Effects of Different Land-Use Types on Soil Properties and Microbial Communities in a Southeastern Tibetan Valley
Abstract
1. Introduction
2. Materials and Methods
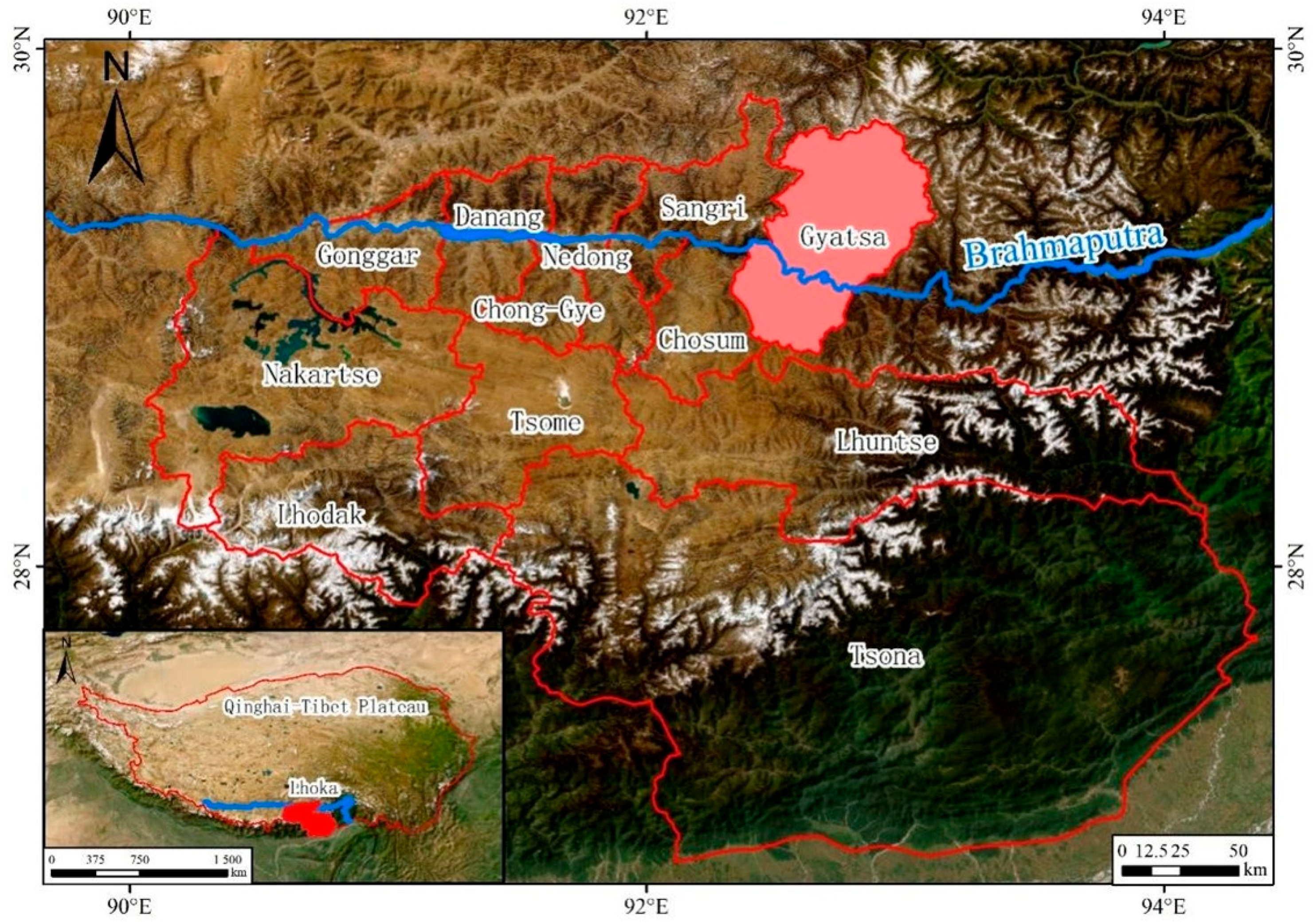
2.1. Study Site Overview
2.2. Experimental Design
2.3. Sample Analysis
2.4. Data Processing and Analysis
3. Results
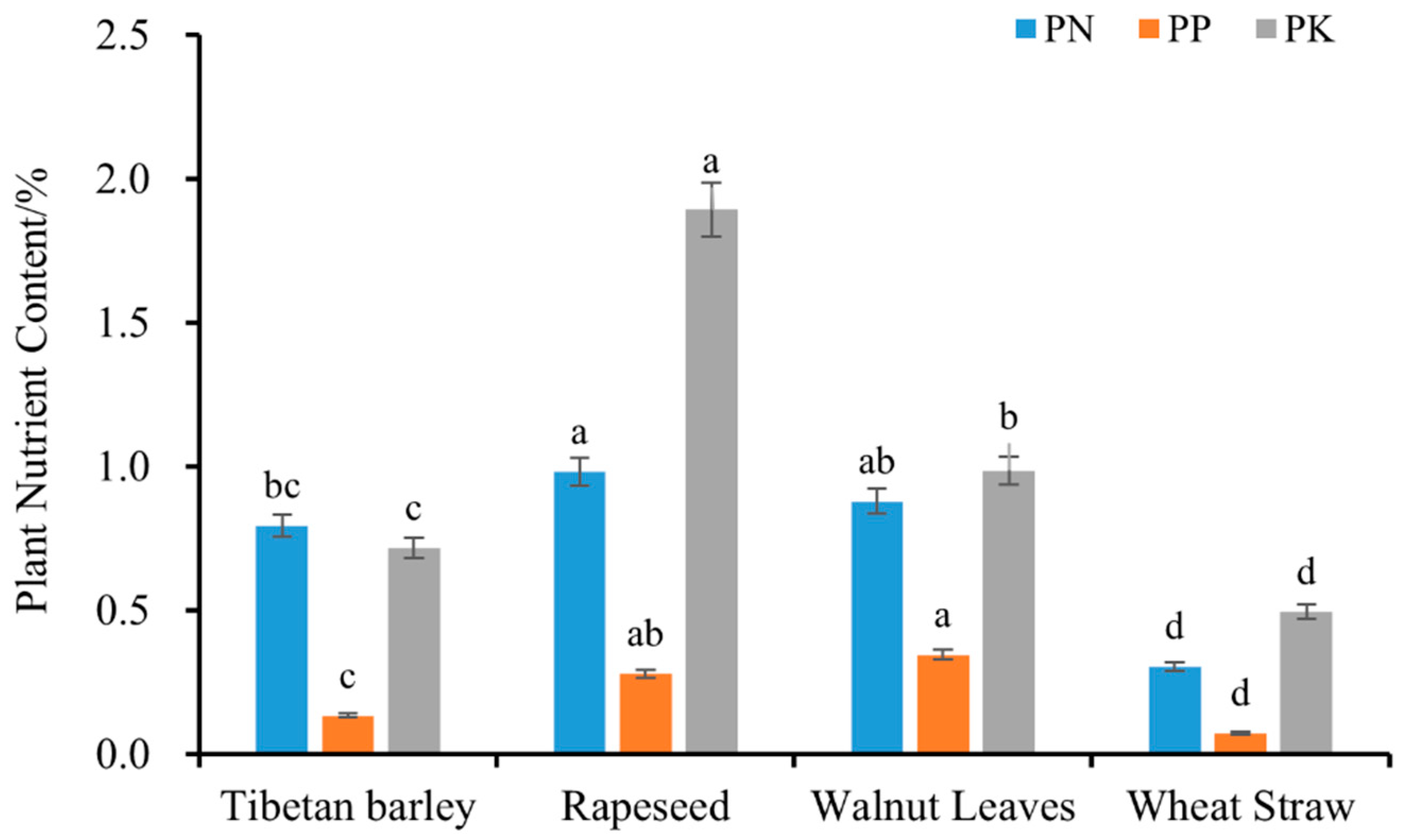
3.1. Soil and Plant Nutrients
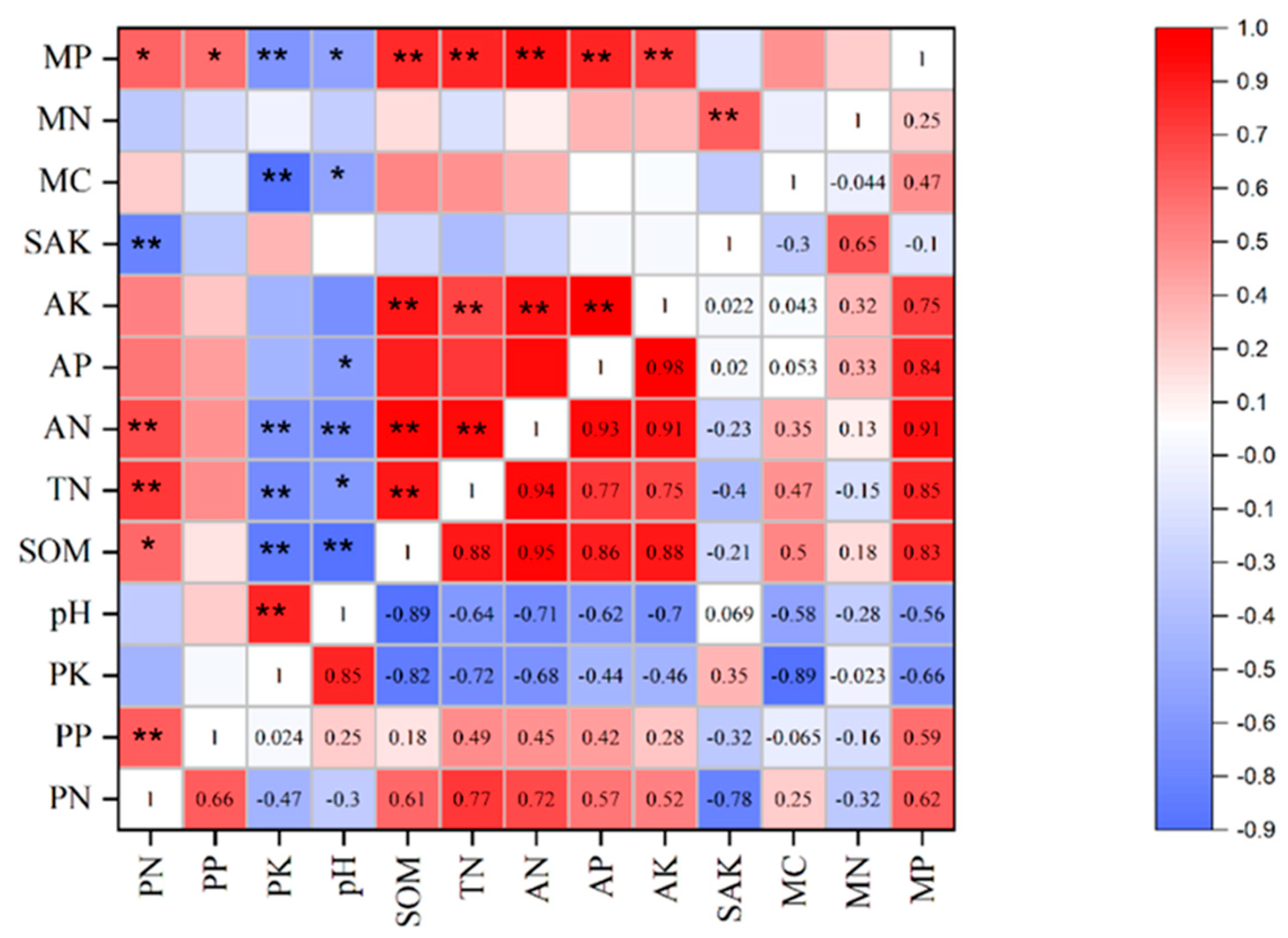
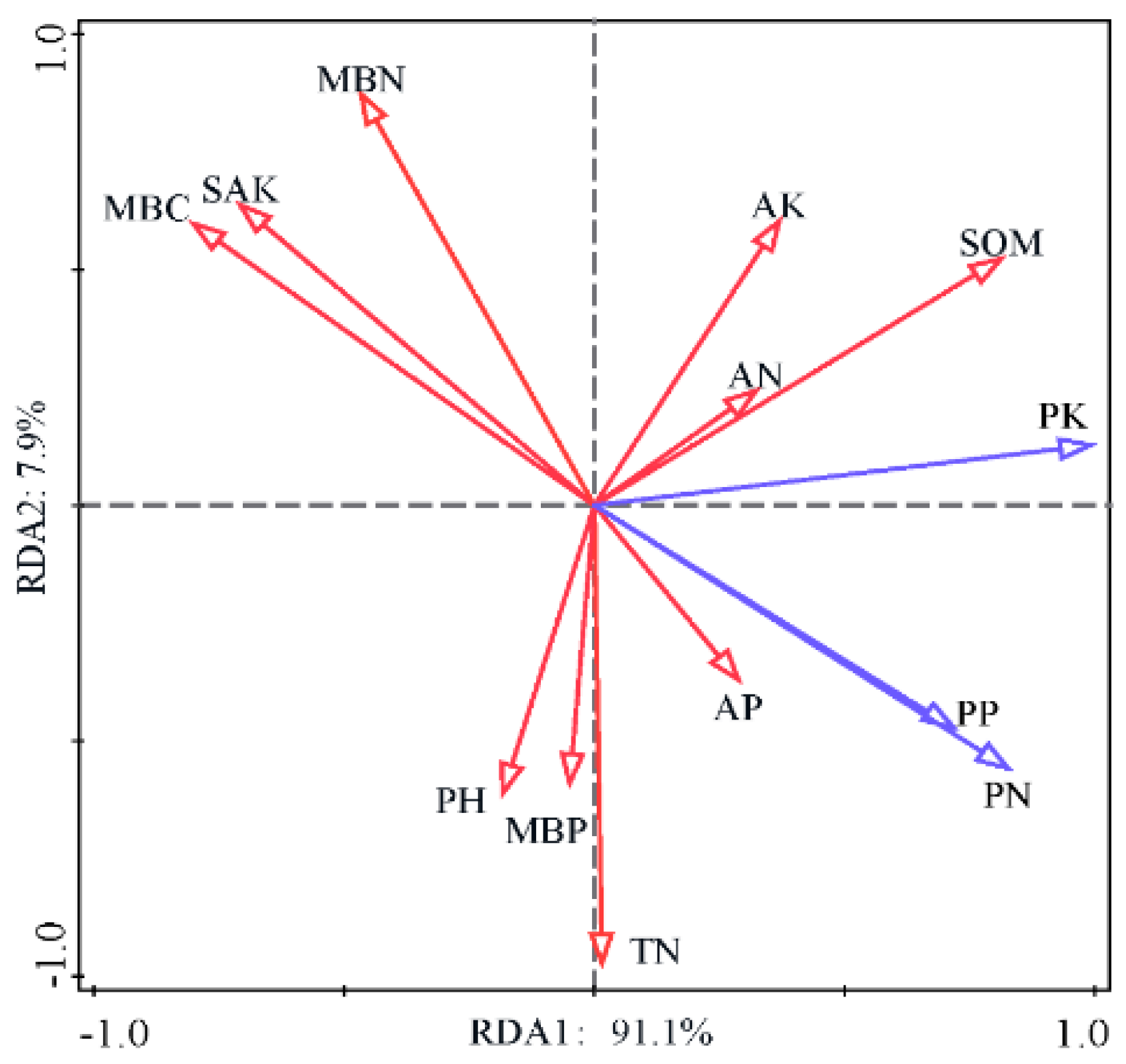
3.2. Relations Between Soil and Plant Nutrients
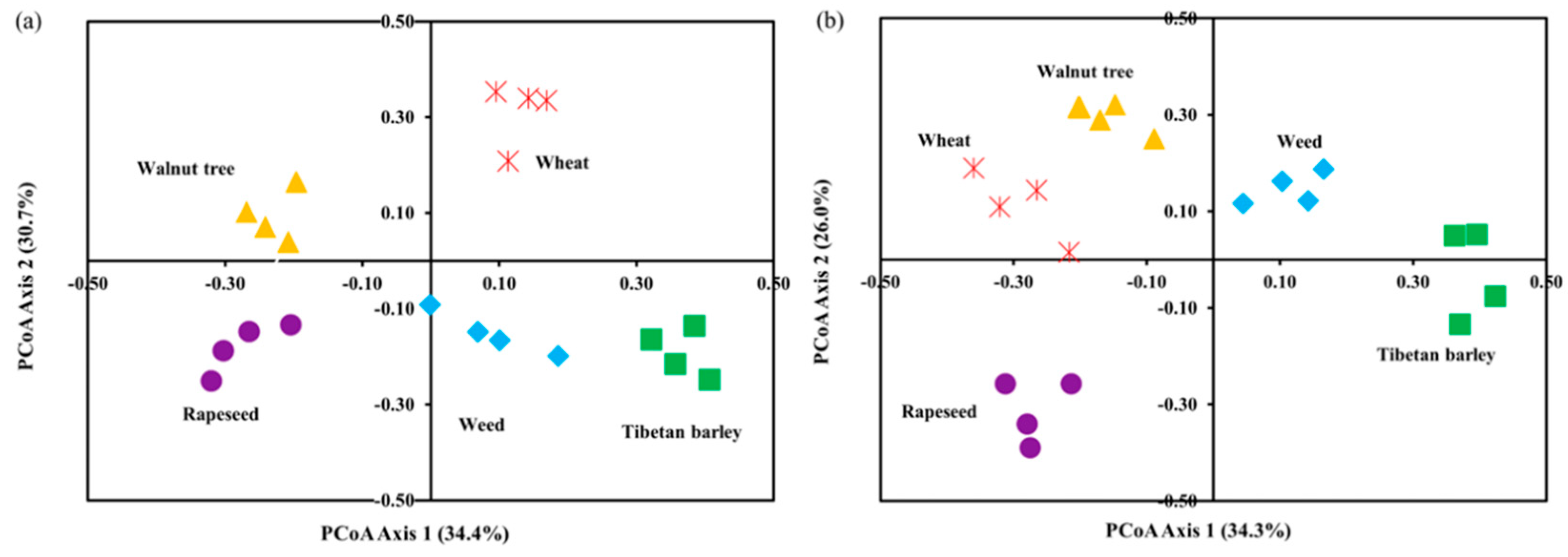
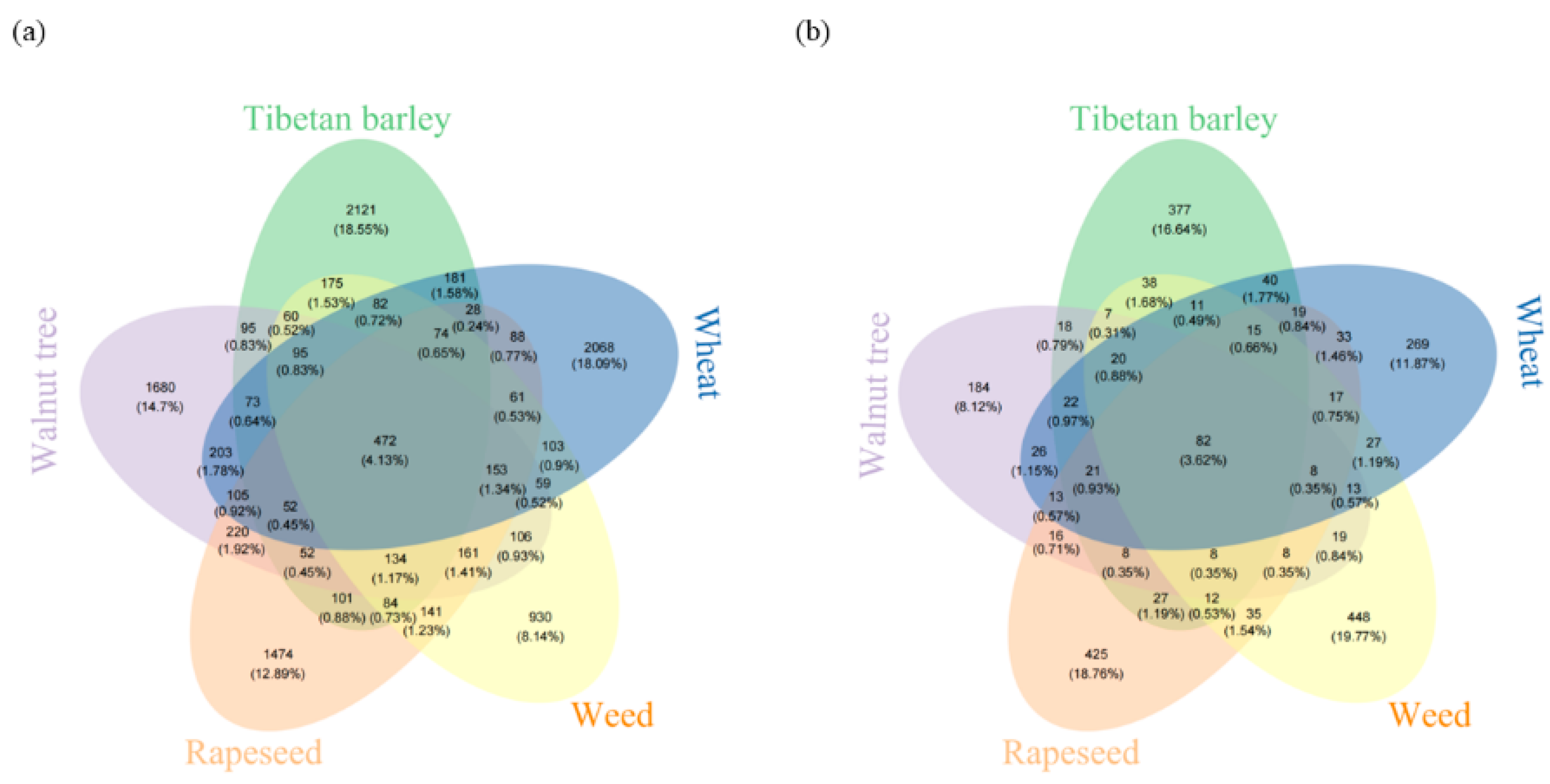
3.3. Microbial Alpha and Beta Diversity
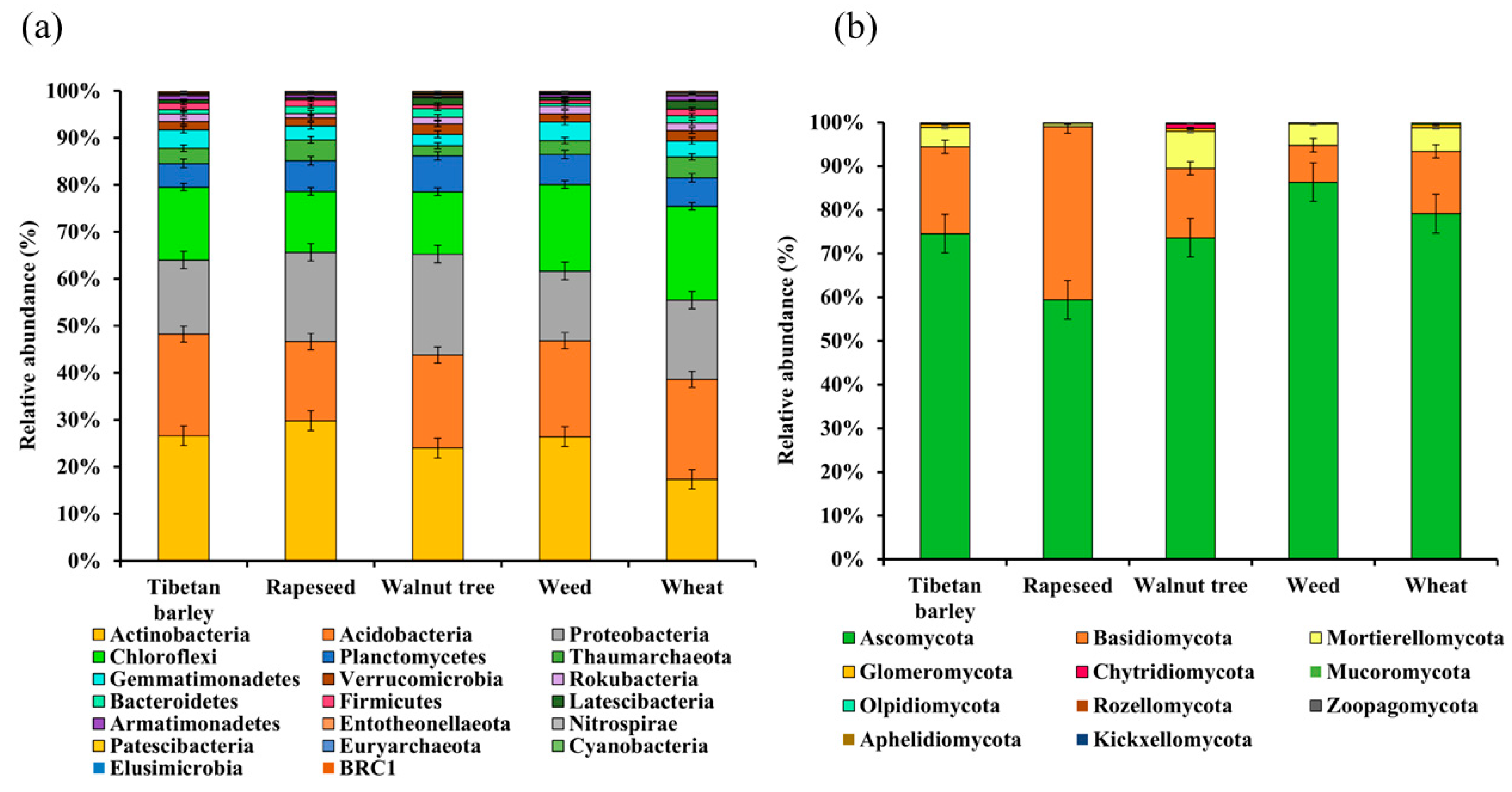
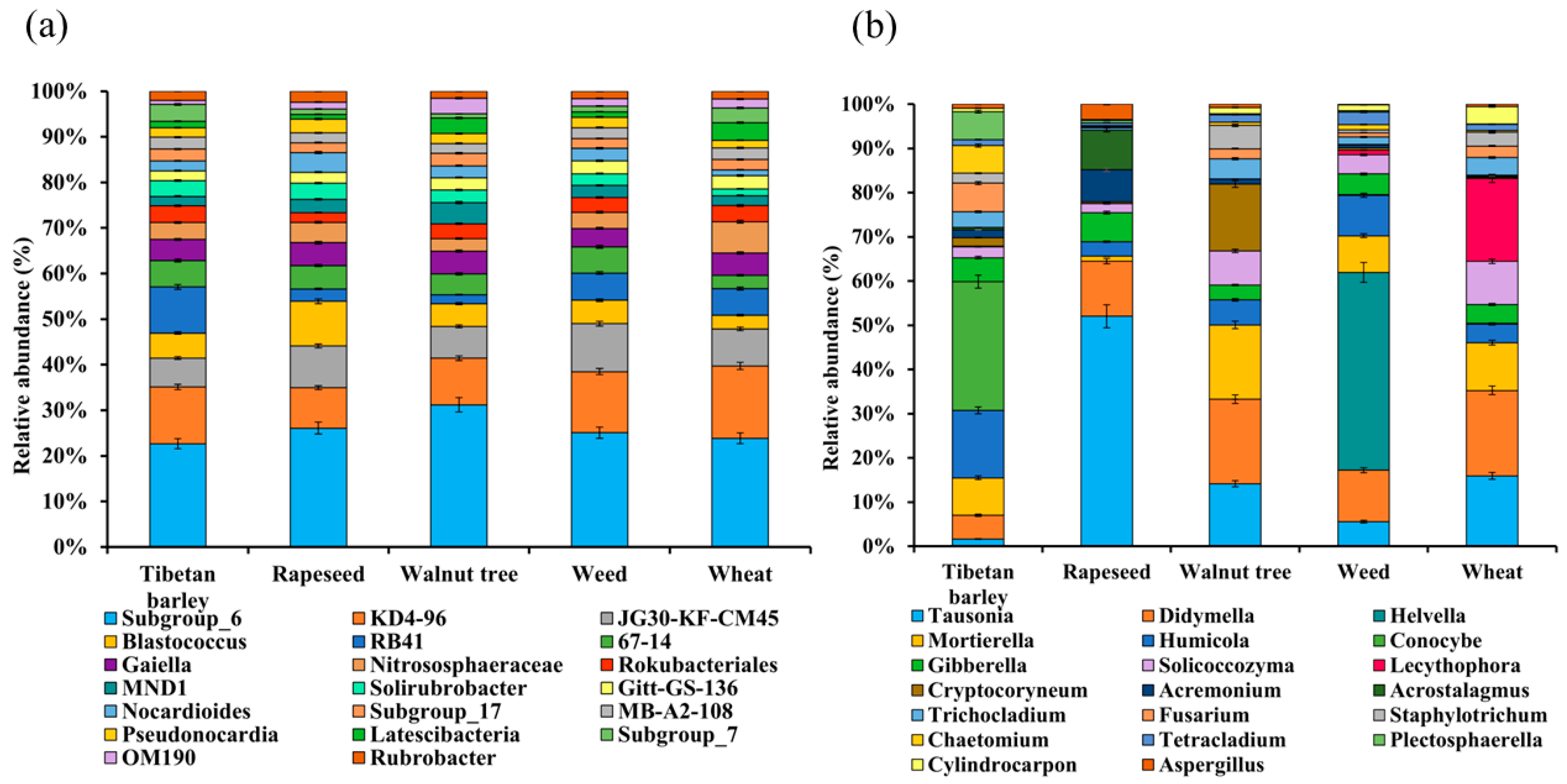
3.4. Microbial Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Sun, Z.K.; Sun, C.Z.; Zhang, T.R.; Liu, J.; Wang, X.N.; Feng, J.; Li, S.C.; Tang, S.M.; Jin, K. Soil microbial community variation among different land use types in the agro-pastoral ecotone of northern China is likely to be caused by anthropogenic activities. Front. Microbiol. 2024, 15, 1390286. [Google Scholar] [CrossRef]
- Muñoz-Arenas, L.C.; Fusaro, C.; Hernández-Guzmán, M.; Dendooven, L.; Estrada-Torres, A.; Navarro-Noya, Y.E. Soil microbial diversity drops with land-use change in a high mountain temperate forest: A metagenomics survey. Environ. Microbiol. Rep. 2020, 12, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, A.T.; Fierer, N.; Turner, B.L.; Whitaker, T.J.; Ostle, N.J.; McNamara, N.P.; Bardgett, R.D.; Leff, W.J.; Salinas, N.; Silman, M.R.; et al. Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 2018, 99, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zhou, Y.; Shi, Z.; Viscarra Rossel, R.A.; Liang, Z.Z.; Wang, H.Z.; Zhou, L.Q.; Yu, W. Interactive effects of elevation and land use on soil bacterial communities in the Tibetan Plateau. Pedosphere 2020, 30, 817–831. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, Z.C.; Yu, Z.Q.; Shen, G.F.; Cheng, H.F.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef]
- Choudhury, B.U.; Ansari, M.A.; Chakraborty, M.; Meetei, T.T. Effect of land-use change along altitudinal gradients on soil micronutrients in the mountain ecosystem of Indian (Eastern) Himalaya. Sci. Rep. 2021, 11, 14279. [Google Scholar] [CrossRef]
- Díaz-Vallejo, E.J.; Seeley, M.; Smith, A.P.; Marín-Spiotta, E. A meta-analysis of tropical land-use change effects on the soil microbiome: Emerging patterns and knowledge gaps. Biotropica 2021, 53, 738–752. [Google Scholar] [CrossRef]
- Yan, R.Y.; Zhao, X.M.; Li, P.H.; Si, Z.Y.; Gao, Y.; Li, J.F. Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet. Land 2023, 12, 1419. [Google Scholar] [CrossRef]
- Vanegas-León, M.L.; Sulzbacher, M.A.; Rinaldi, A.C.; Roy, M.; Selosse, M.A.; Neves, M.A. Are Trechisporales ectomycorrhizal or non-mycorrhizal root endophytes? Mycol. Prog. 2019, 18, 1231–1240. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, P.; Liu, F.; Li, S.Z.; Zhang, J.J.; Dang, Y.; Wang, L.; Wang, S.Y.; Cheng, W.L.; Cai, T.; et al. Plow layer management during the fallow season can enhance the wheat productivity and resource utilization in a semi-arid region. Soil Tillage Res. 2023, 228, 105633. [Google Scholar] [CrossRef]
- Cahanovitc, R.; Livne-Luzon, S.; Angel, R.; Klein, T. Ectomycorrhizal fungal mediate belowground carbon transfer between pines and oaks. ISME J. 2022, 16, 1420–1429. [Google Scholar] [CrossRef]
- Manirakiza, E.; Ziadi, N.; Hamel, C.; Levesque, V.; Antoun, H.; Karam, A. Soil microbial community dynamics after co-application of biochar and paper mill biosolids. Appl. Soil Ecol. 2021, 165, 103960. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Yang, Q.; Wang, H.; Li, Y.; Liu, Y.; Wu, J.; Cai, Z. Organic Fertilizer Substitution Enhances Soil Microbial Diversity and Rice Productivity in Paddy Systems. Soil Biol. Biochem. 2023, 185, 109144. [Google Scholar] [CrossRef]
- Zhao, J.H.; Chen, L.; Zhou, G.X.; Li, F.; Zhang, J.B.; Zhang, C.Z.; Ma, D.H.; Feng, B. Organic-inorganic fertilization promotes paddy soil macroaggregate organic carbon accumulation associated with key bacterial populations in subtropical China. Pedosphere 2024, 34, 941–950. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhai, X.X.; Wang, M.R.; Shi, Y.J.; Chen, Y.M.; Liang, Q.M.; He, B.; Wen, R.H. Biochar amendments improve soil functionalities, microbial community and reduce Pokkah boeng disease of sugarcane. Chem. Biol. Technol. Agric. 2024, 11, 28. [Google Scholar] [CrossRef]
- Yang, Q.E.; Ma, X.D.; Li, M.C.; Zhao, M.S.; Zeng, L.S.; He, M.Z.; Deng, H.; Liao, H.P.; Rengsing, C.; Friman, V.P.; et al. Evolution of triclosan resistance modulates bacterial permissiveness to multidrug resistance plasmids and phages. Nat. Commun. 2024, 15, 3654. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- Mcbain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Price, B.B.; Gilbert, P. Exposure of sink drain micro-cosmos to triclosan: Population dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 2003, 69, 5433–54442. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Sun, H.; Chen, Q.; Jin, H.; Yang, J.; Tao, K. Soil microbial diversity and composition response to degradation of the alpine meadow in the southeastern Qinghai-Tibet Plateau. Environ. Sci. Pollut. Res. 2024, 31, 26076–26088. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, B.X.; Xu, C.Y.; Raza, M.; Wang, Q.; Wang, Q.; Wang, Q.Z.; Fu, Y.N.; Hu, J.Y.; Imoulan, A.; et al. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef] [PubMed]
- Baruch, Z.; Liddicoat, C.; Laws, M.; Marker, L.K.; Morelli, H.; Yan, D.F.; Young, J.M.; Breed, M.F. Characterizing the soil fungal microbiome in metropolitan green spaces across a vegetation biodiversity gradient. Fungal Ecol. 2020, 47, 100939. [Google Scholar] [CrossRef]
- Shu, X.Y.; Hu, Y.F.; Liu, W.J.; Xia, L.L.; Zhang, Y.Y.; Zhou, W.; Liu, W.L.; Zhang, Y.L. Linking between soil properties, bacterial communities, enzyme activities, and soil organic carbon mineralization under ecological restoration in an alpine degraded grassland. Front. Microbiol. 2023, 14, 1131836. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Luo, A.R.; Zhou, C.N. Variation characteristics of forest soil nutrients and their ecological stoichiometry in sejila mountains of southeast Tibet, China. Appl. Ecol. Environ. Res. 2023, 21, 681–697. [Google Scholar] [CrossRef]
- Toonen, J.; Francioli, D.; Hannula, S.E.; Bel, N.; van Bodegom, P.M.; Yang, X.; Bezemer, T.M. Mycorrhizal Fungi Mediate Carbon Transfer Between Tree Species in a Mixed Forest Ecosystem. New Phytol. 2023, 239, 186–198. [Google Scholar] [CrossRef]
- Liu, J.; Kang, L.Y.; Du, L.F.; Liao, S.Q.; Dong, W.; Ma, M.T.; Zou, G.Y.; Li, S.J. Distribution, Accumulation and Translocation of the Heavy Metal Cd in Various Varieties of Edible Rapeseed under Cd Stress. Sustainability 2024, 16, 2876. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, Q.; Tu, C. Responses of soil microbial community composition and enzyme activities to land-use change in the eastern Tibetan Plateau, China. Forests 2019, 10, 483. [Google Scholar] [CrossRef]
- Ishizuka, S.; Hashimoto, S.; Kaneko, S.; Tsuruta, K.; Kida, K.; Aizawa, S.; Hashimoto, T.; Ito, E.; Umemura, M.; Shinomiya, Y.; et al. Soil Carbon Stock Change Due to Afforestation in Japan by Paired-Sampling Method in an Equivalent Mass Basis. Biogeochemistry 2021, 153, 263–281. [Google Scholar] [CrossRef]
- Jeffery, S.; van de Voorde, T.F.; Harris, W.E.; Mommer, L.; Van Groenigen, J.W.; De Deyn, G.B.; Ekelund, F.; Briones, M.J.; Bezemer, T.M. Biochar application differentially affects soil micro-, meso-macro-fauna and plant productivity within a nature restoration grassland. Soil Biol. Biochem. 2022, 174, 108789. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Pan, K.; Justine, M.F.; Zhao, F.; Olatunji, O.A.; Gong, Y.; Li, Y.; Chen, L.; Han, X.; et al. Unraveling Key Functional Bacteria across Land-Use Types on the Tibetan Plateau. Ecosyst. Health Sustain. 2023, 9, 020708. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, M.; Wu, J.; Liu, Y.; Zhang, Y.; Zhao, H.; Cai, T.; Dang, Y.; Wang, L. Effects of Fallow Tillage Practices on Soil Properties and Wheat Yield in a Semi-Arid Region. Agric. Syst. 2024, 214, 103819. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Cai, Y.; Yang, Q.; Gao, C.; Chen, X.; Luo, Y.; Wang, Z. Soil Microbial Community Responses to Long-Term Tillage and Crop Rotation in a Subtropical Rice Ecosystem. Appl. Soil Ecol. 2021, 167, 104098. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, J.; Zhu, K.; Liu, L.; Chen, Y.; Li, J.; Wang, R. Effects of Potassium Fertilization on Rapeseed (Brassica napus L.) Yield and Nutrient Uptake under Drought Stress. Field Crops Res. 2022, 287, 108656. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Wang, Y.; Zhang, Y.; Liu, Y.; Ma, Y.; Zhang, C. Soil Bacterial Community Response to Land-Use Change in Subtropical and Temperate Forests. Appl. Soil Ecol. 2023, 181, 104647. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Yang, J.; Wang, H.; Zhang, Y.; Luo, Y.; Chen, X.; Gao, C. Soil Microbial Community Structure and Functioning in Response to Long-Term Organic and Inorganic Fertilization in a Rice-Wheat Cropping System. Appl. Soil Ecol. 2023, 182, 104705. [Google Scholar] [CrossRef]
- Gu, H.H.; He, Z.Y.; Lu, Z.F.; Liao, S.P.; Zhang, Y.Y.; Li, X.K.; Cong, R.H.; Ren, T. Growth and survival strategies of oilseed rape (Brassica napus L.) leaves under potassium deficiency stress: Trade-offs in potassium ion distribution between vacuoles and chloroplasts. Plant J. 2025, 121, e70009. [Google Scholar] [CrossRef]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree species shape soil bacterial community structure and function in temperate deciduous forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Govednik, A.; Potočnik, Ž.; Eler, K.; Suhadolc, M. Combined effects of long-term tillage and fertilisation regimes on soil organic carbon, microbial biomass, and abundance of the total microbial communities and N-functional guilds. Appl. Soil Ecol. 2023, 188, 104876. [Google Scholar] [CrossRef]
- Zumeaga, H.; Azcárate, F.M.; Concepción, E.D.; Hevia, V.; Díaz, M. Landscape and agri-environmental scheme effects on ant communities in cereal croplands of central Spain. Agric. Ecosyst. Environ. 2021, 312, 107345. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Liu, L.; Gu, X.; Gou, J.; Zhang, M.; Wang, Y.; Zhou, Z. Biochar Amendment Enhances Soil Microbial Diversity and Function in a Degraded Karst Ecosystem. Appl. Soil Ecol. 2022, 178, 104568. [Google Scholar] [CrossRef]
- Jarecke, K.M.; Loecke, T.D.; Burgin, A.J. Coupled soil oxygen and greenhouse gas dynamics under variable hydrology. Soil Biol. Biochem. 2016, 95, 164–172. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, R.; Kumar, S.; Laishram, J.; Meetei, T.T.; Das, A.; Choudhury, B.U. Soil Micronutrient Dynamics and Stocks Under Land-Use Change in the Subtropical Foothills of Eastern Himalaya. Geoderma 2023, 429, 116255. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Pandey, A.; Thakur, A.; Kaur, S. Rhizospheric Microbial Diversity and Plant Growth-Promoting Bacteria for Sustainable Agriculture in Himalayan Agroecosystems. Microbiol. Res. 2021, 252, 126860. [Google Scholar] [CrossRef]

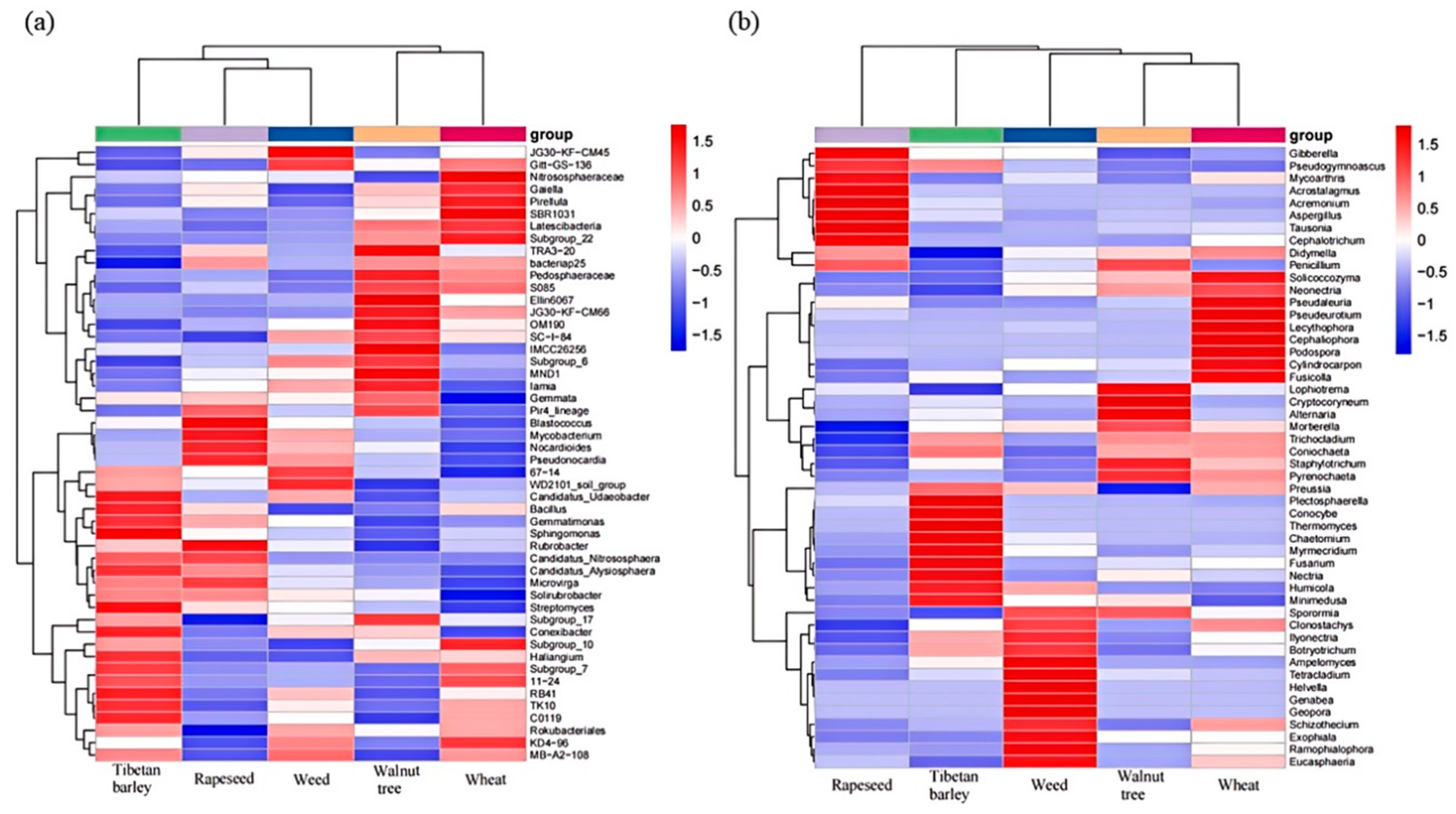
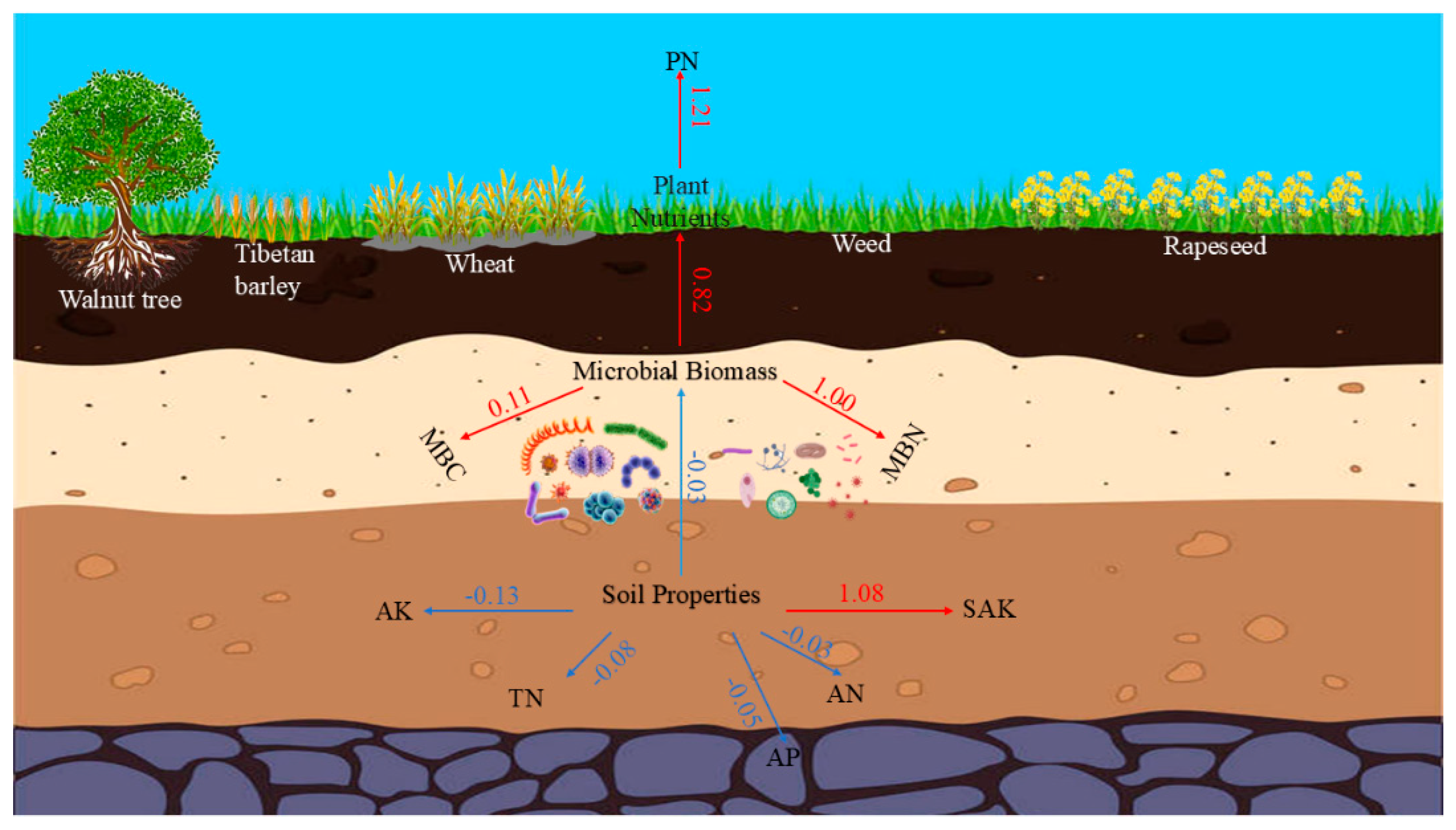
| pH | SOM (g/kg) | TN (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | SAK (mg/kg) | MBP (mg/kg) | MBC (mg/kg) | MBN (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tibetan barley | 7.91 a | 40 b | 0.06 b | 297.3 d | 8.43 d | 72.1 d | 1031.9 c | 23.8 c | 12.9 b | 50.3 d |
| Rapeseed | 7.38 c | 47 a | 0.04 c | 361 c | 24.43 b | 229.4 a | 1006.2 cd | 23.2 c | 8 c | 142.6 c |
| Walnut trees | 7.42 b | 38.9 c | 0.06 b | 398.9 a | 48.73 a | 194.8 b | 1126.2 b | 55.9 a | 8.3 c | 36.3 d |
| Weeds | 7.34 d | 48.1 a | 0.07 a | 369.6 b | 16.16 c | 189.2 c | 1158.6 b | 28.6 b | 13.7 b | 243.2 b |
| Wheat | 7.32 d | 40.3 b | 0.03 d | 352 d | 17.75 c | 212.4 b | 1843.9 a | 27.3 b | 24.8 a | 422.1 a |
| Sample | Richness | Diversity | Coverage | |||
|---|---|---|---|---|---|---|
| Chao1 | Species | Simpson | Shannon | |||
| Bacteria | Tibetan barley | 3911 a | 3880 ab | 0.9984 a | 10.75 ab | 0.9985 a |
| Rapeseed | 3411 c | 3400 d | 0.9976 a | 10.53 d | 0.9993 a | |
| Walnut trees | 3753 b | 3719 c | 0.9986 a | 10.79 a | 0.9985 a | |
| Weeds | 2895 d | 2889 e | 0.9979 a | 10.36 e | 0.9996 a | |
| Wheat | 3920 a | 3894 a | 0.9983 a | 10.67 c | 0.9986 a | |
| Fungi | Tibetan barley | 755 a | 745 ab | 0.9742 b | 6.738 b | 0.9995 a |
| Rapeseed | 482 e | 474 d | 0.8885 e | 4.897 e | 0.9996 a | |
| Walnut trees | 733 c | 723 b | 0.9732 bc | 6.538 c | 0.9995 a | |
| Weeds | 636 d | 636 c | 0.9475 d | 6.502 cd | 0.9999 a | |
| Wheat | 773 ab | 767 a | 0.9801 a | 7.156 a | 0.9997 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; He, W.; Xiang, F.; Zhu, J.; Li, J. Effects of Different Land-Use Types on Soil Properties and Microbial Communities in a Southeastern Tibetan Valley. Agronomy 2025, 15, 2317. https://doi.org/10.3390/agronomy15102317
Zhao X, He W, Xiang F, Zhu J, Li J. Effects of Different Land-Use Types on Soil Properties and Microbial Communities in a Southeastern Tibetan Valley. Agronomy. 2025; 15(10):2317. https://doi.org/10.3390/agronomy15102317
Chicago/Turabian StyleZhao, Ximei, Wenyan He, Fengyun Xiang, Jianqiang Zhu, and Jifu Li. 2025. "Effects of Different Land-Use Types on Soil Properties and Microbial Communities in a Southeastern Tibetan Valley" Agronomy 15, no. 10: 2317. https://doi.org/10.3390/agronomy15102317
APA StyleZhao, X., He, W., Xiang, F., Zhu, J., & Li, J. (2025). Effects of Different Land-Use Types on Soil Properties and Microbial Communities in a Southeastern Tibetan Valley. Agronomy, 15(10), 2317. https://doi.org/10.3390/agronomy15102317






