Field-Based Spatiotemporal Dynamics, Ovarian Maturation and Laboratory Oviposition Behavior of Drosophila suzukii in Peach: Key Insights for Integrated Pest Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Ethics Statement
2.3. Drosophila suzukii Monitoring
2.4. Assessment of Reproductive Status
- : number of females dissected per trap;
- : number of females captured in the given trap;
- : total number of females captured across all traps on that date.
- No ovaries;
- Immature ovaries;
- Ripening eggs in ovarioles;
- Mature eggs in ovarioles;
- Mature eggs outside the ovaries (abdominal).
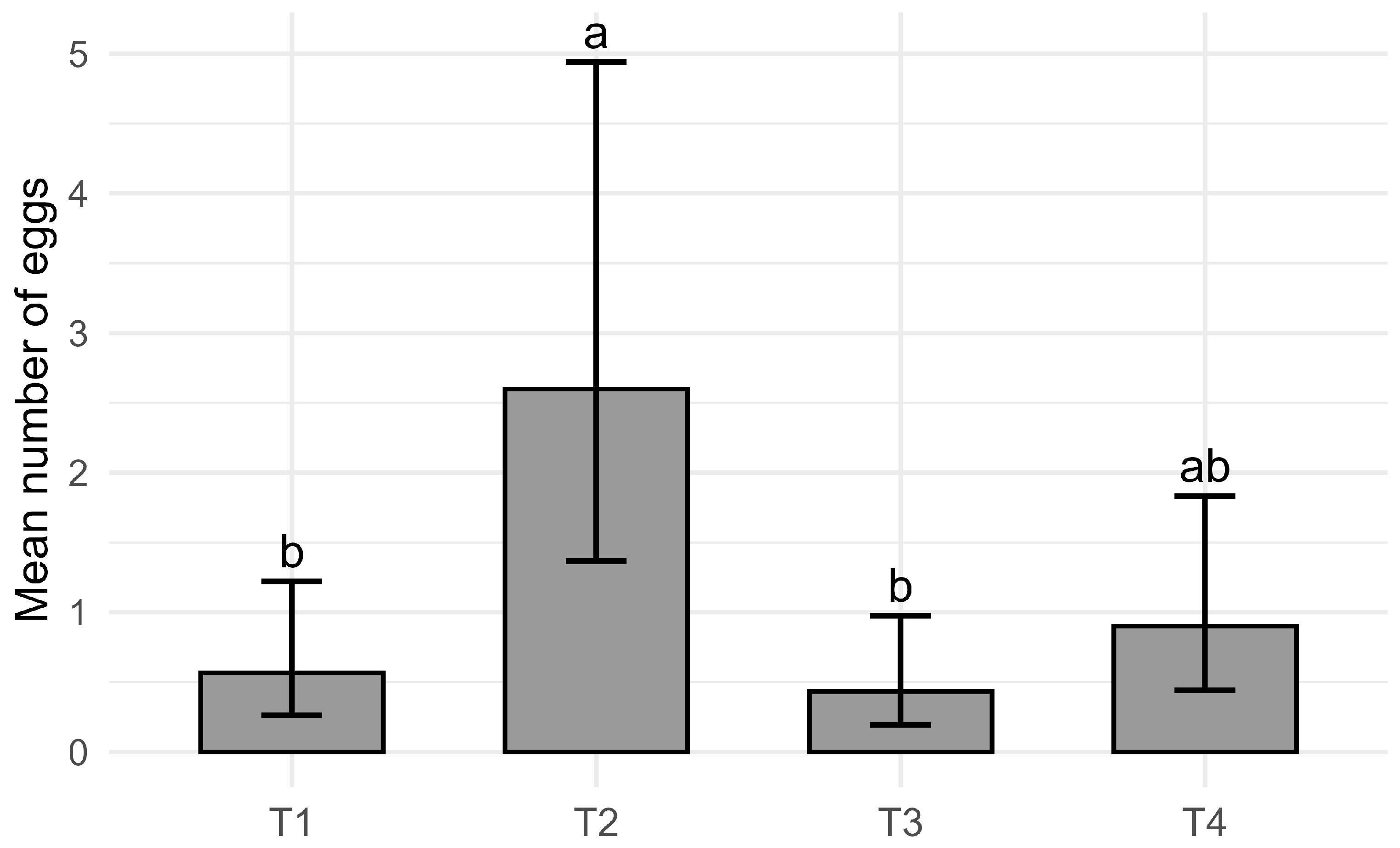
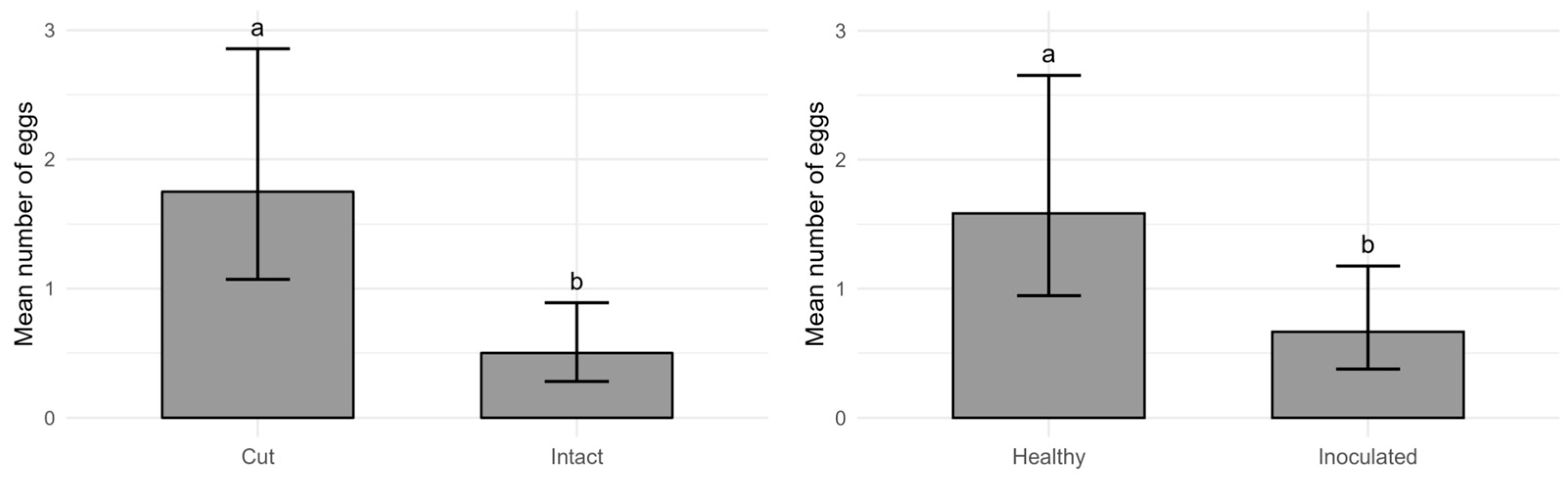
2.5. Laboratory Evaluation of Oviposition Preferences
- T1: Intact, healthy fruit;
- T2: Cut, healthy fruit (2 × 1 cm superficial incision);
- T3: Intact, inoculated fruit (Monilinia fructicola isolated from the University collection);
- T4: Cut, inoculated fruit.
2.6. Statistical Analysis
3. Results
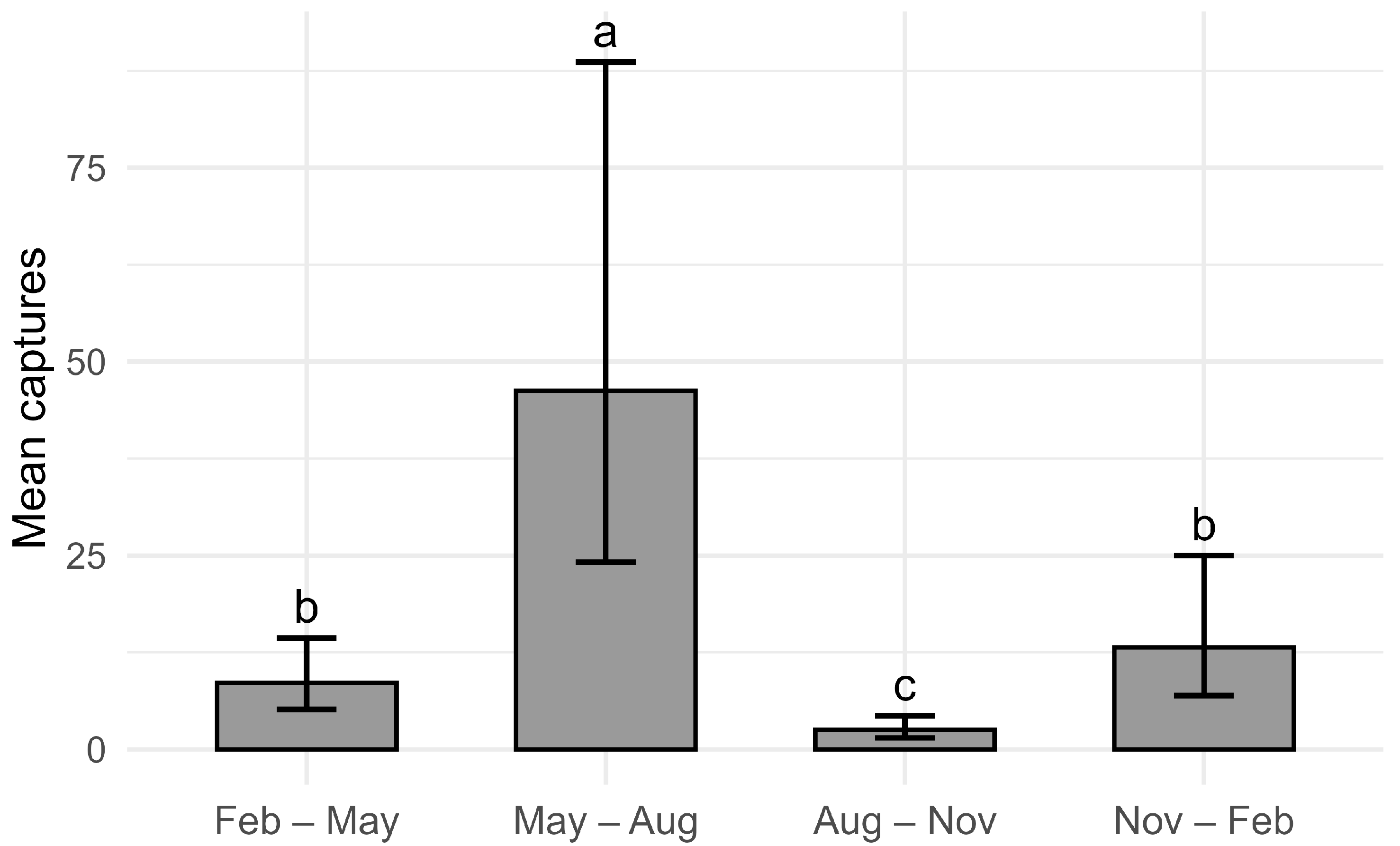
3.1. Seasonal Dynamics of Drosophila suzukii
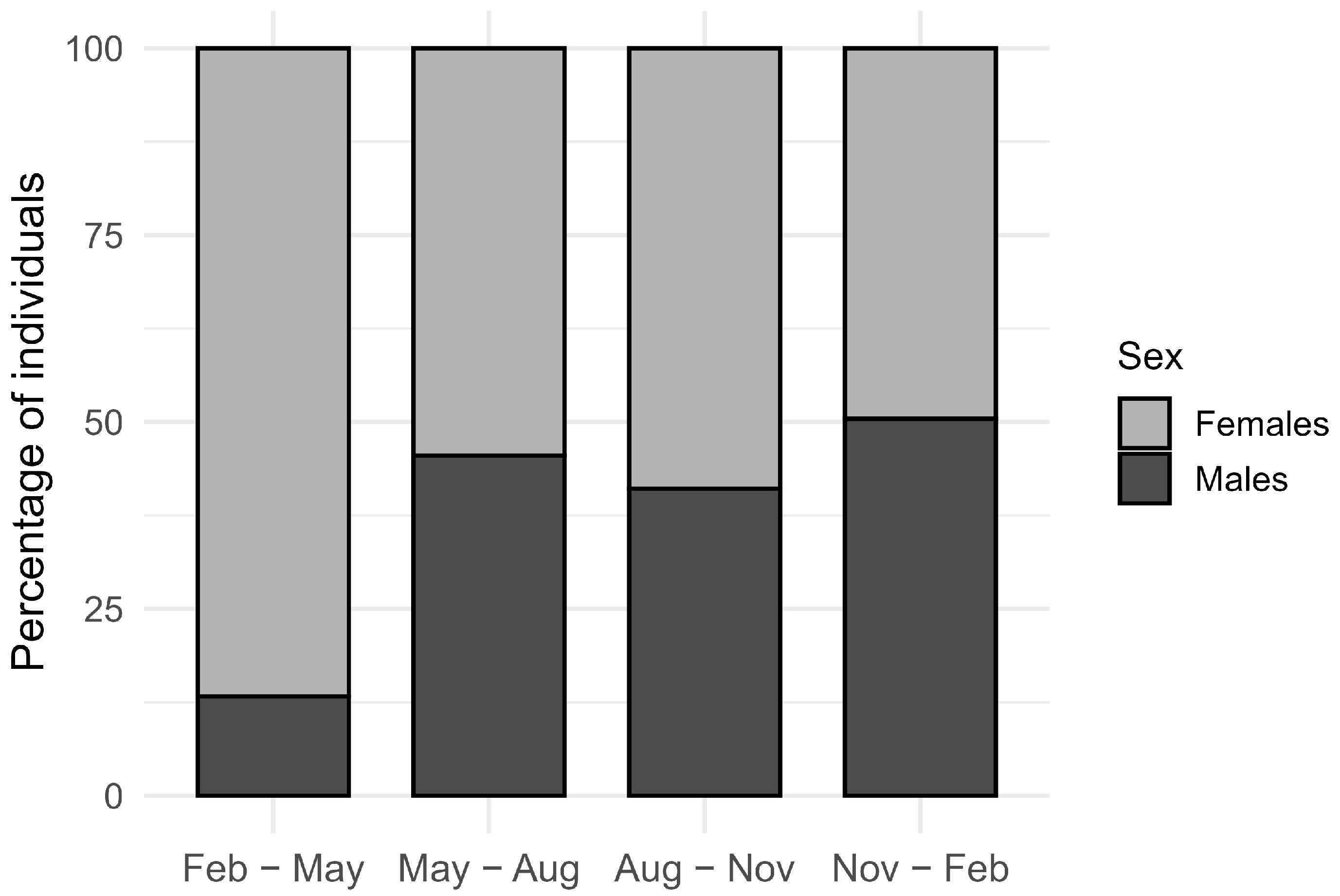
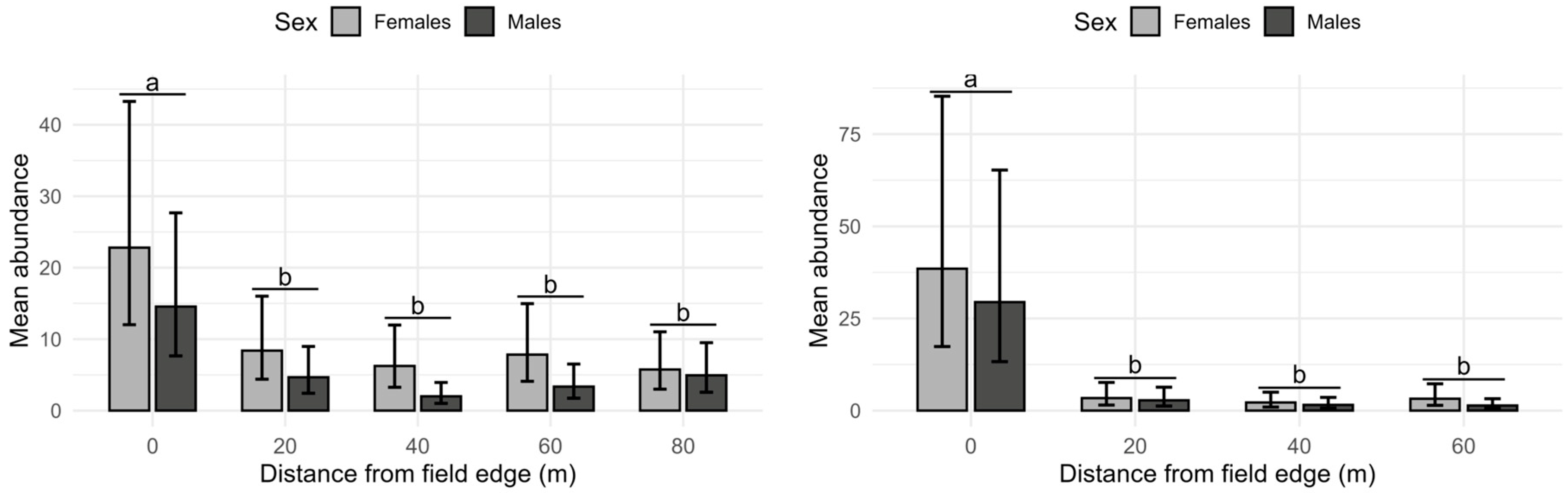
3.2. Spatial Distribution Within the Orchard
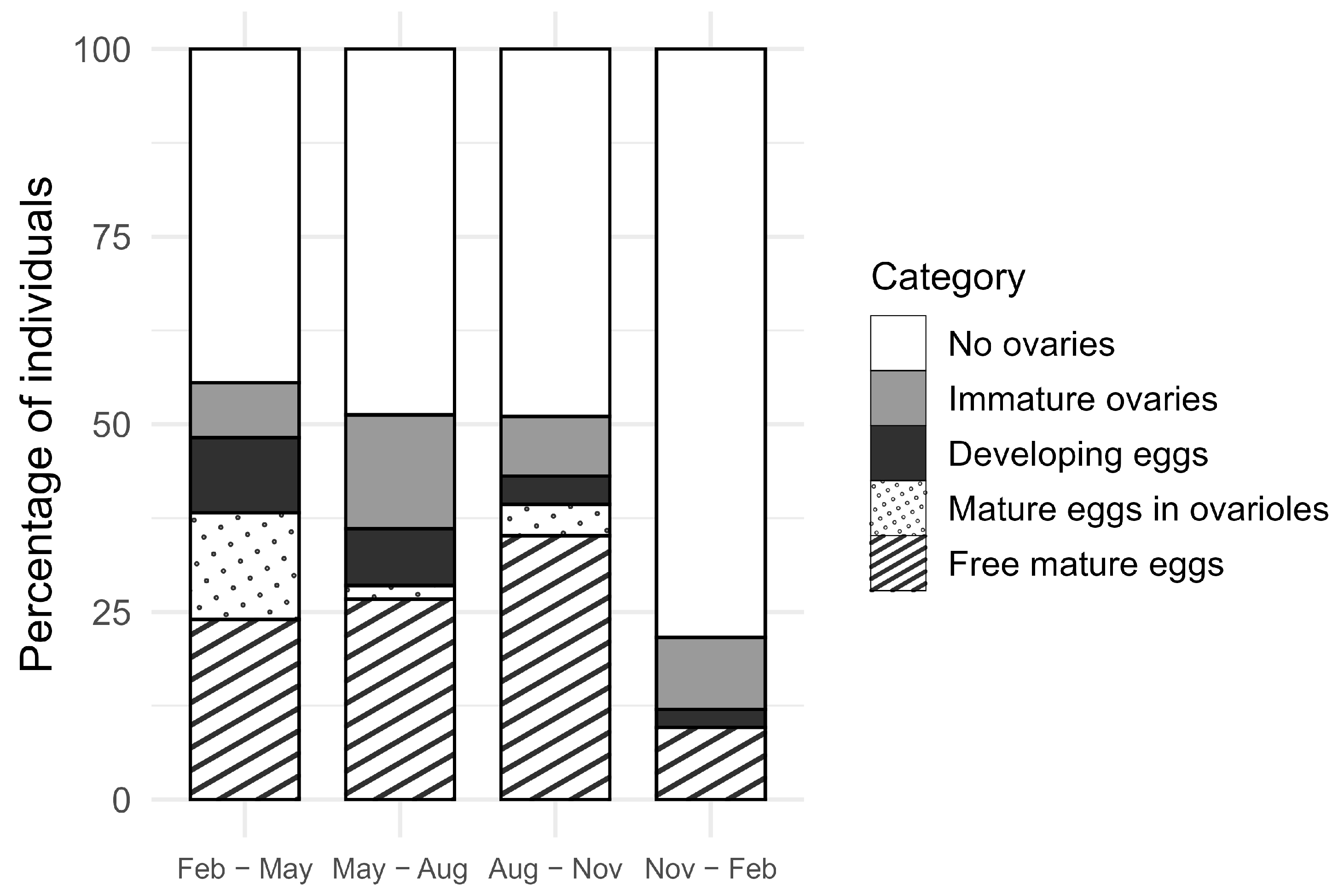
3.3. Drosophila suzukii Reproductive Stages in the Field
3.4. Drosophila suzukii Reproductive Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SWD | Spotted Wing Drosophila |
| IPM | Integrated Pest Management |
References
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent Trends in the Economic Impact of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-62691-4. [Google Scholar]
- Andreazza, F.; Bernardi, D.; Botton, M.; Nava, D.E. Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Peaches: Is It a Problem? Sci. Agric. 2017, 74, 489–491. [Google Scholar] [CrossRef]
- Sario, S.; Melo-Ferreira, J.; Santos, C. Winter Is (Not) Coming: Is Climate Change Helping Drosophila suzukii Overwintering? Biology 2023, 12, 907. [Google Scholar] [CrossRef]
- Abeijon, L.M.; Gómez Llano, J.H.; Robe, L.J.; Ovruski, S.M.; Garcia, F.R.M. Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives. Agronomy 2025, 15, 838. [Google Scholar] [CrossRef]
- Benito, N.P.; Lopes-da-Silva, M.; Santos, R.S.S.D. Potential Spread and Economic Impact of Invasive Drosophila suzukii in Brazil. Pesqui. Agropecu. Bras. 2016, 51, 571–578. [Google Scholar] [CrossRef]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal Cues Induce Phenotypic Plasticity of Drosophila suzukii to Enhance Winter Survival. BMC Ecol. 2016, 16, 11. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. The Economic Impact of Drosophila suzukii: Perceived Costs and Revenue Losses of Swiss Cherry, Plum and Grape Growers. Pest Manag. Sci. 2020, 77, 978–1000. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A Review of the Invasion of Drosophila suzukii in Europe and a Draft Research Agenda for Inte-411 grated Pest Management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Haviland, D.R.; Beers, E.H. Chemical Control Programs for Drosophila suzukii That Comply with International Limitations on Pesticide Residues for Exported Sweet Cherries. J. Integr. Pest Manag. 2012, 3, F1–F6. [Google Scholar] [CrossRef]
- Beers, E.H.; Van Steenwyk, R.A.; Shearer, P.W.; Coates, W.W.; Grant, J.A. Developing Drosophila suzukii Management Programs for Sweet Cherry in the Western United States. Pest Manag. Sci. 2011, 67, 1386–1395. [Google Scholar] [CrossRef]
- Wiman, N.G.; Dalton, D.T.; Anfora, G.; Biondi, A.; Chiu, J.C.; Daane, K.M.; Gerdeman, B.; Gottardello, A.; Hamby, K.A.; Isaacs, R.; et al. Drosophila suzukii Population Response to Environment and Management Strategies. J. Pest Sci. 2016, 89, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Lewis, M.T.; Burrack, H.J.; Grieshop, M.; Isaacs, R.; Rendon, D.; Rogers, M.; Rothwell, N.; Sial, A.A.; Walton, V.M.; et al. Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects 2021, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii Management in Latin America: Current Status and Perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef]
- Parichanon, P.; Farina, P.; Abenaim, L.; Conti, B. Dose-Dependent Effect of Methyl Jasmonate on Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Bull. Entomol. Res. 2025, 1–8. [Google Scholar] [CrossRef]
- Harris, D.W.; Hamby, K.A.; Wilson, H.E.; Zalom, F.G. Seasonal Monitoring of Drosophila suzukii (Diptera: Drosophilidae) in a Mixed Fruit Production System. J. Asia-Pac. Entomol. 2014, 17, 857–864. [Google Scholar] [CrossRef]
- Klick, J.; Yang, W.Q.; Walton, V.M.; Dalton, D.T.; Hagler, J.R.; Dreves, A.J.; Lee, J.C.; Bruck, D.J. Distribution and Activity of Drosophila suzukii in Cultivated Raspberry and Surrounding Vegetation. J. Appl. Entomol. 2016, 140, 37–46. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; Van Der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-Crop Plants Used as Hosts by Drosophila suzukii in Europe. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The Susceptibility of Small Fruits and Cherries to the Spotted-wing Drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in Selection and Utilization of Host Crops in the Field and Laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an Invasive Frugivore: Selection and Utilization of Host Crops by the Invasive Frugivore D. suzukii. Pest. Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef]
- Kinjo, H.; Kunimi, Y.; Ban, T.; Nakai, M. Oviposition Efficacy of Drosophila suzukii (Diptera: Drosophilidae) on Different Cultivars of Blueberry. J. Econ. Entomol. 2013, 106, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Ioriatti, C.; Walton, V.; Dalton, D.; Anfora, G.; Grassi, A.; Maistri, S.; Mazzoni, V. Drosophila suzukii (Diptera: Drosophilidae) and Its Potential Impact to Wine Grapes During Harvest in Two Cool Climate Wine Grape Production Regions. J. Econ. Entomol. 2015, 108, 1148–1155. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.-H.; Zhai, Y.-F.; Chen, H.; Dang, H.-Y.; Qin, D.-Y.; Li, L.-L.; Li, Q.; Yu, Y. Oviposition Suitability of Drosophila suzukii (Diptera: Drosophilidae) for Nectarine Varieties and Its Correlation with the Physiological Indexes. Insects 2019, 10, 221. [Google Scholar] [CrossRef]
- Stewart, T.J.; Wang, X.-G.; Molinar, A.; Daane, K.M. Factors Limiting Peach as a Potential Host for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 1771–1779. [Google Scholar] [CrossRef]
- Mazzetto, F.; Pansa, M.G.; Ingegno, B.L.; Tavella, L.; Alma, A. Monitoring of the Exotic Fly Drosophila suzukii in Stone, Pome and Soft Fruit Orchards in NW Italy. J. Asia-Pac. Entomol. 2015, 18, 321–329. [Google Scholar] [CrossRef]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying Host Potentials: Indexing Postharvest Fresh Fruits for Spotted Wing Drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef]
- Kratschmer, S.; Hauer, J.; Zaller, J.G.; Dürr, A.; Weninger, T. Hedgerow Structural Diversity Is Key to Promoting Biodiversity and Ecosystem Services: A Systematic Review of Central European Studies. Basic Appl. Ecol. 2024, 78, 28–38. [Google Scholar] [CrossRef]
- Weißinger, L.; Schrieber, K.; Breuer, M.; Müller, C. Influences of Blackberry Margins on Population Dynamics of Drosophila suzukii and Grape Infestation in Adjacent Vineyards. J. Appl. Entomol. 2019, 143, 802–812. [Google Scholar] [CrossRef]
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple Lines of Evidence for Reproductive Winter Diapause in the Invasive Pest Drosophila suzukii: Useful Clues for Control Strategies. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Grassi, A.; Gottardello, A.; Dalton, D.T.; Tait, G.; Rendon, D.; Ioriatti, C.; Gibeaut, D.; Rossi Stacconi, M.V.; Walton, V.M. Seasonal Reproductive Biology of Drosophila suzukii (Diptera: Drosophilidae) in Temperate Climates. Environ. Entomol. 2017, 47, 166–174. [Google Scholar] [CrossRef]
- Panel, A.; Zeeman, L.; Van Der Sluis, B.; Van Elk, P.; Pannebakker, B.; Wertheim, B.; Helsen, H. Overwintered Drosophila suzukii Are the Main Source for Infestations of the First Fruit Crops of the Season. Insects 2018, 9, 145. [Google Scholar] [CrossRef]
- Zerulla, F.N.; Schmidt, S.; Streitberger, M.; Zebitz, C.P.W.; Zelger, R. On the Overwintering Ability of Drosophila suzukii in South Tyrol. J. Berry Res. 2015, 5, 41–48. [Google Scholar] [CrossRef]
- Tonina, L.; Grassi, A.; Caruso, S.; Mori, N.; Gottardello, A.; Anfora, G.; Giomi, F.; Vaccari, G.; Ioriatti, C. Comparison of Attractants for Monitoring Drosophila suzukii in Sweet Cherry Orchards in Italy. J. Appl. Entomol. 2018, 142, 18–25. [Google Scholar] [CrossRef]
- Grassi, A.; Anfora, G.; Maistri, S.; Maddalena, G.; De Cristofaro, A.; Savini, G.; Ioriatti, C. Development and Efficacy of Droskidrink, a Food Bait for Trapping Drosophila suzukii. IOBC Bull. 2015, 109, 197–204. [Google Scholar]
- Miller, M.E. A review of the Species of Drosophila (Diptera: Drosophilidae) and Genera of Drosophilidae of Northeastern North America. Can. J. Arthropod Identif. 2017, 31, 1–282. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global Potential Distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef]
- Winkler, A.; Jung, J.; Kleinhenz, B.; Racca, P. A Review on Temperature and Humidity Effects on Drosophila suzukii Population Dynamics. Agric. For. Entomol. 2020, 22, 179–192. [Google Scholar] [CrossRef]
- Holland, J.; Fahrig, L. Effect of Woody Borders on Insect Density and Diversity in Crop Fields: A Landscape-Scale Analysis. Agric. Ecosyst. Environ. 2000, 78, 115–122. [Google Scholar] [CrossRef]
- Den Belder, E.; Elderson, J.; Van Den Brink, W.J.; Schelling, G. Effect of Woodlots on Thrips Density in Leek Fields: A Landscape Analysis. Agric. Ecosyst. Environ. 2002, 91, 139–145. [Google Scholar] [CrossRef]
- Basoalto, E.; Miranda, M.; Knight, A.L.; Fuentes-Contreras, E. Landscape Analysis of Adult Codling Moth (Lepidoptera: Tortricidae) Distribution and Dispersal Within Typical Agroecosystems Dominated by Apple Production in Central Chile. Environ. Entomol. 2010, 39, 1399–1408. [Google Scholar] [CrossRef]
- Reeves, R.B.; Greene, J.K.; Reay-Jones, F.P.F.; Toews, M.D.; Gerard, P.D. Effects of Adjacent Habitat on Populations of Stink Bugs (Heteroptera: Pentatomidae) in Cotton as Part of a Variable Agricultural Landscape in South Carolina. Environ. Entomol. 2010, 39, 1420–1427. [Google Scholar] [CrossRef]
- Santoiemma, G.; Trivellato, F.; Caloi, V.; Mori, N.; Marini, L. Habitat Preference of Drosophila suzukii across Heterogeneous Landscapes. J. Pest Sci. 2019, 92, 485–494. [Google Scholar] [CrossRef]
- Enriquez, T.; Colinet, H. Basal Tolerance to Heat and Cold Exposure of the Spotted Wing Drosophila, Drosophila suzukii. PeerJ 2017, 5, e3112. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Tanaka, K.M.; Yew, J.Y.; Takahashi, A. Drosophila suzukii Avoidance of Microbes in Oviposition Choice. R. Soc. Open Sci. 2021, 8, 201601. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Alborn, H.T. Attraction of Pepper Weevil to Volatiles from Damaged Pepper Plants: Pepper Weevil Attraction to Damaged Pepper Plants. Entomol. Exp. Appl. 2011, 138, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacella, M.; Sperandio, G.; Ruschioni, S.; Ramilli, F.; Corsi, L.; Abulebda, A.M.A.; Battistelli, M.C.; Riolo, P. Field-Based Spatiotemporal Dynamics, Ovarian Maturation and Laboratory Oviposition Behavior of Drosophila suzukii in Peach: Key Insights for Integrated Pest Management. Agronomy 2025, 15, 2415. https://doi.org/10.3390/agronomy15102415
Pacella M, Sperandio G, Ruschioni S, Ramilli F, Corsi L, Abulebda AMA, Battistelli MC, Riolo P. Field-Based Spatiotemporal Dynamics, Ovarian Maturation and Laboratory Oviposition Behavior of Drosophila suzukii in Peach: Key Insights for Integrated Pest Management. Agronomy. 2025; 15(10):2415. https://doi.org/10.3390/agronomy15102415
Chicago/Turabian StylePacella, Matteo, Giorgio Sperandio, Sara Ruschioni, Fabio Ramilli, Lorenzo Corsi, Abdalhadi M.A. Abulebda, Maria Chiara Battistelli, and Paola Riolo. 2025. "Field-Based Spatiotemporal Dynamics, Ovarian Maturation and Laboratory Oviposition Behavior of Drosophila suzukii in Peach: Key Insights for Integrated Pest Management" Agronomy 15, no. 10: 2415. https://doi.org/10.3390/agronomy15102415
APA StylePacella, M., Sperandio, G., Ruschioni, S., Ramilli, F., Corsi, L., Abulebda, A. M. A., Battistelli, M. C., & Riolo, P. (2025). Field-Based Spatiotemporal Dynamics, Ovarian Maturation and Laboratory Oviposition Behavior of Drosophila suzukii in Peach: Key Insights for Integrated Pest Management. Agronomy, 15(10), 2415. https://doi.org/10.3390/agronomy15102415









