Effects of Waterlogging at Different Developmental Stages on Growth, Yield and Physiological Responses of Forage Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Description and Research Design
2.2. Plant Measurements
2.3. Anatomical Observation of Roots
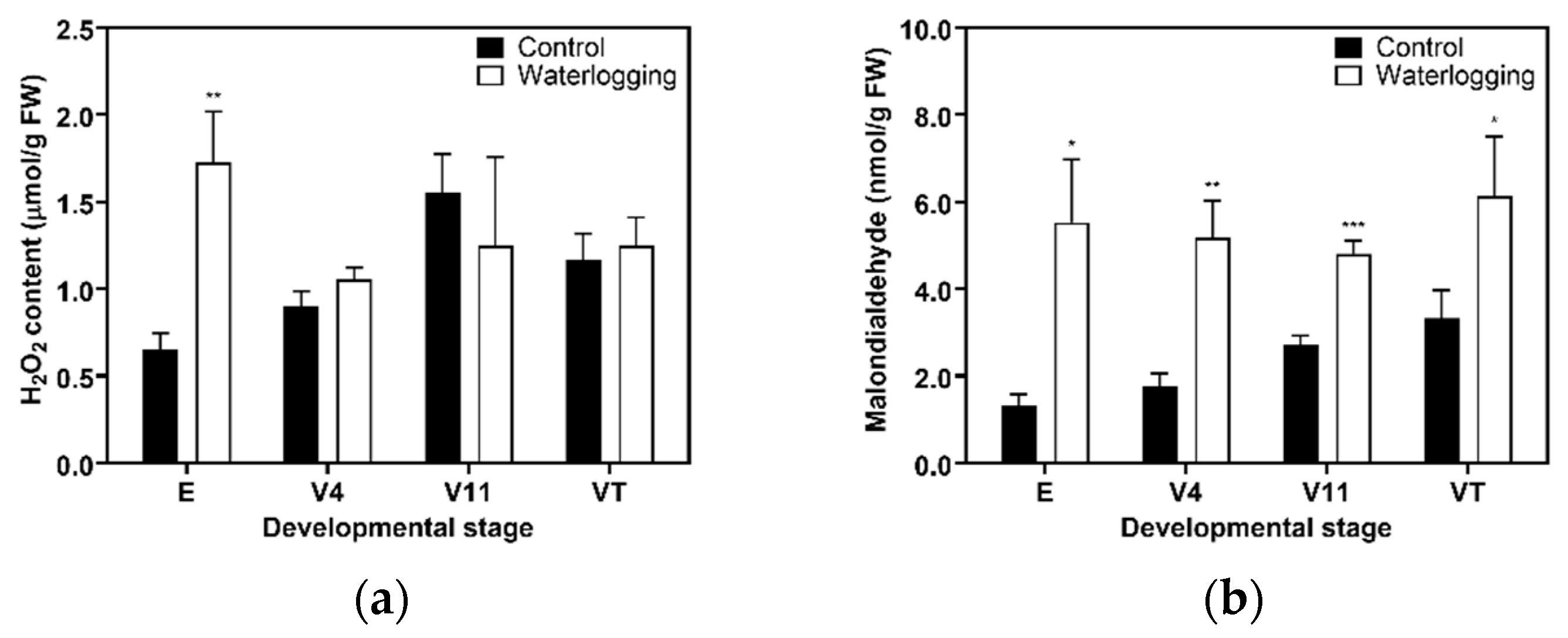
2.4. Root Hydrogen Peroxide (H2O2) and MDA Contents
2.5. RNA Extraction and qRT-PCR Analysis
2.6. Statistical Analysis
3. Results
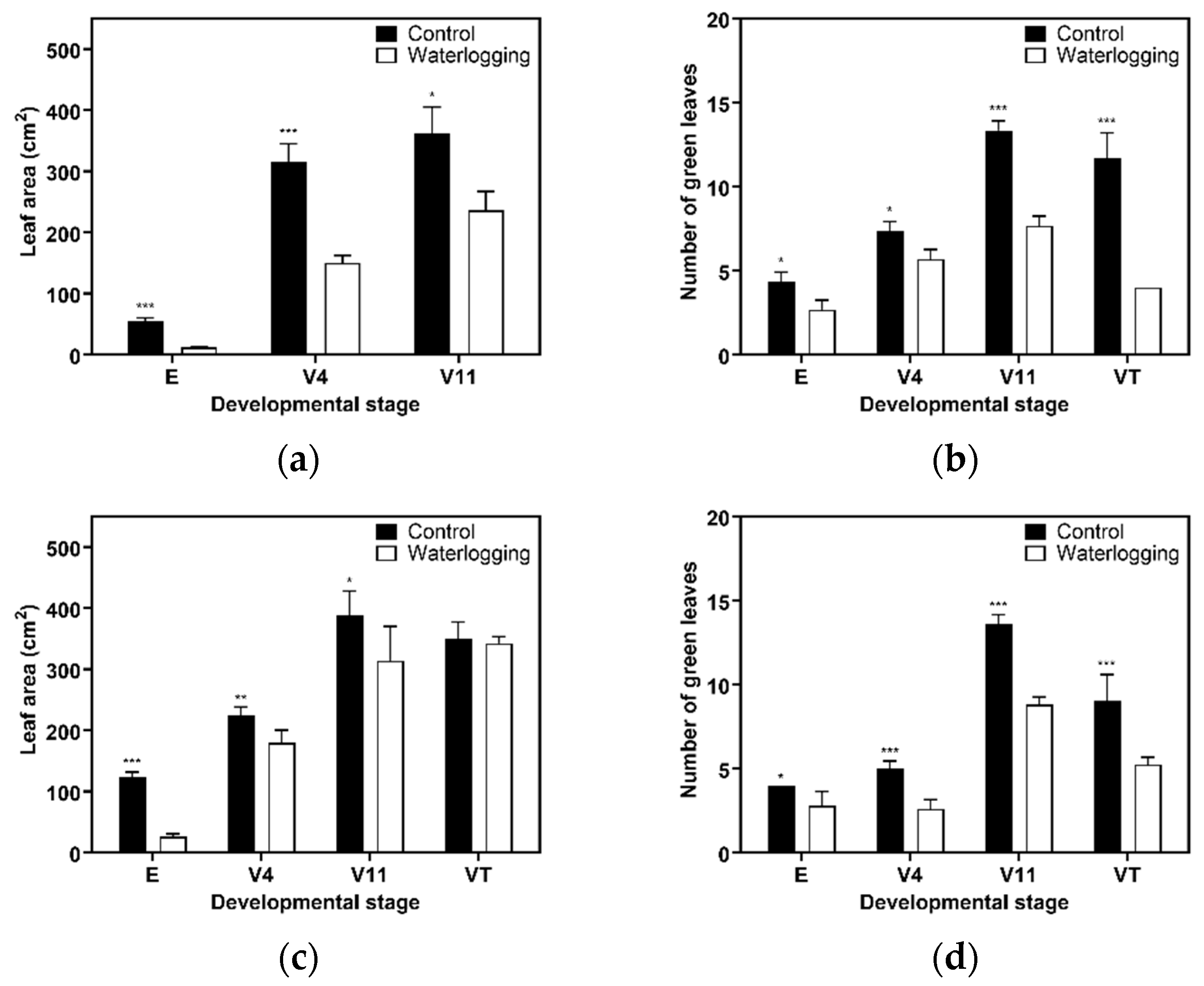
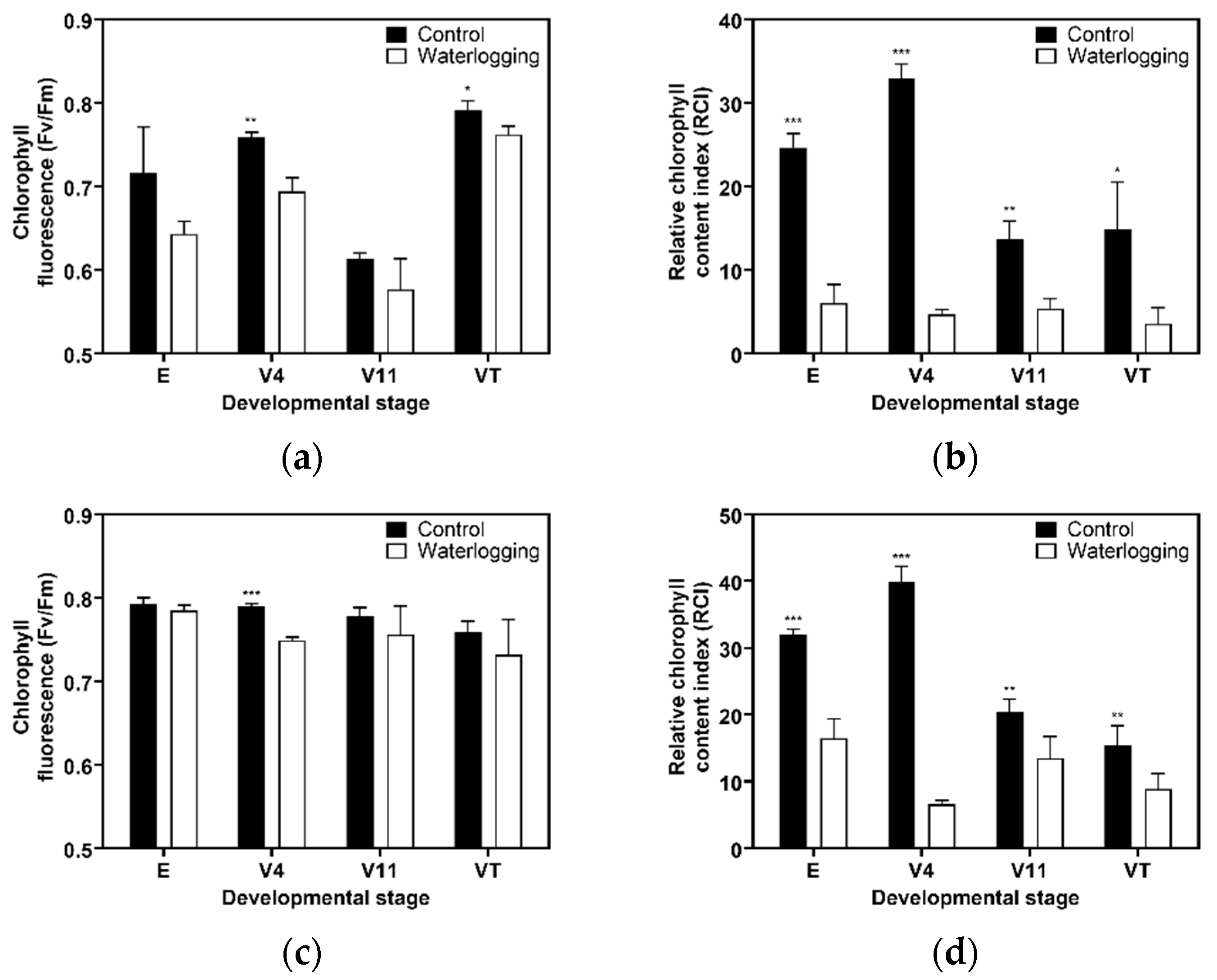
3.1. Growth Dynamics Under Waterlogging
3.2. Aerenchyma Formation and ROS Accumulation in Roots Under Waterlogging
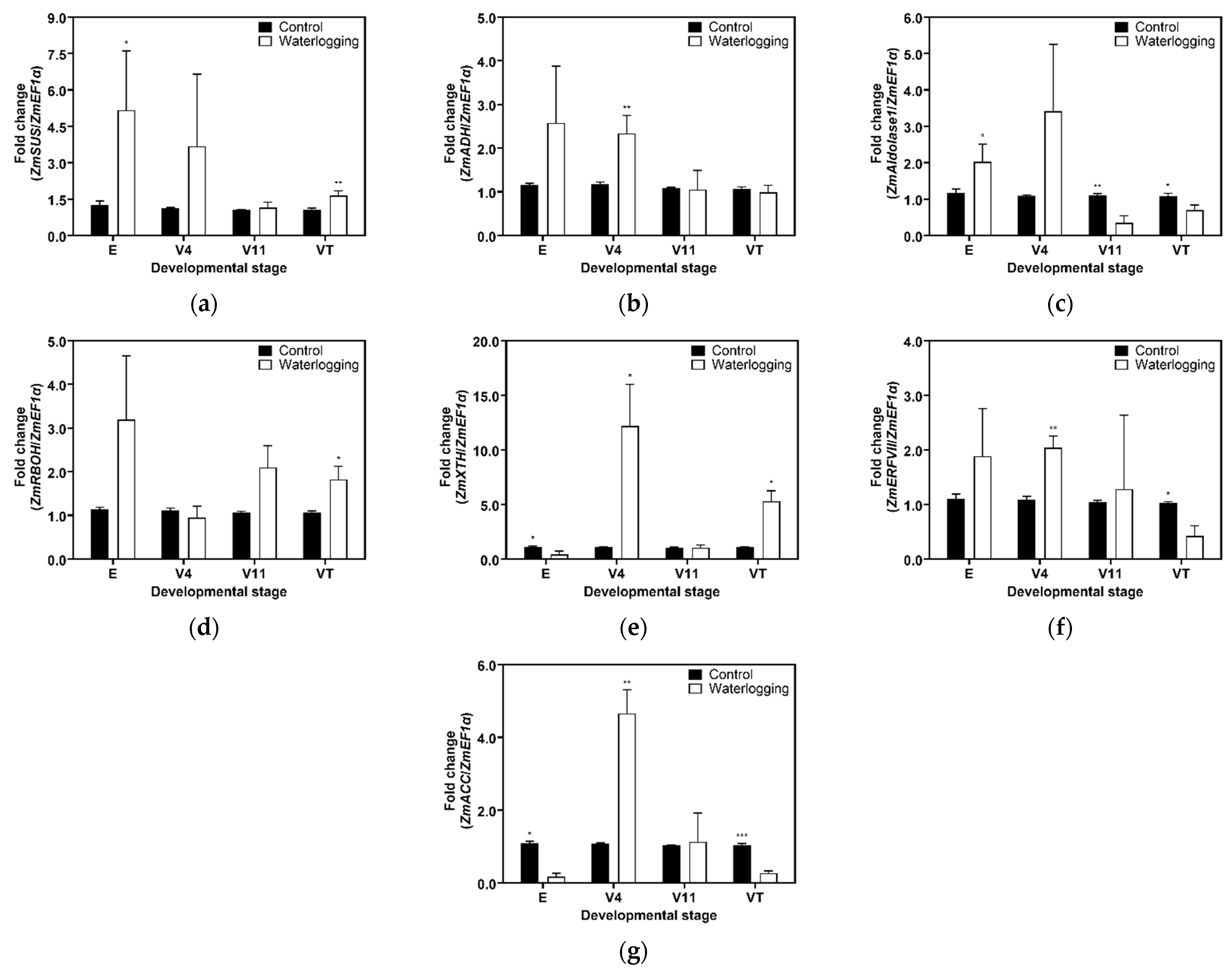
3.3. Expression Analysis of Selected Genes Using Real-Time PCR
3.4. Agronomic Characteristics, Yield, and Forage Quality Change Under Waterlogging
4. Discussion
4.1. Developmental Stage-Specific Sensitivity to 14 Days of Prolonged Waterlogging
4.2. Morpho-Physiological Changes in Forage Maize Roots Under 14 Days of Prolonged Waterlogging at Different Developmental Stages
4.3. Changes in Agronomic Traits at the Harvest Stage Following Prolonged Waterlogging at Different Developmental Stages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acetyl-CoA carboxylase |
| ADF | Acid detergent fiber |
| ADH | Alcohol dehydrogenase |
| CA | Crude ash |
| CF | Crude fat |
| CP | Crude protein |
| Ctrl | Control |
| E | Emergence stage |
| EF1α | Elongation factor 1-alpha |
| ERFVII | Ethylene-response element binding protein |
| Fv/Fm | Chlorophyll fluorescence |
| HSP | Heat shock protein |
| MDA | Malondialdehyde |
| NDF | Neutral detergent fiber |
| NFC | Non-fiber carbohydrate |
| RBOH | Respiratory burst oxidase homolog |
| RCI | Relative chlorophyll content index |
| RFV | Relative feed value |
| ROS | Reactive oxygen species |
| SUS | Sucrose synthase |
| TDNs | Total digestible nutrients |
| V11 | Eleven-leaf stage |
| V4 | Four-leaf stage |
| VT | Tasseling stage |
| WL | Waterlogging |
| XTH | Xyloglucan endotransglycosylase/hydrolase |
| Zm | Zea mays |
References
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2014, 1312, 66–75. [Google Scholar] [CrossRef]
- Amaral-Phillips, D.M.; Roy, N.; Lee, C.; Lehmkuhler, J. Practical Corn Silage Harvest and Storage Guide for Cattle Producers; University of Kentucky Cooperative Extension Service: Lexington, KY, USA, 2023; pp. 1–16. [Google Scholar]
- Karnatam, K.S.; Mythri, B.; Un Nisa, W.; Sharma, H.; Meena, T.K.; Rana, P.; Vikal, Y.; Gowda, M.; Dhillon, B.S.; Sandhu, S. Silage maize as a potent candidate for sustainable animal husbandry development—Perspectives and strategies for genetic enhancement. Front. Genet. 2023, 14, 1150132. [Google Scholar] [CrossRef]
- Allen, M.S.; Coors, J.G.; Roth, G.W. Corn Silage. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 547–608. [Google Scholar]
- Roux, N.; Kaufmann, L.; Bhan, M.; Le Noe, J.; Matej, S.; Laroche, P.; Kastner, T.; Bondeau, A.; Haberl, H.; Erb, K. Embodied HANPP of feed and animal products: Tracing pressure on ecosystems along trilateral livestock supply chains 1986–2013. Sci. Total Environ. 2022, 851, 158198. [Google Scholar] [CrossRef]
- MAFRA. Guidelines for Implementing Agriculture, Food and Rural Affairs Businesses in 2024; Ministry of Agriculture, Food and Rural Affairs: Sejong, South Korea, 2024; pp. 66–116. [Google Scholar]
- Ji, H.C.; Kim, W.H.; Kim, K.Y.; Lee, S.H.; Yoon, S.H.; Lim, Y.C. Effect of different drained conditions on growth, forage production and quality of silage corn at paddy field. J. Korean Soc. Grassl. Forage Sci. 2009, 29, 329–336. [Google Scholar] [CrossRef]
- Kim, L.Y.; Jo, I.S.; Um, K.T.; Min, H.S. Changes of soil characteristics and crop productivity by the paddy-upland rotation system. 1. Changes of soil physical properties. Res. Rept. RDA (S&F) 1990, 32, 1–7. [Google Scholar]
- Nakano, K. Changes in soil physical properties of clayey soil by conversion from ill-drained paddy field into upland field. Bull. Hokuriku Natl. Agric. Exp. Stn. 1978, 21, 63–94. [Google Scholar]
- Ahmed, I.; Ullah, A.; ur Rahman, M.H.; Ahmad, B.; Wajid, S.A.; Ahmad, A.; Ahmed, S. Climate change impacts and adaptation strategies for agronomic crops. In Climate Change and Agriculture; Hussain, S., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Xu, H.; Twine, T.E.; Girvetz, E. Climate change and maize yield in Iowa. PLoS ONE 2016, 11, e0156083. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Iizumi, T.; Hosokawa, N.; Tanoue, M.; Hirabayashi, Y. Flood impacts on global crop production: Advances and limitations. Environ. Res. Lett. 2023, 18, 054007. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Exploring the potential of multiomics and other integrative approaches for improving waterlogging tolerance in plants. Plants 2023, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.G.; Fujinami, H. Recent decadal enhancement of Meiyu–Baiu heavy rainfall over East Asia. Sci. Rep. 2021, 11, 13665. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Perera, E.D.P.; Kudo, S.; Miyamoto, M.; Yamazaki, Y.; Kuribayashi, D.; Sawano, H.; Sayama, T.; Magome, J.; Hasegawa, A.; et al. Assessing flood disaster impacts in agriculture under climate change in the river basins of Southeast Asia. Nat. Hazards 2019, 97, 157–192. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Greenway, H.; Armstrong, W.; Colmer, T.D. Conditions leading to high CO2 (>5 kPa) in waterlogged–flooded soils and possible effects on root growth and metabolism. Ann. Bot. 2006, 98, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Kopyra, M.; Gwóźdź, E.A. The role of nitric oxide in plant growth regulation and responses to abiotic stresses. Acta Physiol. Plant. 2004, 26, 459–473. [Google Scholar] [CrossRef]
- Sasidharan, R.; Bailey-Serres, J.; Ashikari, M.; Atwell, B.J.; Colmer, T.D.; Fagerstedt, K.; Fukao, T.; Geigenberger, P.; Hebelstrup, K.H.; Hill, R.D.; et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017, 214, 1403–1407. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: Separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef]
- Hossain, M.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop Sci. 2011, 5, 1094–1101. [Google Scholar]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant. 2009, 53, 493–504. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma formation in plants. In Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia; van Dongen, J.T., Licausi, F., Eds.; Springer: Vienna, Austria, 2014; pp. 247–265. [Google Scholar] [CrossRef]
- Arora, K.; Panda, K.K.; Mittal, S.; Mallikarjuna, M.G.; Rao, A.R.; Dash, P.K.; Thirunavukkarasu, N. RNAseq revealed the important gene pathways controlling adaptive mechanisms under waterlogged stress in maize. Sci. Rep. 2017, 7, 10950. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.S.; Kim, J.-T.; Bae, H.H.; Son, B.-Y.; Yi, G.; Ha, J.Y.; Kim, S.-L.; Baek, S.-B. Analysis of growth response and gene expression by waterlogging stress on B73 maize. Korean J. Crop Sci. 2020, 65, 104–112. [Google Scholar] [CrossRef]
- Zhou, X.; Han, H.; Li, C.; Guo, S.; Guo, D.; Cheng, J. Physiological characters and yield formation of corn (Zea mays L.) under waterlogging stress in jointing stage. Trans. Chin. Soc. Agric. Eng. 2014, 30, 119–125. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Singh, N.N. Effect of waterlogging on growth, biochemical compositions and reproduction in maize (Zea mays L.). J. Plant Biol. 2001, 28, 61–70. [Google Scholar]
- Huang, C.; Gao, Y.; Qin, A.; Liu, Z.; Zhao, B.; Ning, D.; Ma, S.; Duan, A.; Liu, Z. Effects of waterlogging at different stages and durations on maize growth and grain yields. Agric. Water Manag. 2022, 261, 107334. [Google Scholar] [CrossRef]
- Jung, J.S.; Choi, G.-J.; Choi, B.-R. Effect of waterlogging duration on growth characteristics and productivity of forage corn at different growth stages under paddy field conditions. J. Korean Soc. Grassl. Forage Sci. 2019, 39, 141–147. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops. Special Report No. 48; Iowa State University of Science and Technology, Cooperative Extension Services: Ames, IA, USA, 1986; pp. 1–21. [Google Scholar]
- Hatfield, J.L.; Stanley, C.D.; Carlson, R.E. Evaluation of an electronic foliometer to measure leaf area in corn and soybeans. Agron. J. 1976, 68, 434–436. [Google Scholar] [CrossRef]
- Asha, S.N.; Sultana, N.; Hassan, L.; Akhter, S.; Robin, A.H.K. Response of morphological and biochemical traits of maize genotypes under waterlogging stress. J. Phytol. 2021, 13, 108–121. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis: Apparatus, Reagents, Procedures and some Applications. Agriculture Handbook No. 379; ARS, USDA: Washington, DC, USA, 1970. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; The National Academies Press: Washington, DC, USA, 2001; p. 405. [Google Scholar]
- Menke, K.H.; Huss, W. Tierernährung und Futtermittelkunde; Verlag Eugen: Ulmer, Germany, 1980. [Google Scholar]
- Holland, C.; Kezar, W.; Kautz, W.P.; Lazowski, E.J.; Mahanna, W.C.; Reinhart, R. Pioneer Forage Manual: A Nutritional Guide; Pioneer Hi-Bred International, Inc.: Des Moines, IA, USA, 1990. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Mano, Y.; Omori, F.; Takamizo, T.; Kindiger, B.; Bird, R.M.; Loaisiga, C.H. Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 2006, 281, 269–279. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Fixation, embedding, and sectioning of plant tissues. Cold Spring Harb. Protoc. 2008, 3, pdb.prot4941. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Hong, C.-Y.; Liu, L.-F.; Kao, C.H. Relative importance of Na+ and Cl− in NaCl-induced antioxidant systems in roots of rice seedlings. Physiol. Plant. 2004, 122, 86–94. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, C.; Lan, H.; Gao, S.; Liu, H.; Liu, J.; Cao, M.; Pan, G.; Rong, T.; Zhang, S. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 2014, 9, e95445. [Google Scholar] [CrossRef]
- Thomas, T.; Purushothaman, J.; Janarthanan, R.; Anusuya, N.; Medisetti, P.G.; Karthick, J.; Nadaradjan, S.; Thirumeni, S. Identification of rice genotypes for seedling stage multiple abiotic stress tolerance. Plant Physiol. Rep. 2020, 25, 697–706. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. Chapter 10—Senescence and crop performance. In Crop Physiology, 2nd ed.; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 223–249. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Tian, L.-X.; Zhang, Y.-C.; Chen, P.-L.; Zhang, F.-F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.-L. How does the waterlogging regime affect crop yield? a global meta-analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- Pociecha, E.; Kościelniak, J.; Filek, W. Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor). Acta Physiol. Plant. 2008, 30, 529–535. [Google Scholar] [CrossRef]
- Zhu, M.; Li, F.H.; Shi, Z.S. Morphological and photosynthetic response of waxy corn inbred line to waterlogging. Photosynthetica 2016, 54, 636–640. [Google Scholar] [CrossRef]
- Ren, B.; Yu, W.; Liu, P.; Zhao, B.; Zhang, J. Responses of photosynthetic characteristics and leaf senescence in summer maize to simultaneous stresses of waterlogging and shading. Crop J. 2023, 11, 269–277. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, S.G.; Lee, J.S.; Go, T.-H.; Shon, J.; Kang, S.; Lee, J.-S.; Bae, H.H.; Son, B.-Y.; Shim, K.-B.; et al. Impact of the consecutive days of visible wilting on growth and yield during tassel initiation in maize (Zea mays L.). J. Crop Sci. Biotechnol. 2016, 18, 219–229. [Google Scholar] [CrossRef]
- Kaur, G.; Zurweller, B.; Motavalli, P.P.; Nelson, K.A. Screening corn hybrids for soil waterlogging tolerance at an early growth stage. Agriculture 2019, 9, 33. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Rafique, S.; Rai, P.K.; Singh, N.N.; Srinivasan, G. Tolerance to excess moisture in maize (Zea mays L.): Susceptible crop stages and identification of tolerant genotypes. Field Crops Res. 2004, 90, 189–202. [Google Scholar] [CrossRef]
- Liu, M.Y.; Sun, J.; Wang, K.Y.; Liu, D.; Li, Z.Y.; Zhang, J. Spermidine enhances waterlogging tolerance via regulation of antioxidant defence, heat shock protein expression and plasma membrane H+-ATPase activity in Zea mays. J. Agron. Crop Sci. 2014, 200, 199–211. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Niu, Q.; Qiu, H.; Tian, G.; Lu, H.; Zhou, X. Effects of combined high temperature and waterlogging stress at booting stage on root anatomy of rice (Oryza sativa L.). Water 2020, 12, 2524. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2008; pp. 286–287. [Google Scholar]
- Lee, H.Y.; Yoon, G.M. Regulation of ethylene biosynthesis by phytohormones in etiolated rice (Oryza sativa L.) seedlings. Mol. Cells 2018, 41, 311–319. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Niu, S.; Cui, D. The genetic relationship among plant-height traits found using multiple-trait QTL mapping of a dent corn and popcorn cross. Genome 2007, 50, 357–364. [Google Scholar] [CrossRef]
- Popović, A.; Kravić, N.; Branković-Radojčić, D.; Golijan, J.; Mladenović, M.; Vančetović, J.; Babić, V. Variability in ratio between ear and plant height among maize top cross hybrids. Serb. Assoc. Plant Breed. Seed Prod. 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Azrai, M.; Efendi, R.; Muliadi, A.; Aqil, M.; Suwarti; Zainuddin, B.; Syam, A.; Junaedi; Syah, U.T.; Dermail, A.; et al. Genotype by environment interaction on tropical maize hybrids under normal irrigation and waterlogging conditions. Front. Sustain. Food Syst. 2022, 6, 913211. [Google Scholar] [CrossRef]
- Daku, E.K.; Salack, S.; Worou, O.N.; Ogunjobi, K. Maize response to temporary floods under ambient on-farm conditions of the West African Sahel. Environ. Res. Commun. 2022, 4, 045004. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, C.; Song, X.; Jiang, Y. Interactive short-term effects of waterlogging and salinity stress on growth and carbohydrate, lipid peroxidation, and nutrients in two Perennial ryegrass cultivars. J. Am. Soc. Hortic. Sci. 2017, 142, 110–118. [Google Scholar] [CrossRef]
- Agualongo, D.A.P.; Da-Silva, C.J.; Garcia, N.; de Oliveira, F.K.; Shimoia, E.P.; Posso, D.A.; de Oliveira, A.C.B.; de Oliveira, D.d.S.C.; do Amarante, L. Waterlogging priming alleviates the oxidative damage, carbohydrate consumption, and yield loss in soybean (Glycine max) plants exposed to waterlogging. Funct. Plant Biol. 2022, 49, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.q.; Meng, J.l.; Zeng, B.; Wu, M. Improved flooding tolerance and carbohydrate status of flood-tolerant plant Arundinella anomala at lower water temperature. PLoS ONE 2018, 13, e0192608. [Google Scholar] [CrossRef]


| Gene | Gene ID | Accession | Primer Sequence (5’–3’) | Amplicon Size (bp) |
|---|---|---|---|---|
| Sucrose synthase | GRMZM2G152908 | NM_001111853 | F: AGCTCCTGTACAGCCAAACC R: CCCGTTCAGGTTGTACTGCT | 266 |
| Aldolase | GRMZM2G057823 | NM_001111866.2 | F: GGAGAATGGTCTGGTGCCAA R: ACCTCAGGGGTCACCTTCTT | 213 |
| Alcohol dehydrogenase (ADH) | GRMZM2G152981 | NM_001146840.1 | F: TGTGTGGAACCTACCGTGTG R: CGAGTCCAAACACTGCAACG | 298 |
| Respiratory burst oxidase homolog (RBOH) | GRMZM2G034896 | NM_001322022.1 | F: AAGGTTGCAGTGTATCCGGG R: ACGTGAAGTCCAATCACCCC | 201 |
| Xyloglucan endotransglycosylase/hydrolase (XTH) | GRMZM2G060837 | NM_001137402.1 | F: TACGGCAATGGCAGCACTAG R: TTGACCTTGTACTTGCCCCC | 254 |
| Ethylene-response element binding protein (ERF VII) | GRMZM2G018398 | NM_001155219.2 | F: CCATTGCTCCCATTCCCACT R: TCGAAGGTCCAGAGGTCCAT | 250 |
| Acetyl-CoA carboxylase (ACC) | GRMZM5G858094 | NM_001111903.1 | F: GCTGCTTAGACGCGATACCT R: ATATTGGGGAGCCAGGGACT | 213 |
| Elongation factor 1-alpha | GRMZM2G153541 | NM_001112117 | F: TGGGCTACTGGTCTTACTACTGA R: ACATACCCACGCTTCAGATCCT | 135 [46] |
| Year | Treatment 1 | Stem Height (cm) | Ear Height (cm) | Stem Diameter (mm) | Dry Matter (g/plant) | |
|---|---|---|---|---|---|---|
| Total | Ear | |||||
| 2022 | Ctrl | 229.5 ± 10.6 a | 95.5 ± 7.78 a | 21.35 ± 0.49 a | 234.80 ± 43.84 a | 80.55 ± 27.51 a |
| E | 198.3 ± 11.0 b | 82.0 ± 8.7 b | 18.10 ± 2.07 ab | 179.87 ± 29.45 ab | 34.67 ± 8.04 b | |
| V4 | 192.3 ± 4.1 b | 99.3 ± 4.1 a | 15.12 ± 3.11 b | 139.07 ± 18.23 b | 19.9 ± 4.64 b | |
| V11 | 184.5 ± 1.5 b | 100.5 ± 3.5 a | 21.45 ± 0.05 a | 157.00 ± 6.00 b | 17.15 ± 1.45 b | |
| VT | 231.3 ± 6.7 a | 102.7 ± 2.3 a | 21.10 ± 0.96 a | 156.7 ± 10.70 b | 3.87 ± 3.03 b | |
| 2023 | Ctrl | 219.8 ± 5.8 ab | 100.3 ± 8.5 a | 22.84 ± 2.13 a | 367.76 ± 76.87 a | 113.78 ± 1.2 a |
| E | 213.8 ± 14.1 b | 87.8 ± 10.0 b | 15.74 ± 1.14 b | 161.98 ± 23.52 b | 30.27 ± 2.69 c | |
| V4 | 198.2 ± 26.8 b | 83.2 ± 9.2 b | 14.96 ± 1.28 b | 151.76 ± 32.58 b | 21.43 ± 6.59 c | |
| V11 | 196.2 ± 22.9 b | 99.5 ± 5.4 a | 22.28 ± 0.59 a | 157.24 ± 16.77 b | 1.24 ± 0.75 d | |
| VT | 238.6 ± 10.5 a | 109.4 ± 5.5 a | 21.20 ± 1.17 a | 189.69 ± 11.38 b | 54.08 ± 13.62 b | |
| Year | Treat 1 | CP 2 (%) | CF 2 (%) | CA 2 (%) | ADF 2 (%) | NDF 2 (%) | NFC (%) | TDN 2 (%) | RFV 2 |
|---|---|---|---|---|---|---|---|---|---|
| 2022 | Ctrl | 4.90 ± 2.16 a | 1.60 ± 0.41 a | 4.21 ± 0.42 b | 37.74 ± 2.30 c | 62.46 ± 3.25 c | 26.8 ± 1.16 a | 59.09 ± 1.81 a | 88.87 ± 7.34 a |
| E | 3.54 ± 0.18 a | 1.12 ± 0.06 b | 4.61 ± 0.42 b | 41.74 ± 1.40 ab | 67.30 ± 1.10 ab | 23.4 ± 1.69 b | 55.93 ± 1.10 bc | 77.97 ± 2.78 bc | |
| V4 | 4.25 ± 0.55 a | 1.04 ± 0.15 b | 5.75 ± 0.4 a | 40.51 ± 0.58 ab | 64.93 ± 0.24 bc | 24.0 ± 0.34 b | 56.90 ± 0.46 bc | 82.15 ± 0.52 ab | |
| V11 | 4.22 ± 0.79 a | 1.38 ± 0.34 ab | 4.62 ± 0.99 b | 39.83 ± 0.72 bc | 67.01 ± 2.02 ab | 22.8 ± 2.41 b | 57.44 ± 0.57 ab | 80.40 ± 2.91 bc | |
| VT | 4.59 ± 0.87 a | 1.02 ± 0.06 b | 4.87 ± 0.5 ab | 42.99 ± 1.23 a | 70.05 ± 1.60 a | 19.5 ± 1.07 c | 54.94 ± 0.97 c | 73.63 ± 2.70 c | |
| 2023 | Ctrl | 3.84 ± 0.13 b | 1.92 ± 0.44 a | 2.88 ± 0.35 b | 37.34 ± 1.66 b | 64.61 ± 2.13 b | 26.7 ± 3.27 a | 59.40 ± 1.31 a | 86.21 ± 4.78 a |
| E | 3.49 ± 0.12 b | 1.34 ± 0.11 b | 3.32 ± 0.25 b | 38.81 ± 0.48 b | 63.52 ± 0.73 b | 27.2 ± 0.77 a | 58.24 ± 0.38 a | 85.93 ± 0.64 a | |
| V4 | 3.47 ± 0.30 b | 1.62 ± 0.14 ab | 3.43 ± 0.29 b | 39.17 ± 0.78 b | 64.68 ± 0.75 b | 26.8 ± 0.96 a | 57.95 ± 0.61 a | 83.98 ± 1.65 a | |
| V11 | 5.06 ± 0.12 a | 1.52 ± 0.07 ab | 4.4 ± 0.36 a | 43.50 ± 1.41 a | 70.48 ± 2.55 a | 18.6 ± 3.01 b | 54.53 ± 1.11 b | 72.70 ± 4.08 b | |
| VT | 5.04 ± 0.52 a | 1.86 ± 0.08 a | 3.12 ± 0.19 b | 44.65 ± 1.14 a | 69.67 ± 0.10 a | 18.4 ± 1.33 b | 53.63 ± 0.90 b | 72.25 ± 1.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, C.-W.; Yoon, I.-K.; Kim, M.-J.; Jung, J.-S.; Rahman, M.A.; Lee, B.-H. Effects of Waterlogging at Different Developmental Stages on Growth, Yield and Physiological Responses of Forage Maize. Agronomy 2025, 15, 2389. https://doi.org/10.3390/agronomy15102389
Min C-W, Yoon I-K, Kim M-J, Jung J-S, Rahman MA, Lee B-H. Effects of Waterlogging at Different Developmental Stages on Growth, Yield and Physiological Responses of Forage Maize. Agronomy. 2025; 15(10):2389. https://doi.org/10.3390/agronomy15102389
Chicago/Turabian StyleMin, Chang-Woo, Il-Kyu Yoon, Min-Jun Kim, Jeong-Sung Jung, Md Atikur Rahman, and Byung-Hyun Lee. 2025. "Effects of Waterlogging at Different Developmental Stages on Growth, Yield and Physiological Responses of Forage Maize" Agronomy 15, no. 10: 2389. https://doi.org/10.3390/agronomy15102389
APA StyleMin, C.-W., Yoon, I.-K., Kim, M.-J., Jung, J.-S., Rahman, M. A., & Lee, B.-H. (2025). Effects of Waterlogging at Different Developmental Stages on Growth, Yield and Physiological Responses of Forage Maize. Agronomy, 15(10), 2389. https://doi.org/10.3390/agronomy15102389







