | 100 ± 0.0 e | 100 ± 0.0 c | 100 ± 0.0 b |
| T− | 20 ± 0.33 a | 50 ± 0.22 a | 53.33 ± 0.36 a |
| FDG (Day) | GRI | MGT (Day) | GP (%) | GMR | |
|---|---|---|---|---|---|
| MW | 6 (2) | 0.99 (2) | 18.83 (3) | 100 (1) | 2 |
| Myco | 7 (4) | 0.99 (2) | 18.77 (2) | 100 (1) | 2.25 |
| MW + Myco | 7 (4) | 0.77 (4) | 19.63 (4) | 100 (1) | 3.25 |
| T+ | 4 (1) | 1.45 (1) | 17.00 (1) | 100 (1) | 1 |
| T− | 6 (2) | 0.44 (5) | 19.34 (5) | 53.33 (5) | 4.25 |
| WSL (cm) | TNL | SVI | SMR | |
|---|---|---|---|---|
| MW | 21. 2 ± 6.67 a (4) | 32.66 ± 7.77 ab (3) | 21.2 (4) | 3.66 |
| Myco | 23.47 ± 6.37 a (2) | 29.33 ± 7.23 bc (4) | 23.47 (2) | 2.66 |
| MW + Myco | 23.46 ± 8.52 a (2) | 34 ± 9.5 bc (2) | 23.46 (2) | 2 |
| T+ | 37.9 ± 6.32 b (1) | 40.06 ± 7.66 c (1) | 37.9 (1) | 1 |
| T− | 18 ± 10.53 a (5) | 21.5 ± 9.98 a (5) | 9.6 (5) | 5 |
| pH | EC (mS/cm) | TOC (%) | N (%) | AP (mg/g sol) | |
|---|---|---|---|---|---|
| S0 | 8.05 ± 0.07 | 0.17 ± 0.04 | 0.06 ± 0.01 | 0.01 ± 0.00 | 0.1 ± 0.02 |
| MW | 7.99 ± 0.16 a | 0.61 ± 0.2 a | 0.75 ± 0.1 a | 0.04 ± 0.03 a | 0.18 ± 0.03 a |
| Myco | 8.12 ± 0.15 a | 0.49 ± 0.19 a | 0. 58 ± 0.26 a | 0.05 ± 0.004 a | 0.17 ± 0.01 a |
| MW + Myco | 7.97 ± 0.07 a | 0.7 ± 0.32 a | 0.46 ± 0.26 a | 0.02 ± 0.009 a | 0.19 ± 0.03 a |
| T+ | 8.22 ± 0.33 a | 0.5 ± 0.2 a | 0.4 ± 0.1 a | 0.03 ± 0.009 a | 0.21 ± 0.01 a |
| T− | 8. 29 ± 0.04 a | 0.39 ± 0.07 a | 0.64 ± 0.05 a | 0.04 ± 0.007 a | 0.2 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabidi, Z.; Manaut, N.; Douma, M. Enhancing Almond Seed Germination and Growth Through Microbial Priming: A Biostimulation Strategy for Sustainable Agriculture. Agronomy 2025, 15, 2434. https://doi.org/10.3390/agronomy15102434
Bouabidi Z, Manaut N, Douma M. Enhancing Almond Seed Germination and Growth Through Microbial Priming: A Biostimulation Strategy for Sustainable Agriculture. Agronomy. 2025; 15(10):2434. https://doi.org/10.3390/agronomy15102434
Chicago/Turabian StyleBouabidi, Zineb, Najat Manaut, and Mountasser Douma. 2025. "Enhancing Almond Seed Germination and Growth Through Microbial Priming: A Biostimulation Strategy for Sustainable Agriculture" Agronomy 15, no. 10: 2434. https://doi.org/10.3390/agronomy15102434
APA StyleBouabidi, Z., Manaut, N., & Douma, M. (2025). Enhancing Almond Seed Germination and Growth Through Microbial Priming: A Biostimulation Strategy for Sustainable Agriculture. Agronomy, 15(10), 2434. https://doi.org/10.3390/agronomy15102434





