Limitations in the Valorization of Food Waste as Fertilizer: Cytogenotoxicity Assessment of Apple and Tomato Juices By-Products
Abstract
1. Introduction
- (a)
- to determine the mitotic index of the embryonic roots of wheat sprouts treated with apple and tomato pomaces;
- (b)
- to determine the level of genotoxicity induced by the treatments with apple and tomato pomaces;
- (c)
- to determine the germination of caryopses, root length, and stem length of wheat sprouts under the influence of treatments with apple and tomato pomaces;
- (d)
- to establish correlations between the mitotic index and the level of genotoxicity on the one hand and the germination rate of caryopses, root length, and stem length of wheat sprouts on the other hand.
2. Materials and Methods
2.1. Extracts Preparation
2.2. Experimental Procedure
2.3. Experiment Design
2.4. Cytogenetic Parameters
2.5. Determination of the Germination Rate
2.6. Measurement of the Embryonic Root and Shoot Lengths
2.7. Correlations Between Cytogenetic Parameters and Biometric Parameters
2.8. Statistical Analysis
3. Results
3.1. Evaluation of the Cytogenetic Parameters
3.1.1. Mitotic Index
3.1.2. Genetic Abnormalities
3.2. Evaluation of the Length of the Embryonic Root and Shoot
3.3. Correlations Between Cytogenetic Parameters and Biometric Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | control; |
| APE | apple pomace extract; |
| APE 1 | apple pomace extract 0.05%; |
| APE 2 | apple pomace extract 0.5%; |
| TPE | tomato pomace extract |
| TPE 1 | tomato pomace extract 0.05% |
| TPE 2 | tomato pomace extract 0.5% |
| MI | mitotic index; |
| GI | genotoxic index. |
References
- Coman, I.V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 5; Volume 91, pp. 157–225. ISBN 9780128204702. [Google Scholar] [CrossRef]
- Russ, W.; Meyer-Pittroff, R. Utilizing waste products from the food production and processing industries. Crit. Rev. Food Sci. Nutr. 2004, 44, 57–62. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.A.; Varvara, R.A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Valorisation of agri-food waste to fertilisers is a challenge in implementing the circular economy concept in practice. Environ. Pollut. 2022, 312, 119906. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Ungureanu, G.; Enache, I.-M.; Cara, I.G.; Motrescu, I.; Patras, A. Insights into the environmental benefits of using apple pomace for biosorption of lead from contaminated water. Heliyon 2024, 10, e36811. [Google Scholar] [CrossRef] [PubMed Central]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Tsoupras, A.; Gkika, D.A.; Markopoulos, T.; Curran, R.; Scallon, C.; Karali, M.; Kyzas, G.Z. Apple Products (Apple Juice and Cider) and by-Products (Apple Pomace): Bioactive Compounds and Biological Properties. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–42. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, E.A. Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic Antioxidant Profiles in the Whole Fruit, Flesh and Peel of Apple Cultivars Grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Aplications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Huc-Mathis, D.; Journet, C.; Fayolle, N.; Bosc, V. Emulsifying properties of food by-products: Valorizing apple pomace and oat bran. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 84–91. [Google Scholar] [CrossRef]
- Torbica, A.; Škrobot, D.; Janić Hajnal, E.; Belović, M.; Zhang, N. Sensory and physico-chemical properties of wholegrain wheat bread prepared with selected food by-products. LWT—Food Sci. Technol. 2019, 114, 108414. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, H.; Wang, Q.; Liu, J. Sensory and physical quality characteristics of bread fortified with apple pomace using fuzzy mathematical model. Int. J. Food Sci. Technol. 2017, 52, 1092–1100. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; La Pointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, C.M.T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Bortolini, D.G.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Effect of addition of phenolic compounds recovered from apple pomace on cider quality. LWT—Food Sci. Technol. 2019, 100, 348–354. [Google Scholar] [CrossRef]
- Choi, I.S.; Lee, Y.G.; Khanal, S.K.; Park, B.J.; Bae, H.-J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products e a review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Binoy, G.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersicon esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Chabi, I.B.; Omiyalé, O.J.; Dèdéhou, S.E.C.A.; Ayégnon, B.P.; Idrissou, I.; Boya, B.; Kpoclou, Y.E.; Kayodé, A.P.P. Tomato Seed (Solanum lycopersicum) Meal Derived From Agrifood Waste as Functional Ingredient: Nutritional Value, Antioxidant and Antimicrobial Activities, and Functional Properties. J. Food Process. Preserv. 2024, 2024, 8824581. [Google Scholar] [CrossRef]
- Beecher, G.R. Nutrient Content of Tomatoes and Tomato Products. Proc. Soc. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Giovanelli, G.; Pagliarini, E. Antioxidant Composition of Tomato Products Typically Consumed in Italy. Ital. J. Food Sci. 2009, 21, 305–316. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Simonne, H.A.; Behe, B.K.; Marshall, M.M. Consumers Prefer Low-Priced and High-Lycopene-Content Fresh-Market Tomatoes. Hort Technol. 2006, 16, 674–681. [Google Scholar] [CrossRef]
- Fadupin, T.G.; Osadola, O.T.; Atinmo, T. Lycopene Content of Selected Tomato Based Products, Fruits and Vegetables Consumed in South Western Nigeria. Afr. J. Biomed. Res. 2012, 15, 187–191. [Google Scholar]
- Cadoni, E.; De Giorgi, M.R.; Medda, E.; Poma, G. Supercritical CO2 extraction of lycopene and β-carotene from ripe tomatoes. Dyes Pigments 1999, 44, 27–32. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzym. Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Løvdal, T.; Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of Tomato Surplus and Waste Fractions: A Case Study Using Norway, Belgium, Poland, and Turkey as Examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- Badolati, N.; Masselli, R.; Maisto, M.; Di Minno, A.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Genotoxicity Assessment of Three Nutraceuticals Containing Natural Antioxidants Extracted from Agri-Food Waste Biomasses. Foods 2020, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Kirkland, D.; Kasper, P.; Martus, H.J.; Müller, L.; van Benthem, J.; Madia, F.; Corvi, R. Updated Recommended Lists of Genotoxic and Non-Genotoxic Chemicals for Assessment of the Performance of New or Improved Genotoxicity Tests. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 795, 7–30. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple pomace as a source of bioactive polyphenol compounds in gluten-free breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Phachonpai, W.; Muchimapura, S.; Tong-Un, T.; Sripanidkulchai, B.; Wannanon, P. Acute toxicity study of tomato pomace extract in Rodent. Online J. Biol. Sci. 2013, 1, 28–34. [Google Scholar] [CrossRef]
- Radić, K.; Galić, E.; Vinković, T.; Golub, N.; Čepo, D.V. Tomato Waste as a Sustainable Source of Antioxidants and Pectins: Processing, Pretreatment and Extraction Challenges. Sustainability 2024, 16, 9158. [Google Scholar] [CrossRef]
- Musto, G.; Schiano, E.; Iannuzzo, F.; Tenore, G.G.; Novellino, E.; Stornaiuolo, M. Genotoxicity Assessment of Nutraceuticals Extracted from Thinned Nectarine (Prunus persica L.) and Grape Seed (Vitis vinifera L.) Waste Biomass. Foods 2023, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Berbel, J.; Posadillo, A. Review and analysis of alternatives for the valorisation of agro-industrial olive oil by-products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Schmidt, L.; Prestes, O.D.; Rossini Augusti, P.; Fonseca Moreira, J.C. Phenolic compounds and contaminants in olive oil and pomace—A narrative review of their biological and toxic effects. Food Biosci. 2023, 53, 102626. [Google Scholar] [CrossRef]
- Mekersi, N.; Kadi, K.; Casini, S.; Addad, D.; Bazri, K.E.; Marref, S.E.; Lekmine, S.; Amari, A. Effects of single and combined olive mill wastewater and olive mill pomace on the growth, reproduction, and survival of two earthworm species (Aporrectodea trapezoides, Eisenia fetida). Appl. Soil Ecol. 2021, 168, 104123. [Google Scholar] [CrossRef]
- Padureanu, S.; Patras, A. Biological Response of Triticum aestivum L. to the Abiotic Stress Induced by Winemaking Waste. Agronomy 2022, 12, 1371. [Google Scholar] [CrossRef]
- Corvi, R.; Madia, F. In vitro genotoxicity testing—Can the performance be enhanced? Food Chem. Toxicol. 2017, 106 B, 600–608. [Google Scholar] [CrossRef]
- Saks, M.; Upreti, S.; Rajendra, S.V.; Dang, R. Genotoxicity: Mechanisms, Testing Guidelines and Methods. Glob. J. Pharm. Pharm. Sci. 2017, 1, 133–138. [Google Scholar] [CrossRef]
- Musto, G.V.; Laurenzi, V.; Annunziata, G.; Novellino, E.; Stornaiuolo, M. Genotoxic Assessment of Nutraceuticals Obtained from Agricultural Biowaste: Where Do We “AMES”? Antioxidants 2022, 11, 1197. [Google Scholar] [CrossRef]
- Jitareanu, A.; Caba, I.C.; Trifan, A.; Padureanu, S.; Agoroaei, L. Triticum aestivum assay—A useful tool for environmental monitoring and toxicity assessment. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1005–1018. [Google Scholar] [CrossRef]
- Maravilla, A.J.; Rosato, M.; Rosselló, J.A. Preparation of Mitotic Chromosomes with the Squash Technique. In Plant Cytogenetics and Cytogenomics; Heitkam, T., Garcia, S., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2023; Volume 2672, pp. 141–149. [Google Scholar] [CrossRef]
- Graña, E. Mitotic Index. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A., Reigosa, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 231–240. [Google Scholar] [CrossRef]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Kato, T.A.; Haskins, J.S. Mitotic Index Analysis. In Chromosome Analysis; Gotoh, E., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2023; Volume 2519. [Google Scholar] [CrossRef]
- Akinboro, A.; Bakare, A.A. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J. Ethnopharmacol. 2007, 112, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Souza, T.; da Silva Figueira Barone, L.; Lacerda, D.; dos Santos Vergilio, C.; de Oliveira, B.C.V.; de Almeida, M.G.; Thompson, F.; de Rezende, C.E. Cytogenotoxicity of the water and sediment of the Paraopeba River immediately after the iron ore mining dam disaster (Brumadinho, Minas Gerais, Brazil). Sci. Total Environ. 2021, 775, 145193. [Google Scholar] [CrossRef]
- Janssen, A.; van der Burg, M.; Szuhai, K.; Kops, G.J.P.L.; Medema, R.H. Chromosome Segregation Errors as a Cause of DNA Damage and Structural Chromosome Aberrations. Science 2011, 333, 1895–1898. [Google Scholar] [CrossRef]
- Türkoğlu, Ş. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2007, 626, 4–14. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Goedecke, W.; Obe, G. Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis 2000, 15, 289–302. [Google Scholar] [CrossRef]
- Gömürgen, A.N. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia 2005, 70, 119–128. [Google Scholar] [CrossRef]
- Pickett-Heaps, J.D.; Tippit, D.H.; Cohn, S.A.; Spurk, T.P. Microtubule Dynamics in the Spindle. Theoretical Aspects of Assembly/Disassembly Reactions In Vivo. J. Theor. Biol. 1986, 118, 153–169. [Google Scholar] [CrossRef]
- Anglada, T.; Rodriguez-Munoz, M.; Pulido-Artola, N.; Genesca, A. Engineering Chromosome Bridges Through CRISPR/Cas9 to Decipher the Impact of Intercentromeric Distance on Resolution Dynamics. FASEB J. 2025, 39, e70599. [Google Scholar] [CrossRef]
- Ryu Endo, T.; Gill, B.S. Somatic karyotype, heterochromatin distribution, and nature of chromosome differentiation in common wheat, Triticum aestivum L. em Thell. Chromosoma 1984, 89, 361–369. [Google Scholar] [CrossRef]
- Najafi, S.; Ulker, M.; Altuner, F.; Oral, E.; Ozdemir, B.; Jamal Salih, S.; Selem, E. Karyological analysis on wheat Tir (Triticum aestivum var. Aestivum L. spp. Leucospermum Körn.) ecotypes in Lake Van Basin. Int. J. Agric. Technol. 2022, 18, 1093–1102. [Google Scholar]
- Yi, H.; Meng, Z. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat. Res. 2003, 537, 109–114. [Google Scholar] [CrossRef] [PubMed]
- El-Ghamery, A.A.; Mousa, M.A. Investigation on the effect of benzyladenine on the germination, radicle growth and meristematic cells of Nigella sativa L. and Allium cepa L. Ann. Agric. Sci. 2017, 62, 11–21. [Google Scholar] [CrossRef]
- Barman, M.; Roy, S.; Ray, S. Mitotic abnormality inducing effects of leaf aqueous extract of Clerodendrum inerme Gaertn. on Allium cepa root apical meristem cells. Cytologia 2021, 86, 113–118. [Google Scholar] [CrossRef]
- Ma, T.H.; Xu, Z.; Xu, C.; McConnell, H.; Rabago, E.V.; Arreola, G.A.; Zhang, H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 1995, 334, 185–195. [Google Scholar] [CrossRef]
- Khanna, N.; Sharma, S. Allium Cepa Root Chromosomal Aberration Assay: A Review. Indian J. Pharm. Biol. 2013, 1, 105–119. [Google Scholar] [CrossRef]
- Huang, S.; Chueh, P.J.; Lin, Y.W.; Shih, T.S.; Chuang, S.M. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol. Appl. Pharmacol. 2009, 241, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Nouska, C.; Ciurla, L.; Patras, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products. Foods 2025, 14, 2224. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.M.; Ullah, M.F.; Shamim, U.; Bhatt, S.H.; Azmi, A.S. Catalytic therapy of cancer by ascorbic acid involves redox cycling of exogenous/endogenous copper ions and generation of reactive oxygen species. Chemotherapy 2010, 56, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Rody, H.V.S.; da C. Gontijo, D.; de M. Coelho, V.P.; Ventrella, M.C.; de Pádua, R.M.; Fietto, L.G.; Leite, J.P.V. Mutagenic activity and chemical composition of phenolic-rich extracts of leaves from two species of Ficus medicinal plants. J. Toxicol. Environ. Health A 2018, 81, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Teerarak, M.; Laosinwattana, C.; Charoenying, P. Evaluation of allelopathic, decomposition and cytogenetic activities of Jasminum officinale L. f. var. grandiflorum (L.) Kob. on bioassay plants. Bioresour. Technol. 2010, 101, 5677–5684. [Google Scholar] [CrossRef]
- Alcaraz, M.; Olivares, A.; Achel, D.G.; García-Gamuz, J.A.; Castillo, J.; Alcaraz-Saura, M. Genoprotective Effect of Some Flavonoids against Genotoxic Damage Induced by X-rays In Vivo: Relationship between Structure and Activity. Antioxidants 2021, 11, 94. [Google Scholar] [CrossRef]
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]


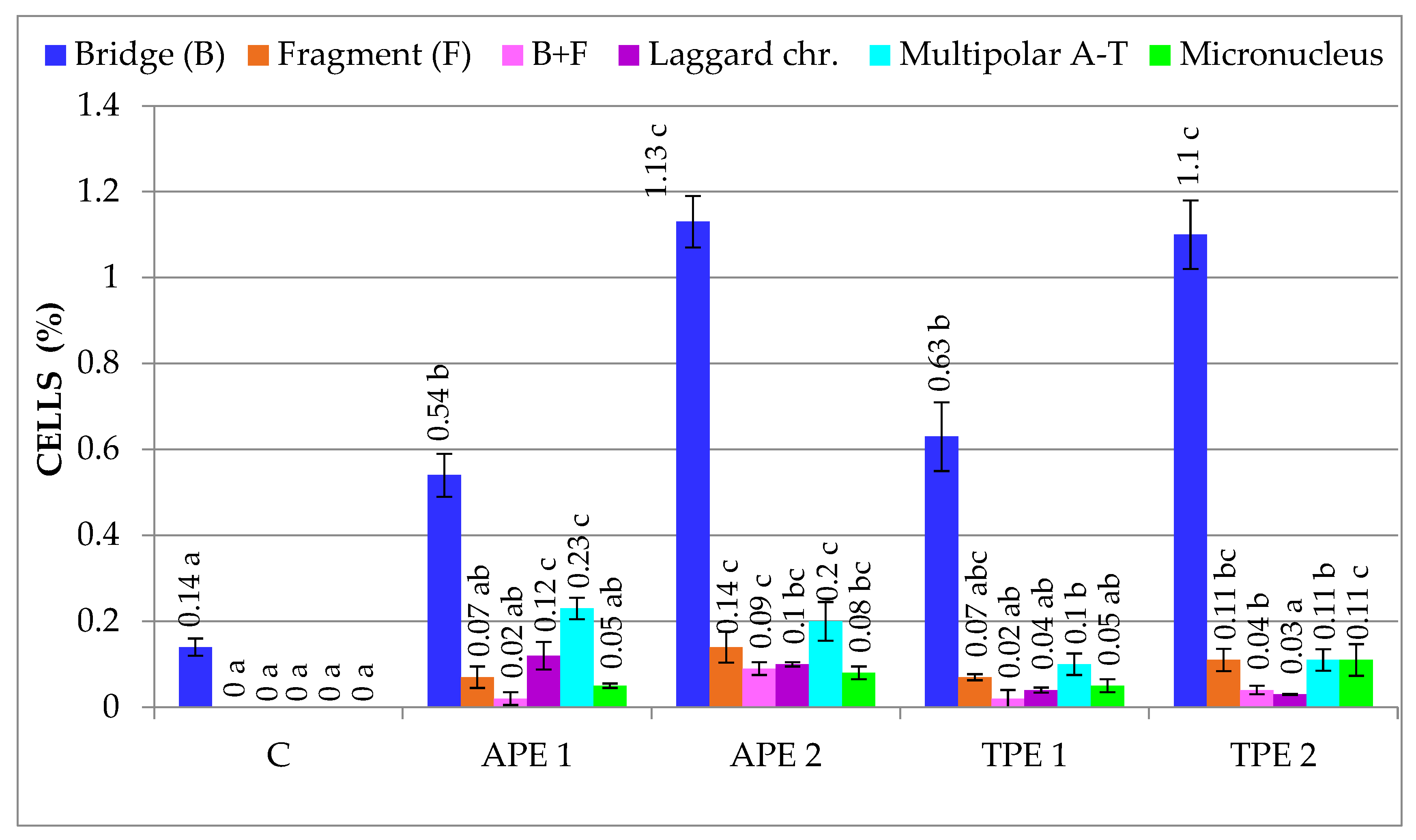
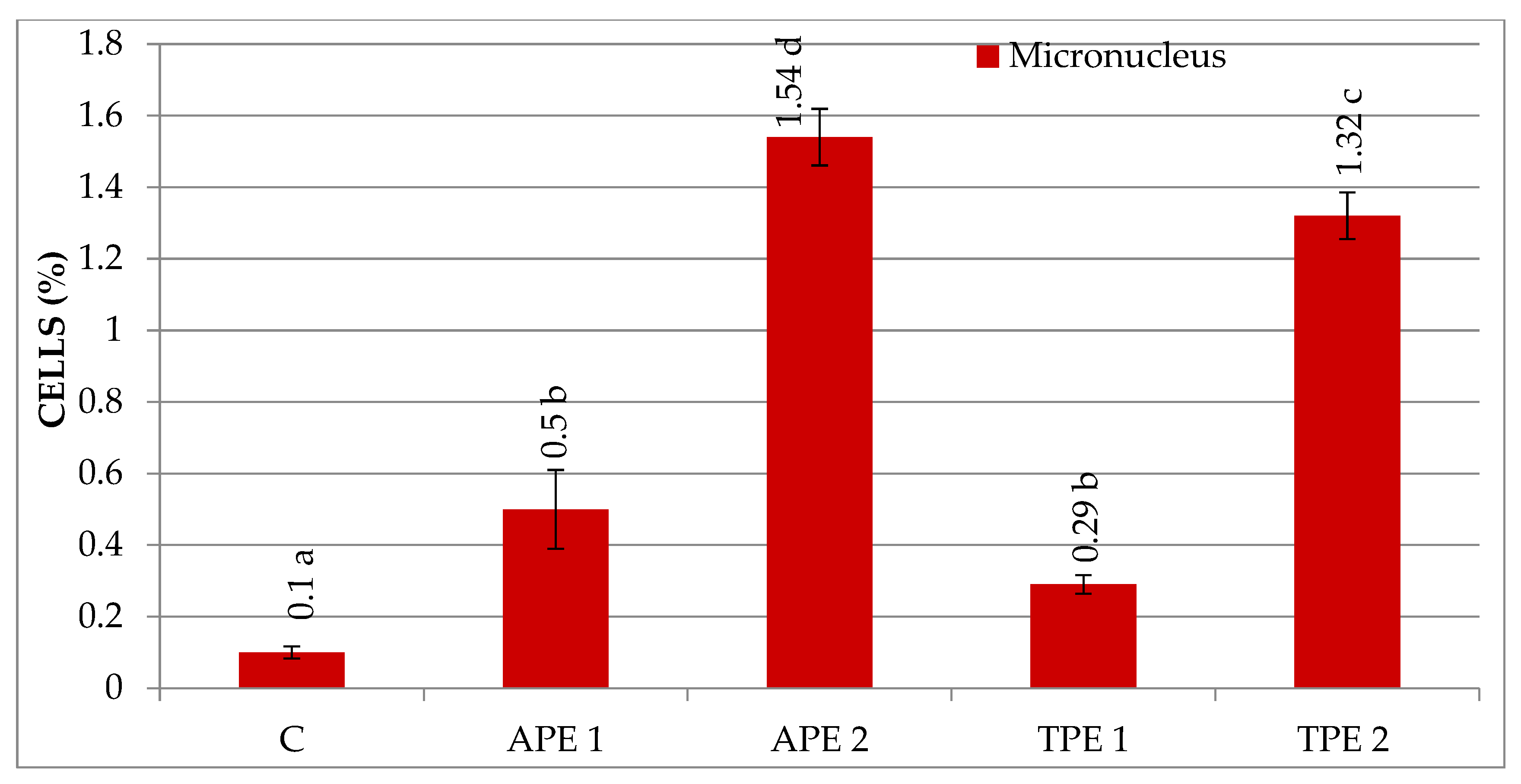
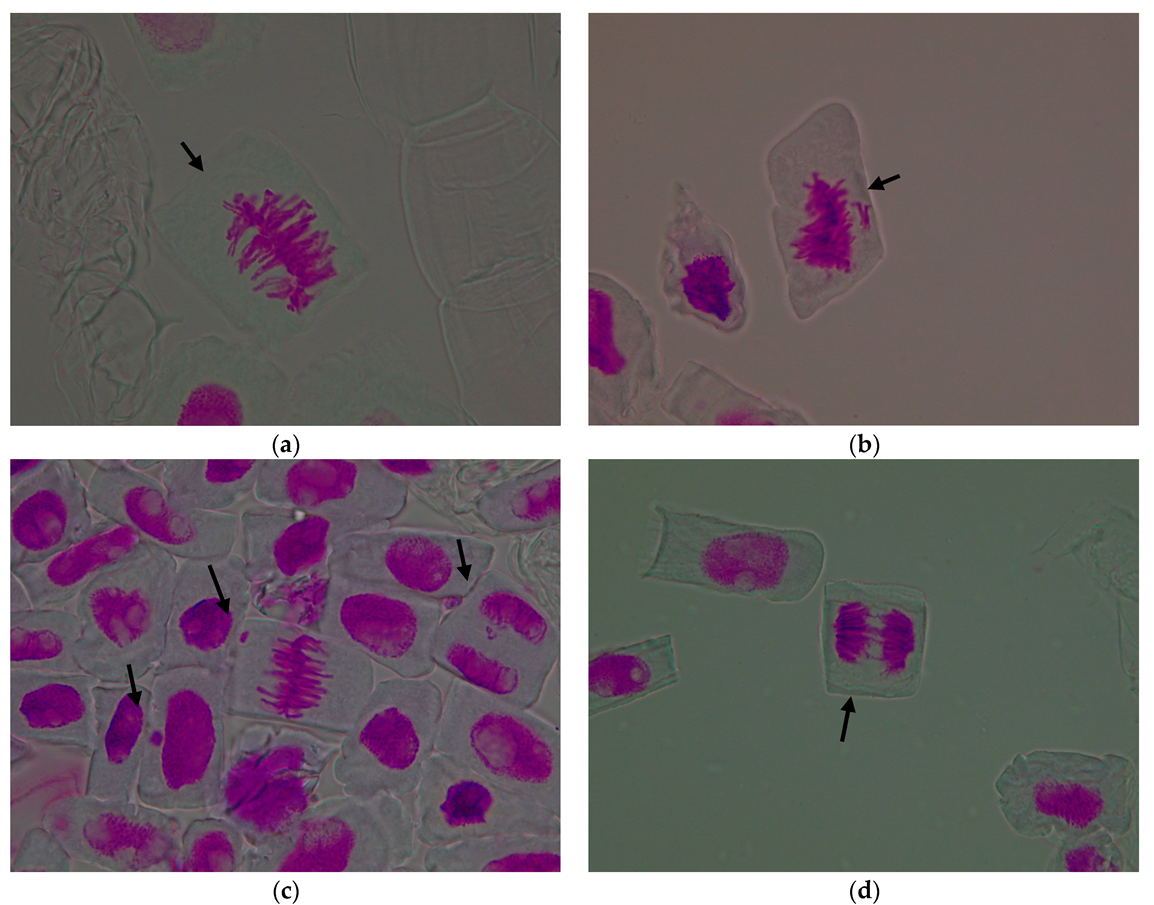
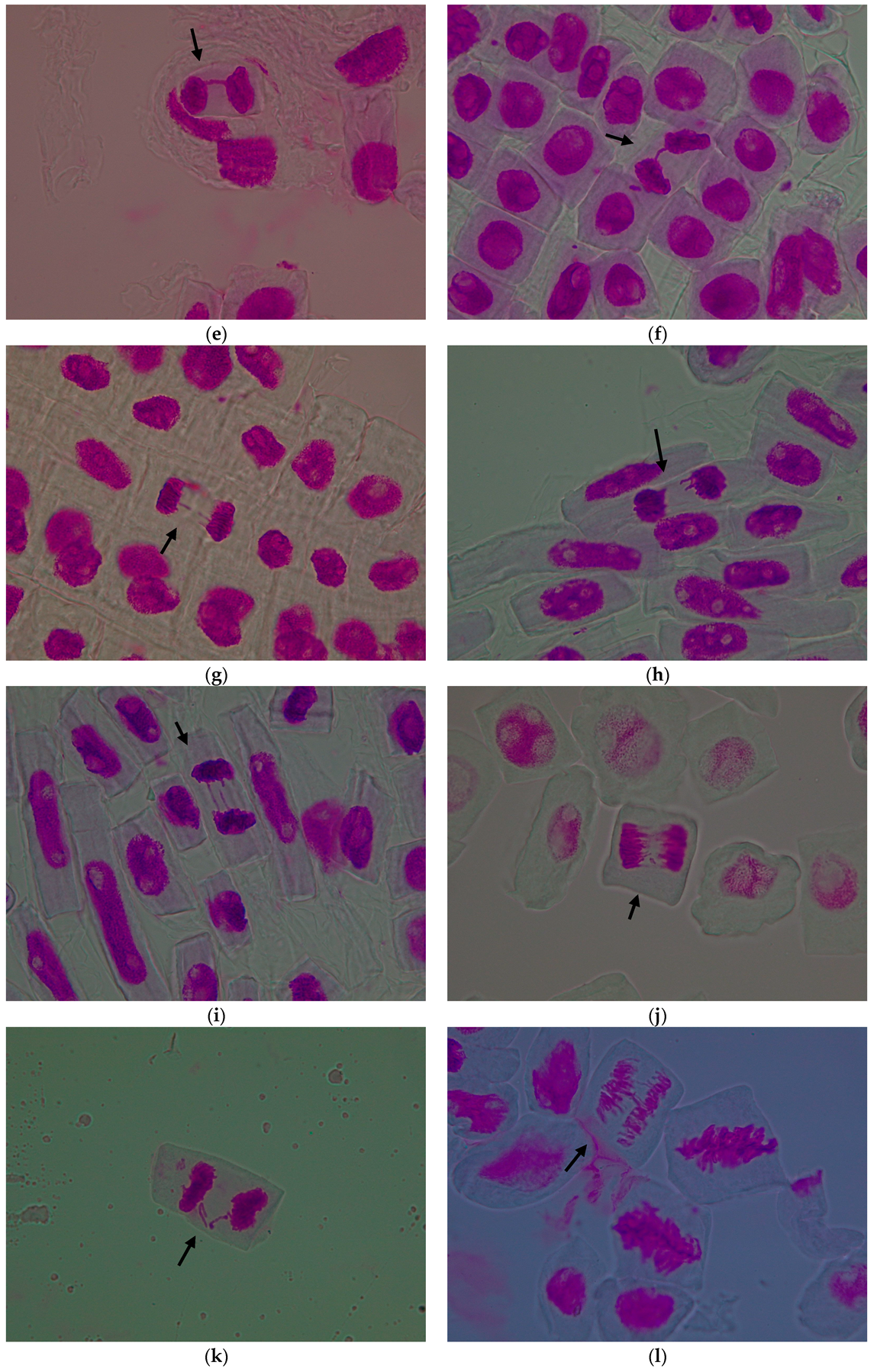
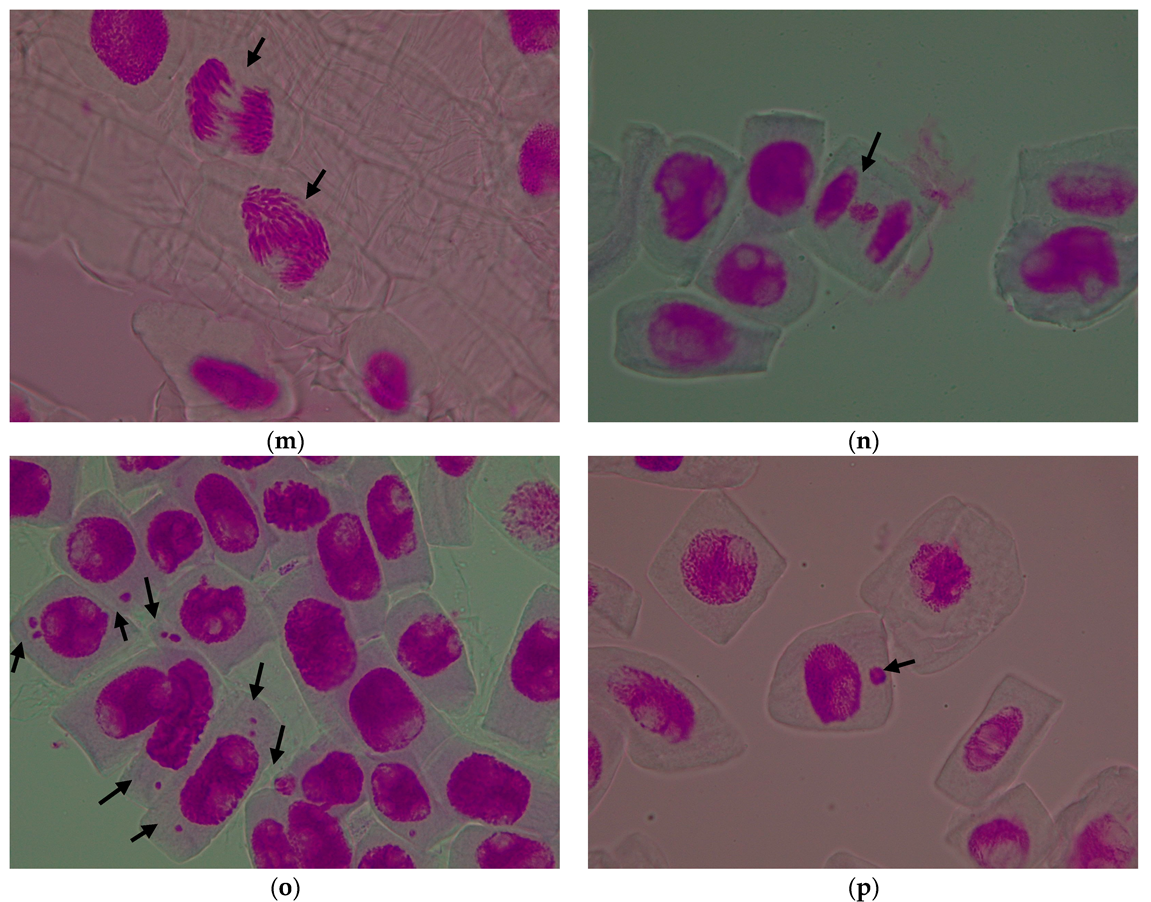
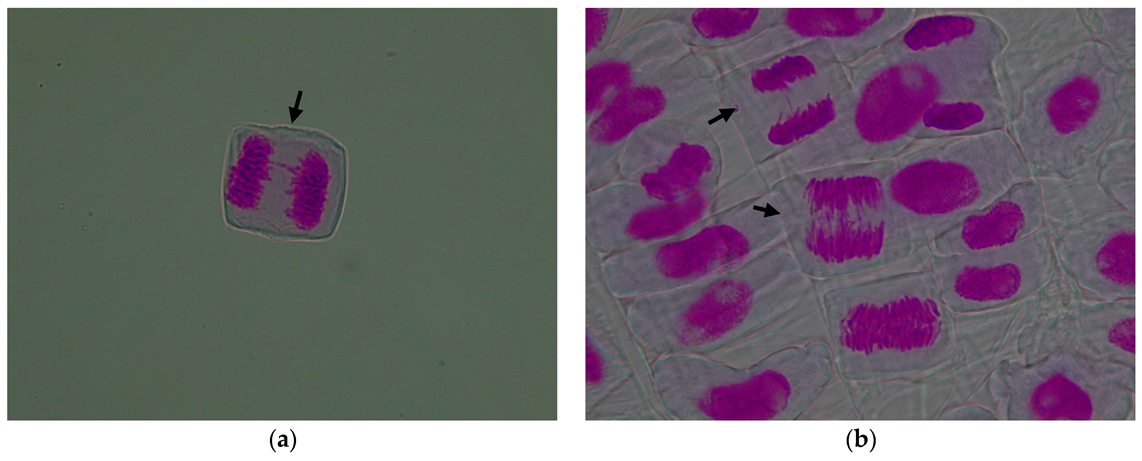
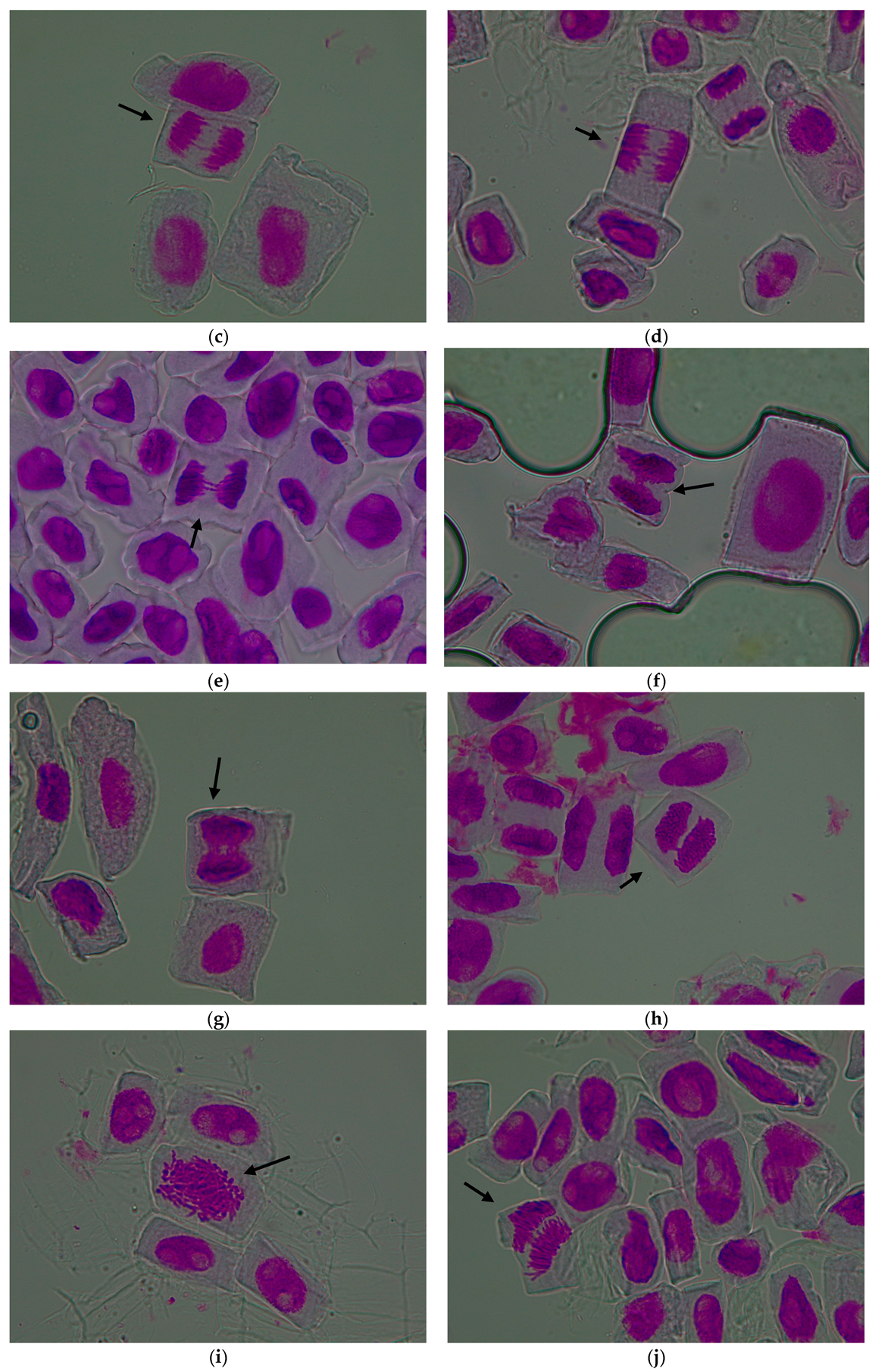
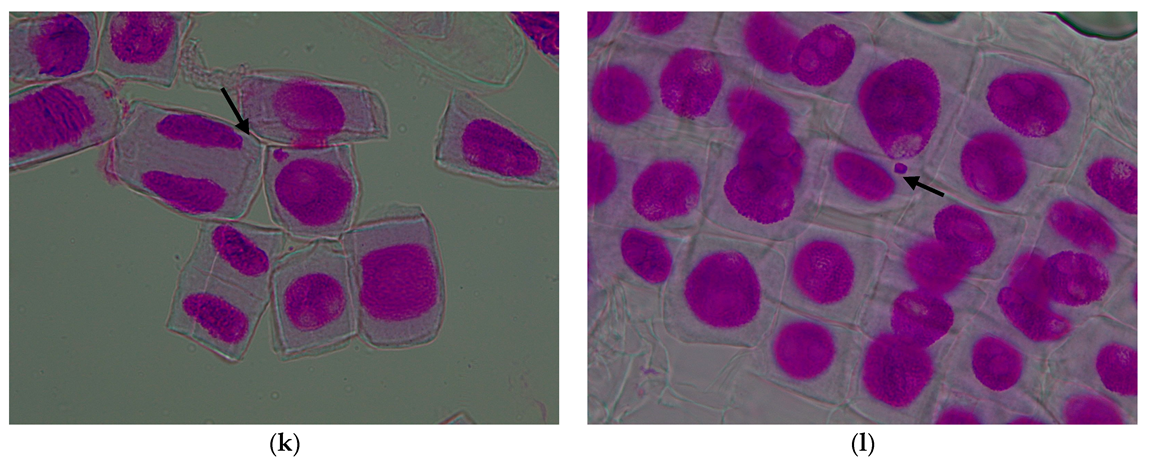
| Treatment Variant with Pomace Extract | Mean Number of Cells/Variant | Mitotic Index (%) | Cells in Prophase (%) | Cells in Metaphase (%) | Cells in Anaphase (%) | Cells in Telophase (%) |
|---|---|---|---|---|---|---|
| C | 11,623.00 | 15.49 ± 2.49 b | 5.93 ± 0.89 b | 3.08 ± 0.20 b | 2.28 ± 0.76 a | 4.20 ± 1.36 a |
| APE 1 | 11,835.00 | 14.96 ± 3.01 b | 5.89 ± 0.85 b | 3.05 ± 0.67 b | 2.23 ± 0.35 a | 3.78 ± 1.65 a |
| APE 2 | 11,728.66 | 8.66 ± 0.07 a | 2.53 ± 0.40 a | 1.88 ± 0.23 a | 1.52 ± 0.04 a | 2.73 ± 0.57 a |
| TPE 1 | 11,774.66 | 15.16 ± 2.01 b | 5.39 ± 0.43 b | 3.44 ± 0.68 b | 1.98 ± 0.56 a | 4.35 ± 0.84 a |
| TPE 2 | 12,272.66 | 10.33 ± 0.68 a | 2.45 ± 0.15 a | 2.21 ± 0.13 a | 1.92 ± 0.46 a | 3.75 ± 0.20 a |
| Treatment Variant with Pomace Extract | Cells in Metaphase (%) | Cells in A-T (%) | Cells in Interphase | GI (%) | |||
|---|---|---|---|---|---|---|---|
| Normal Metaphase (%) | Aberrant Metaphase (%) | Normal A-T (%) | Aberrant A-T (%) | Normal Interphase (%) | Aberrant Interphase (%) | ||
| C | 3.08 ± 0.20 b | 0.00 ± 0.00 a | 6.35 ± 1.65 b | 0.14 ± 0.03 a | 84.41 ± 2.50 a | 0.10 ± 0.02 a | 0.24 ± 0.03 a |
| APE 1 | 2.98 ± 0.67 b | 0.07 ± 0.03 b | 4.99 ± 1.93 b | 1.02 ± 0.08 b | 84.70 ± 3.00 a | 0.33 ± 0.31 b | 1.43 ± 0.13 b |
| APE 2 | 1.61 ± 0.26 a | 0.27 ± 0.04 c | 2.52 ± 0.49 a | 1.74 ± 0.16 d | 89.80 ± 0.08 b | 1.54 ± 0.08 c | 3.54 ± 0.26 d |
| TPE 1 | 3.40 ± 0.70 b | 0.03 ± 0.01 ab | 5.42 ± 0.14 b | 0.91 ± 0.17 b | 84.55 ± 1.99 a | 0.29 ± 0.03 ab | 1.23 ± 0.17 b |
| TPE 2 | 2.14 ± 0.13 a | 0.07 ± 0.02 b | 4.16 ± 0.50 ab | 1.51 ± 0.03 c | 88.35 ± 0.75 b | 1.32 ± 0.07 c | 2.90 ± 0.11 c |
| Treatment Variant with Pomace Extract | Root Length (mm) | ||
|---|---|---|---|
| After 48 h | After 72 h | After 96 h | |
| C | 11.14 ± 0.36 b | 30.97 ± 0.68 b | 53.46 ± 0.54 c |
| APE 1 | 11.23 ± 0.17 b | 29.58 ± 2.85 ab | 48.28 ± 4.91 b |
| APE 2 | 10.10 ± 0.32 a | 27.24 ± 1.39 a | 39.79 ± 1.48 a |
| TPE 1 | 11.36 ± 0.30 b | 30.02 ± 0.38 ab | 50.28 ± 1.11 bc |
| TPE 2 | 10.24 ± 0.27 a | 28.50 ± 0.74 ab | 42.57 ± 0.40 a |
| Treatment Variant with Pomace Extract | Shoot Length (mm) | ||
|---|---|---|---|
| After 48 h | After 72 h | After 96 h | |
| C | 3.80± 0.17 b | 16.87± 0.11 a | 35.11± 0.94 d |
| APE 1 | 3.86± 0.13 b | 15.97± 0.73 a | 33.74± 0.29 c |
| APE 2 | 3.57± 0.23 ab | 15.62± 0.75 a | 28.55± 0.53 a |
| TPE 1 | 3.50± 0.41 ab | 16.22± 0.73 a | 34.25± 0.46 cd |
| TPE 2 | 3.30± 0.18 a | 15.05± 1.83 a | 31.85± 0.41 b |
| Correlated Parameters | Correlation Coefficient (R2) | ||
|---|---|---|---|
| After 48 h | After 72 h | After 96 h | |
| MI (%) correlated to Germination Rate (%) | 0.8551 | 0.8163 | 0.9532 |
| GI (%) correlated to Germination Rate (%) | −0.8162 | −0.9429 | −0.9779 |
| MI (%) correlated to Root Length (%) | 0.9608 | 0.8938 | 0.9255 |
| GI (%) correlated to Root Length (%) | −0.805 | −0.9757 | −0.9938 |
| MI (%) correlated to Shoot Length (%) | 0.3516 | 0.6092 | 0.906 |
| GI (%) correlated to Shoot Length (%) | −0.3652 | −0.7636 | −0.8976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padureanu, S.; Patras, A. Limitations in the Valorization of Food Waste as Fertilizer: Cytogenotoxicity Assessment of Apple and Tomato Juices By-Products. Agronomy 2025, 15, 2364. https://doi.org/10.3390/agronomy15102364
Padureanu S, Patras A. Limitations in the Valorization of Food Waste as Fertilizer: Cytogenotoxicity Assessment of Apple and Tomato Juices By-Products. Agronomy. 2025; 15(10):2364. https://doi.org/10.3390/agronomy15102364
Chicago/Turabian StylePadureanu, Silvica, and Antoanela Patras. 2025. "Limitations in the Valorization of Food Waste as Fertilizer: Cytogenotoxicity Assessment of Apple and Tomato Juices By-Products" Agronomy 15, no. 10: 2364. https://doi.org/10.3390/agronomy15102364
APA StylePadureanu, S., & Patras, A. (2025). Limitations in the Valorization of Food Waste as Fertilizer: Cytogenotoxicity Assessment of Apple and Tomato Juices By-Products. Agronomy, 15(10), 2364. https://doi.org/10.3390/agronomy15102364






