Chemical Composition and Insecticidal Activity of Eschweilera jefensis Organic Extracts Against Aphis gossypii
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts
2.3. Bioassay
2.4. GC-MS-Based Metabolomic Analysis of the E. jefensis Extracts
2.5. Molecular Networking
2.6. Heatmap of the Compounds Identified in the Leaf and Stem Extracts of Eschweilera jefensis
3. Results
3.1. Insecticidal Activity
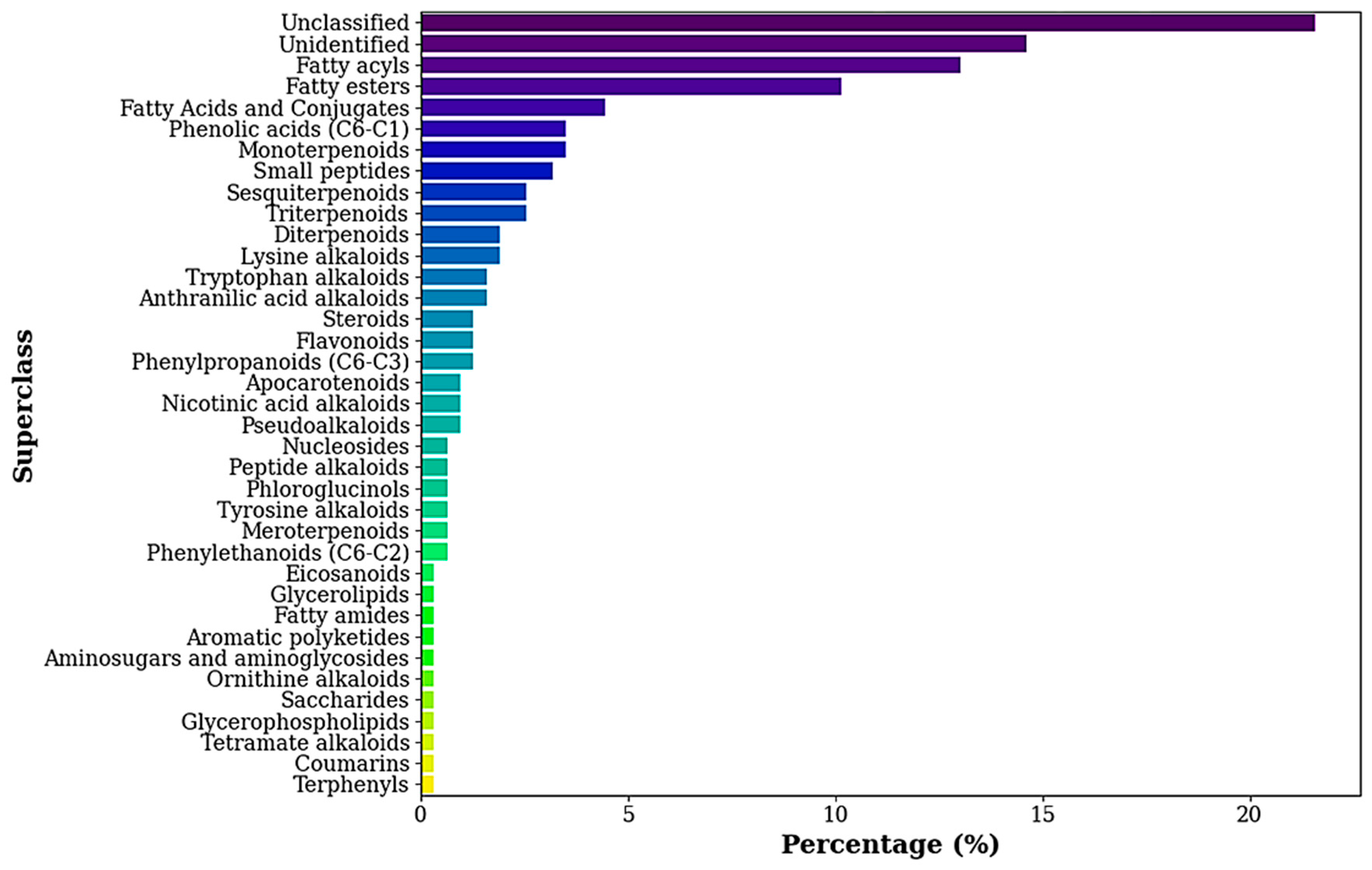
3.2. GC-MS-Based Metabolomic Analysis
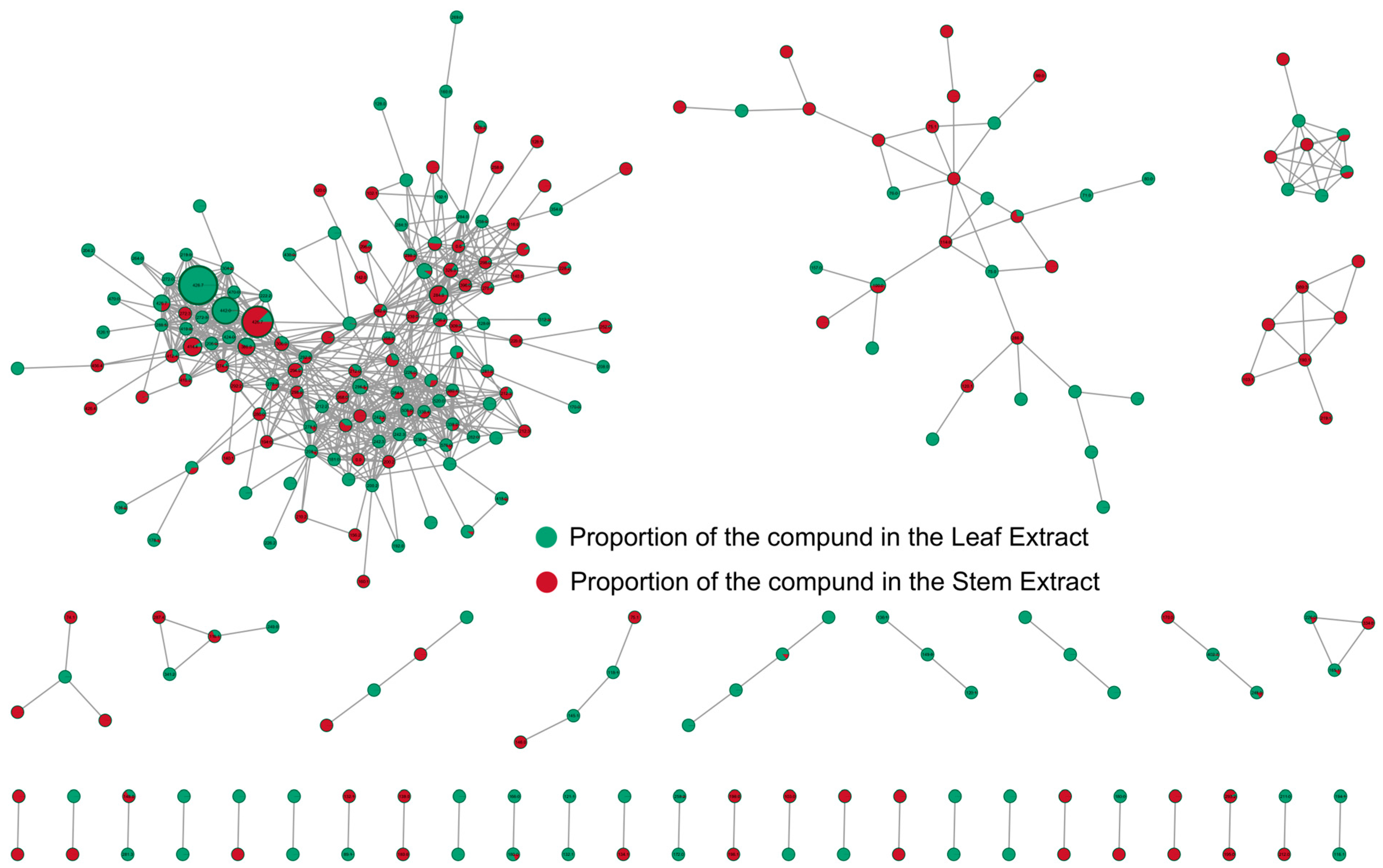
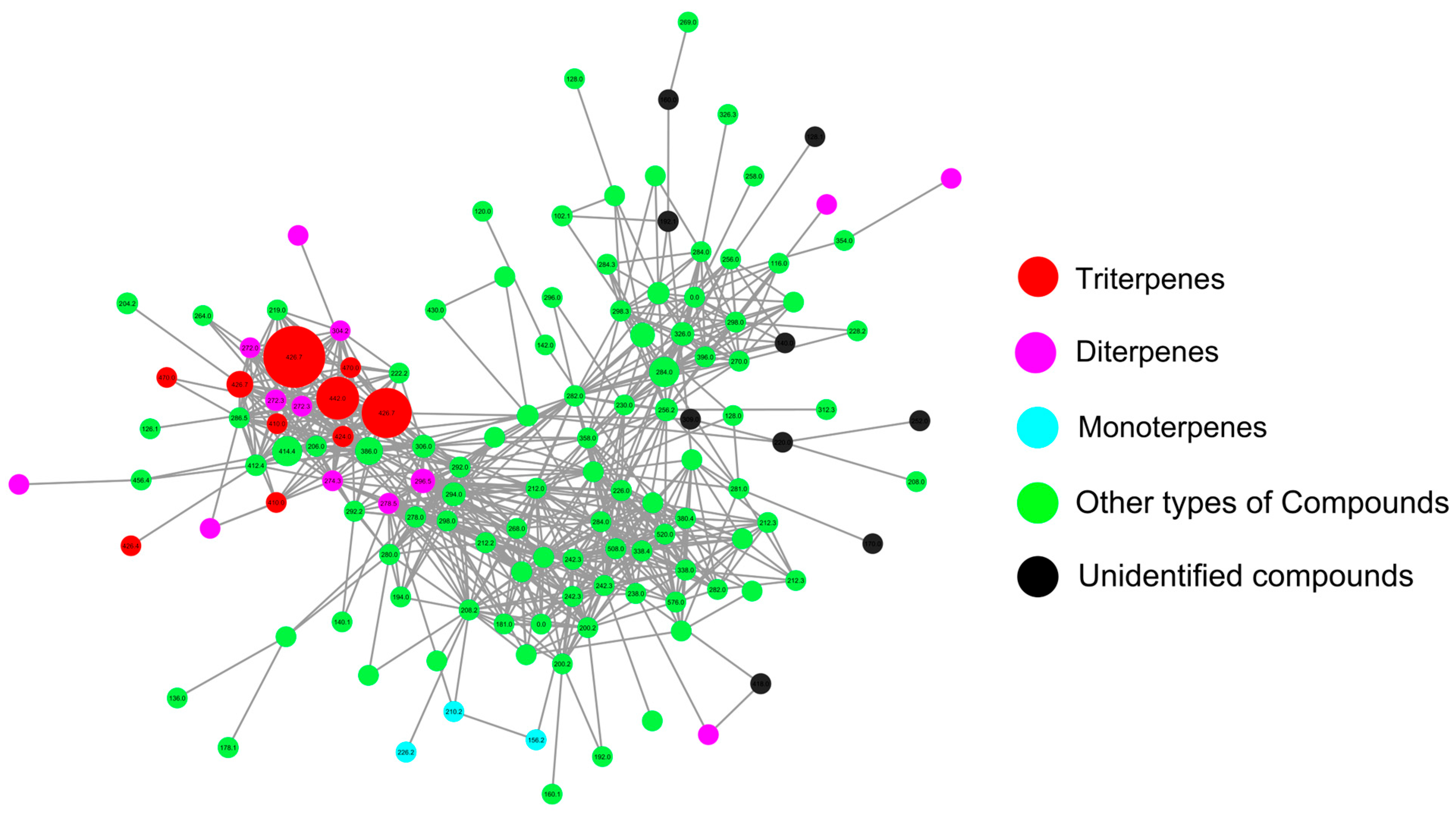
3.3. Molecular Networking
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIST20 | National Institute of Standards and Technology Database 20 |
| GC-MS | Gas chromatography–mass spectrometry |
| GNPS | Global Natural Product Social Molecular Networking |
References
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical Pesticides for an Eco-Friendly and Sustainable Agriculture: New Challenges and Prospects. In Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2022; pp. 69–96. [Google Scholar] [CrossRef]
- Jiang, C.-J.; Sun, Y.; Xu, S.; Liu, X.; Xie, X. From Waste to Weapon: The Potential of Medicinal Plant Waste Extracts for Eco-Friendly Crop Disease Management. Front. Sustain. Food Syst. 2025, 9, 1556604. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Chérigo, L.; Fernández, J.; Martínez, R.; Martínez-Luis, S. Metabolomic Analysis Uncovers the Presence of Pimarenyl Cation-Derived Diterpenes as Insecticidal Constituents of Sphagneticola trilobata. Plants 2025, 14, 2219. [Google Scholar] [CrossRef]
- Batista, G.E.; Mori, S.A.; Harrison, J.S. New Species of Eschweilera and a First Record of Cariniana (Lecythidaceae) from Panama. Phytoneuron 2017, 62, 1–16. [Google Scholar]
- Wang, Z.-J.; Liang, C.-R.; Shang, Z.-Y.; Yu, Q.-T.; Xue, C.-B. Insecticide Resistance and Resistance Mechanisms in the Melon Aphid, Aphis gossypii, in Shandong, China. Pestic. Biochem. Physiol. 2021, 172, 104768. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Ahmed, M.; Peiwen, Q.; Gu, Z.; Liu, Y.; Sikandar, A.; Hussain, D.; Javeed, A.; Shafi, J.; Iqbal, M.F.; An, R.; et al. Insecticidal Activity and Biochemical Composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi Extracts against Cabbage Aphid (Brevicoryne brassicae L.). Sci. Rep. 2020, 10, 522. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Cherigo, L.; Liao-Luo, J.; Fernández, J.; Martínez-Luis, S. Isolation of Alpha-Glucosidase Inhibitors from the Panamanian Mangrove Plant Mora oleifera (Triana Ex Hemsl.) Ducke. Pharmaceuticals 2024, 17, 890. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Laponogov, I.; Zhang, Z.; Doran, S.L.F.; Belluomo, I.; Veselkov, D.; Bittremieux, W.; Nothias, L.F.; Nothias-Esposito, M.; Maloney, K.N.; et al. Auto-Deconvolution and Molecular Networking of Gas Chromatography–Mass Spectrometry Data. Nat. Biotechnol. 2021, 39, 169–173. [Google Scholar] [CrossRef]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.-F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef]
- Carneiro, T.; Medeiros Da Nobrega, R.V.; Nepomuceno, T.; Bian, G.-B.; De Albuquerque, V.H.C.; Filho, P.P.R. Performance Analysis of Google Colaboratory as a Tool for Accelerating Deep Learning Applications. IEEE Access 2018, 6, 61677–61685. [Google Scholar] [CrossRef]
- Phillips, M.A.; Croteau, R.B. Resin-Based Defenses in Conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef]
- Trapp, S.; Croteau, R. Defensive Resin Biosynthesis in Conifers. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef]
- Budiman, A.; Hafidz, N.P.M.; Azzahra, R.S.S.; Amaliah, S.; Sitinjak, F.Y.; Rusdin, A.; Subra, L.; Aulifa, D.L. Advancing the Physicochemical Properties and Therapeutic Potential of Plant Extracts Through Amorphous Solid Dispersion Systems. Polymers 2024, 16, 3489. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Kory, N. Principles and Functions of Metabolic Compartmentalization. Nat. Metab. 2022, 4, 1232–1244. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products a Biosynthetic Approach, 3rd ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Baskar, K.; Duraipandiyan, V.; Ignacimuthu, S. Bioefficacy of the Triterpenoid Friedelin against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2014, 70, 1877–1883. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, X.; Cao, K.; Zhu, G.; Fang, W.; Chen, C.; Wu, J.; Guo, J.; Xu, Q.; et al. Betulin, Synthesized ByPpCYP716A1, Is a Key Endogenous Defensive Metabolite of Peach against Aphids. J. Agric. Food Chem. 2022, 70, 12865–12877. [Google Scholar] [CrossRef]
- Wang, J.; Klakong, M.; Zhu, Q.; Pan, J.; Duan, Y.; Wang, L.; Li, Y.; Dang, J.; Jing, D.; Zhou, H. An Aphid-Resistant Plant Metabolite as a Candidate Aphicide: Insight into the Bioactivity and Action Mode of Betulin against Aphids. eLife 2025, 14, RP107598. [Google Scholar] [CrossRef]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-Based Plant Defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef]
- Hodges, J.D.; Elam, W.W.; Watson, W.F.; Nebeker, T.E. Oleoresin Characteristics and Susceptibility of Four Southern Pines to Southern Pine Beetle (Coleoptera: Scolytidae) Attacks. Can. Entomol. 1979, 111, 889–896. [Google Scholar] [CrossRef]
- Xie, Y.; Isman, M.B.; Feng, Y.; Wong, A. Diterpene Resin Acids: Major Active Principles in Tall Oil against Variegated Cutworm, Peridroma saucia (Lepidoptera: Noctuidae). J. Chem. Ecol. 1993, 19, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M. Unlocking the Genetic Potential: Strategies for Enhancing Secondary Metabolite Biosynthesis in Plants. J. Saudi Soc. Agric. Sci. 2024, 23, 542–554. [Google Scholar] [CrossRef]
| Plant Extract | Time (h) | LC50 (μg/L) | 95% F.L. | Slope ± SE | χ2 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Stem | 24 | 66.5 | 27.63 | 332.7 | 1.63 ± 0.23 | 0.16 |
| 48 | 36.8 | 23.26 | 109.16 | 1.24 ± 0.27 | 1.10 | |
| 72 | 31.0 | 18.61 | 87.35 | 0.99 ± 0.22 | 0.88 | |
| Leaves | 24 | 37.3 | 16.27 | 195.94 | 0.43 ± 0.14 | 0.99 |
| 48 | 28.4 | 19.21 | 90.14 | 1.03 ± 0.22 | 0.91 | |
| 72 | >25 * | * | * | * | * | |
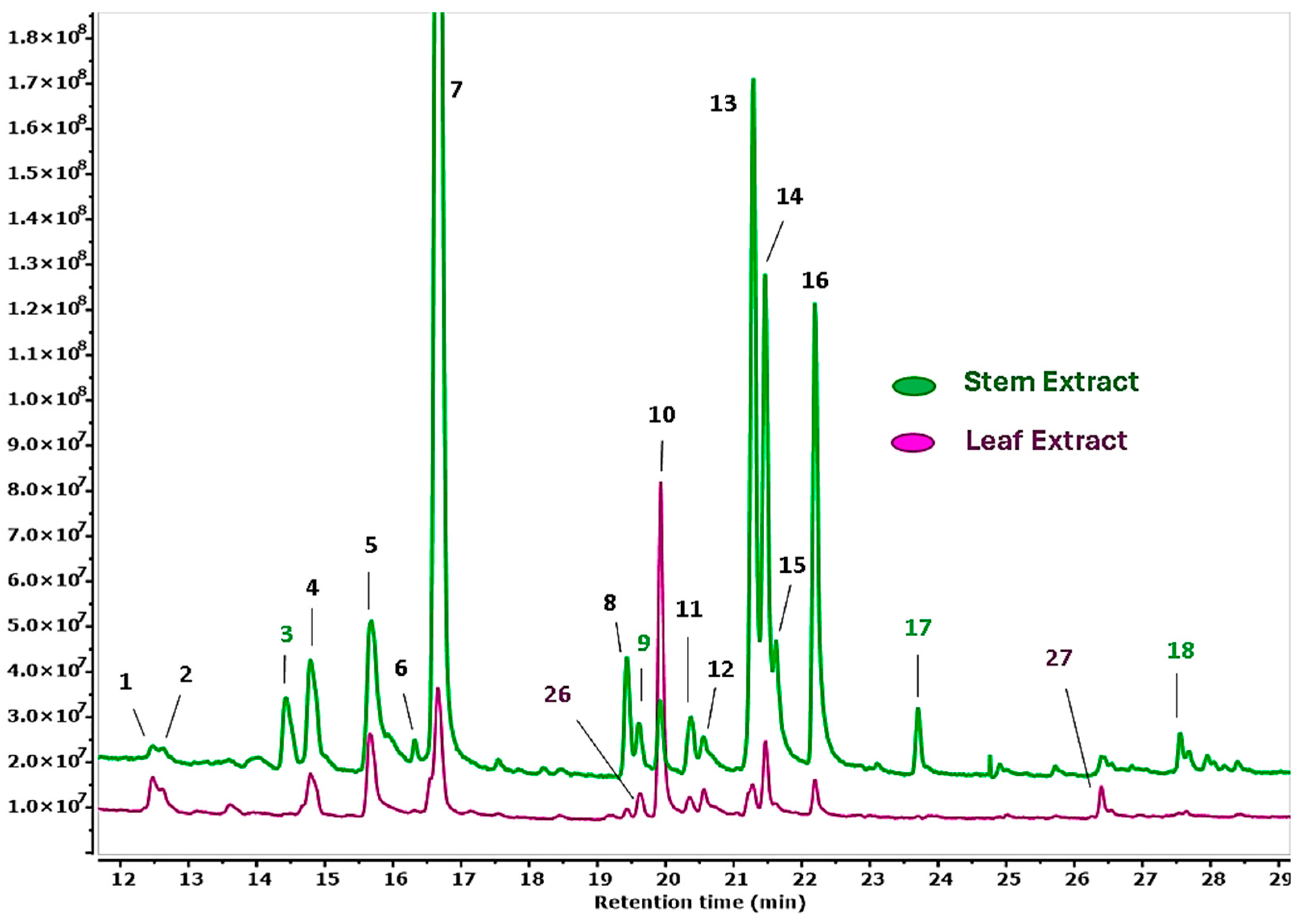
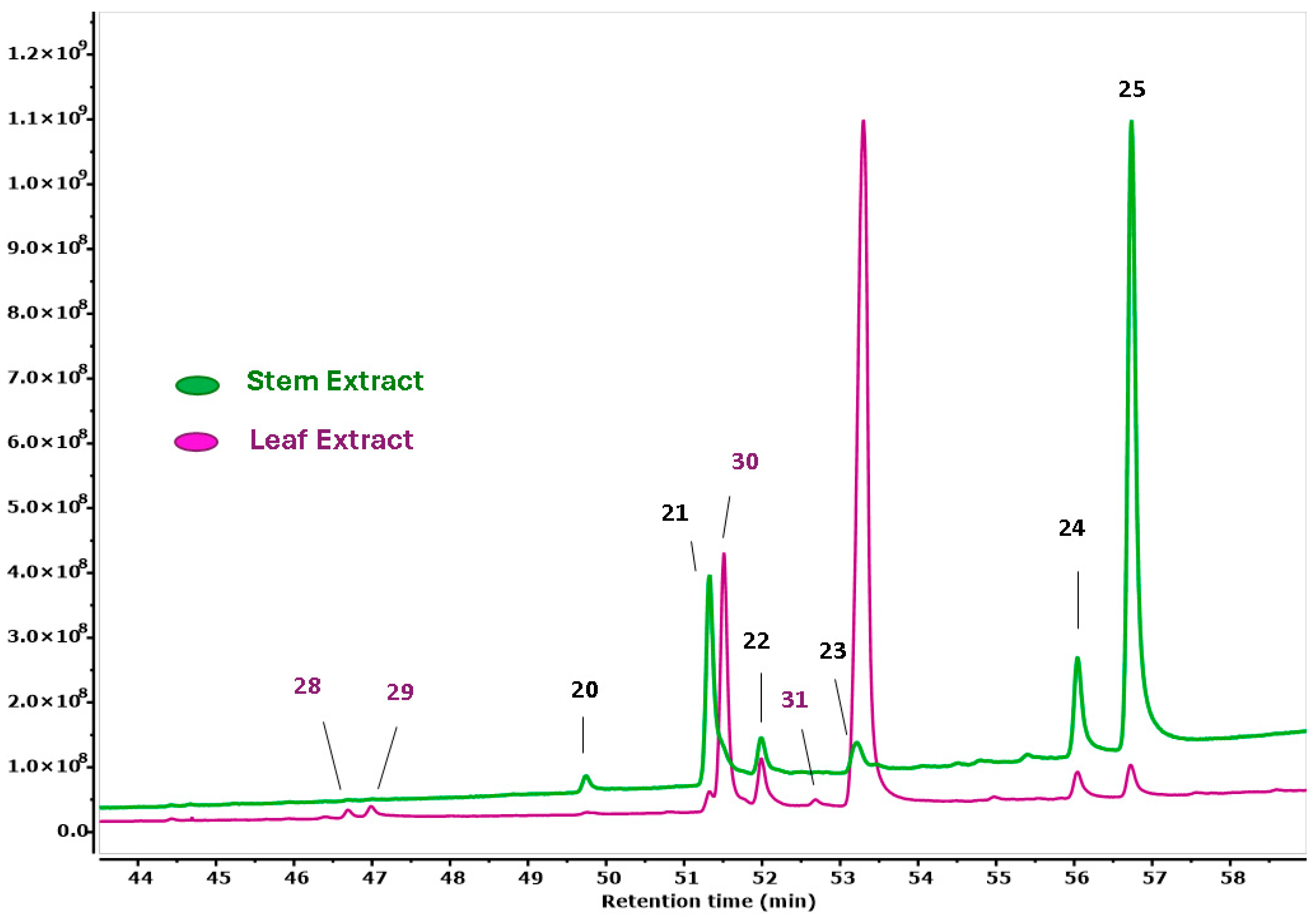
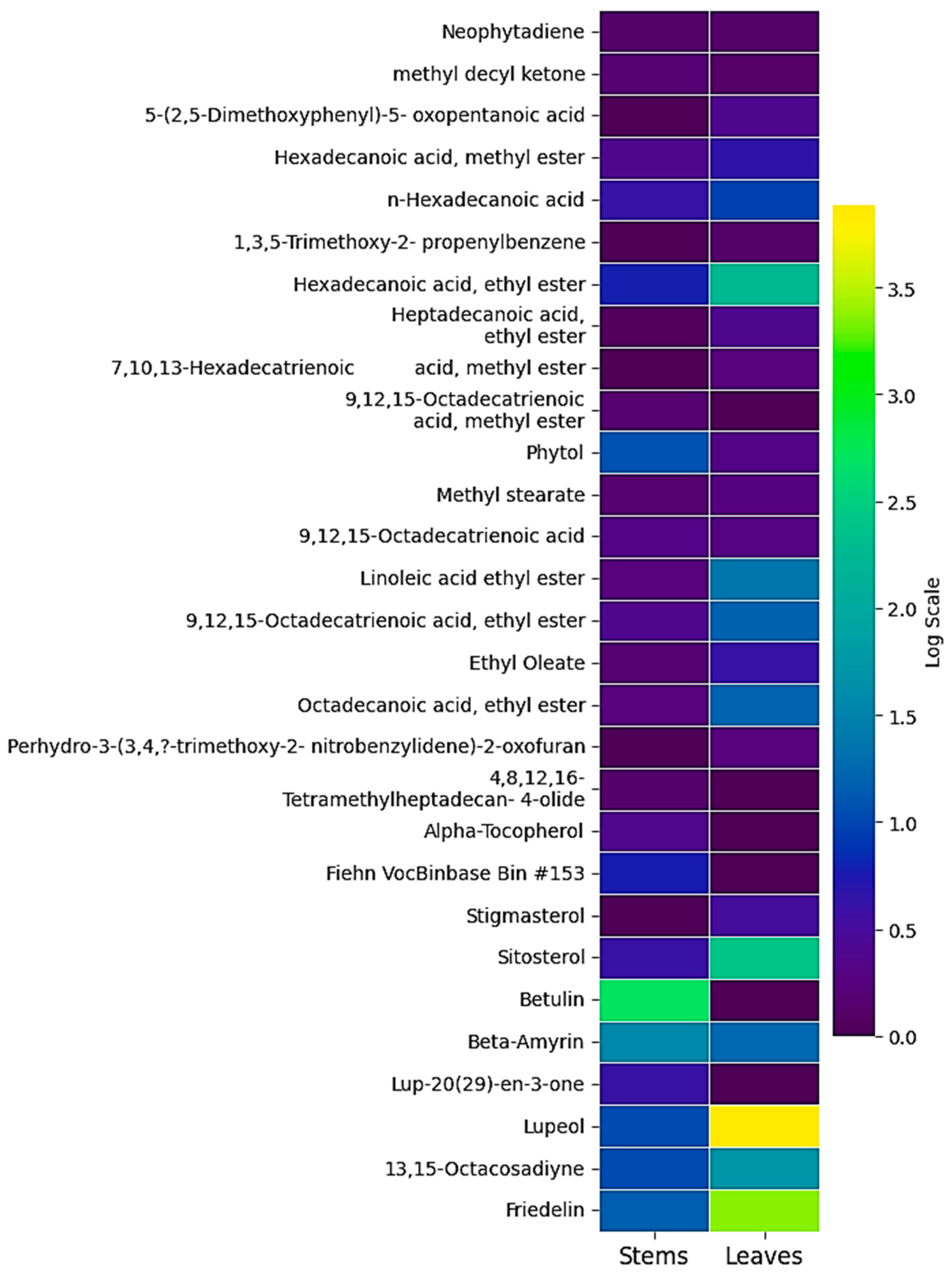
| No. | Compound | RT (min) | A% | MW (g/mol) | MF |
|---|---|---|---|---|---|
| 1 | Neophytadiene | 12.47 | 0.13 | 278.5 | C20H38 |
| 2 | Methyl decyl ketone | 12.63 | 0.12 | 184.32 | C15H26O |
| 3 | 5-(2,5-Dimethoxyphenyl)-5-oxopentanoic acid | 14.43 | 0.49 | 252.26 | C13H16O5 |
| 4 | Hexadecanoic acid, methyl ester | 14.79 | 0.91 | 270.45 | C17H34O2 |
| 5 | Hexadecanoic acid | 15.67 | 1.63 | 256.42 | C16H32O2 |
| 6 | 1,3,5-Trimethoxy-2-propenylbenzene | 16.32 | 0.14 | 208.25 | C16H16O3 |
| 7 | Hexadecanoic acid, ethyl ester | 16.66 | 8.50 | 284.47 | C18H36O2 |
| 8 | Heptadecanoic acid, ethyl ester | 19.43 | 0.49 | 298.50 | C19H38O2 |
| 9 | 7,10,13-Hexadecatrienoic acid, methyl ester | 19.61 | 0.29 | 264.40 | C17H28O2 |
| 10 | Phytol | 19.92 | 0.36 | 296.50 | C20H40O |
| 11 | Methyl stearate | 20.37 | 0.31 | 298.50 | C19H38O2 |
| 12 | 9,12,15-Octadecatrienoic acid | 20.56 | 0.34 | 278.42 | C18H30O2 |
| 13 | Linoleic acid ethyl ester | 21.28 | 2.93 | 308.50 | C20H36O2 |
| 14 | 9,12,15-Octadecatrienoic acid, ethyl ester | 21.47 | 2.28 | 306.48 | C20H34O2 |
| 15 | Ethyl Oleate | 21.62 | 0.88 | 310.50 | C20H38O2 |
| 16 | Octadecanoic acid, ethyl ester | 22.20 | 2.34 | 312.53 | C20H40O2 |
| 17 | Perhydro-3-(3,4,a-trimethoxy-2-nitrobenzylidene)-2-oxofuran | 23.71 | 0.28 | 309.27 | C14H15NO7 |
| 18 | Eicosanoic acid, ethyl ester | 27.55 | 0.15 | 340.58 | C22H44O2 |
| 19 | Lichexanthone | 33.92 | 0.09 | 286.28 | C16H14O5 |
| 20 | Stigmasterol | 49.74 | 0.69 | 412.70 | C29H48O2 |
| 21 | Sitosterol | 51.33 | 10.01 | 302.50 | C20H30O2 |
| 22 | Beta-Amyrin | 51.99 | 2.51 | 426.70 | C30H50O |
| 23 | Lupeol | 53.28 | 47.56 | 426.72 | C30H50O |
| 24 | 13,15-Octacosadiyne | 56.04 | 4.51 | 386.70 | C28H50 |
| 25 | Friedelin | 56.73 | 27.73 | 426.700 | C30H50O |
| No. | Compound | RT (min) | A% | MW (g/mol) | MF |
|---|---|---|---|---|---|
| 1 | Neophytadiene | 12.47 | 0.13 | 278.5 | C20H38 |
| 2 | methyl decyl ketone | 12.63 | 0.20 | 184.32 | C12H24O |
| 4 | Hexadecanoic acid, methyl ester | 14.79 | 0.48 | 270.45 | C17H34O2 |
| 5 | n-Hexadecanoic acid | 15.67 | 0.89 | 256.42 | C16H32O2 |
| 7 | Hexadecanoic acid, ethyl ester | 16.66 | 1.19 | 284.47 | C18H36O2 |
| 8 | Heptadecanoic acid, ethyl ester | 19.43 | 0.06 | 298.50 | C19H38O2 |
| 26 | 9,12,15-Octadecatrienoic acid, methyl ester | 19.63 | 0.18 | 292.45 | C19H32O2 |
| 10 | Phytol | 19.93 | 1.90 | 296.50 | C20H40O |
| 11 | Methyl stearate | 20.35 | 0.18 | 298.50 | C19H38O2 |
| 12 | 9,12,15-Octadecatrienoic acid | 20.56 | 0.36 | 278.42 | C18H30O2 |
| 13 | Linoleic acid ethyl ester | 21.28 | 0.29 | 308.50 | C20H36O2 |
| 14 | 9,12,15-Octadecatrienoic acid, ethyl ester | 21.47 | 0.47 | 306.48 | C20H34O2 |
| 15 | Ethyl Oleate | 21.62 | 0.19 | 310.50 | C20H38O2 |
| 16 | Octadecanoic acid, ethyl ester | 22.19 | 0.29 | 312.53 | C20H40O2 |
| 27 | 4,8,12,16-Tetramethylheptadecan-4-olide | 26.40 | 0.16 | 324.54 | C21H40O2 |
| 28 | Alpha-Tocopherol | 46.69 | 0.46 | 430.71 | C29H50O2 |
| 29 | Fiehn VocBinbase Bin #153 | 46.98 | 1.14 | - | - |
| 21 | Sitosterol | 51.33 | 0.83 | 302.50 | C20H30O2 |
| 30 | Betulin | 51.51 | 13.81 | 442.70 | C20H50O2 |
| 22 | Beta-Amyrin | 51.99 | 3.79 | 426.70 | C30H50O |
| 31 | Lup-20(29)-en-3-one | 52.68 | 0.82 | 424.70 | C30H48O |
| 23 | Lupeol | 53.21 | 1.81 | 426.72 | C30H50O |
| 24 | 13,15-Octacosadiyne | 56.04 | 1.83 | 386.70 | C28H50 |
| 25 | Friedelin | 56.73 | 2.19 | 426.700 | C30H50O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chérigo, L.; Fernández, J.; Martínez, R.; Santos, E.; Martínez-Luis, S. Chemical Composition and Insecticidal Activity of Eschweilera jefensis Organic Extracts Against Aphis gossypii. Agronomy 2025, 15, 2374. https://doi.org/10.3390/agronomy15102374
Chérigo L, Fernández J, Martínez R, Santos E, Martínez-Luis S. Chemical Composition and Insecticidal Activity of Eschweilera jefensis Organic Extracts Against Aphis gossypii. Agronomy. 2025; 15(10):2374. https://doi.org/10.3390/agronomy15102374
Chicago/Turabian StyleChérigo, Lilia, Juan Fernández, Ramy Martínez, Emmanuel Santos, and Sergio Martínez-Luis. 2025. "Chemical Composition and Insecticidal Activity of Eschweilera jefensis Organic Extracts Against Aphis gossypii" Agronomy 15, no. 10: 2374. https://doi.org/10.3390/agronomy15102374
APA StyleChérigo, L., Fernández, J., Martínez, R., Santos, E., & Martínez-Luis, S. (2025). Chemical Composition and Insecticidal Activity of Eschweilera jefensis Organic Extracts Against Aphis gossypii. Agronomy, 15(10), 2374. https://doi.org/10.3390/agronomy15102374






