Potential Contributions of Residual Soil Nitrogen to Subsequent Ratoon Sugarcane Crops in the Wet Subtropics
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Soil Indicators
2.2. Determination of Root Indicators
2.3. Statistical Analysis and Graphing of Data
3. Results
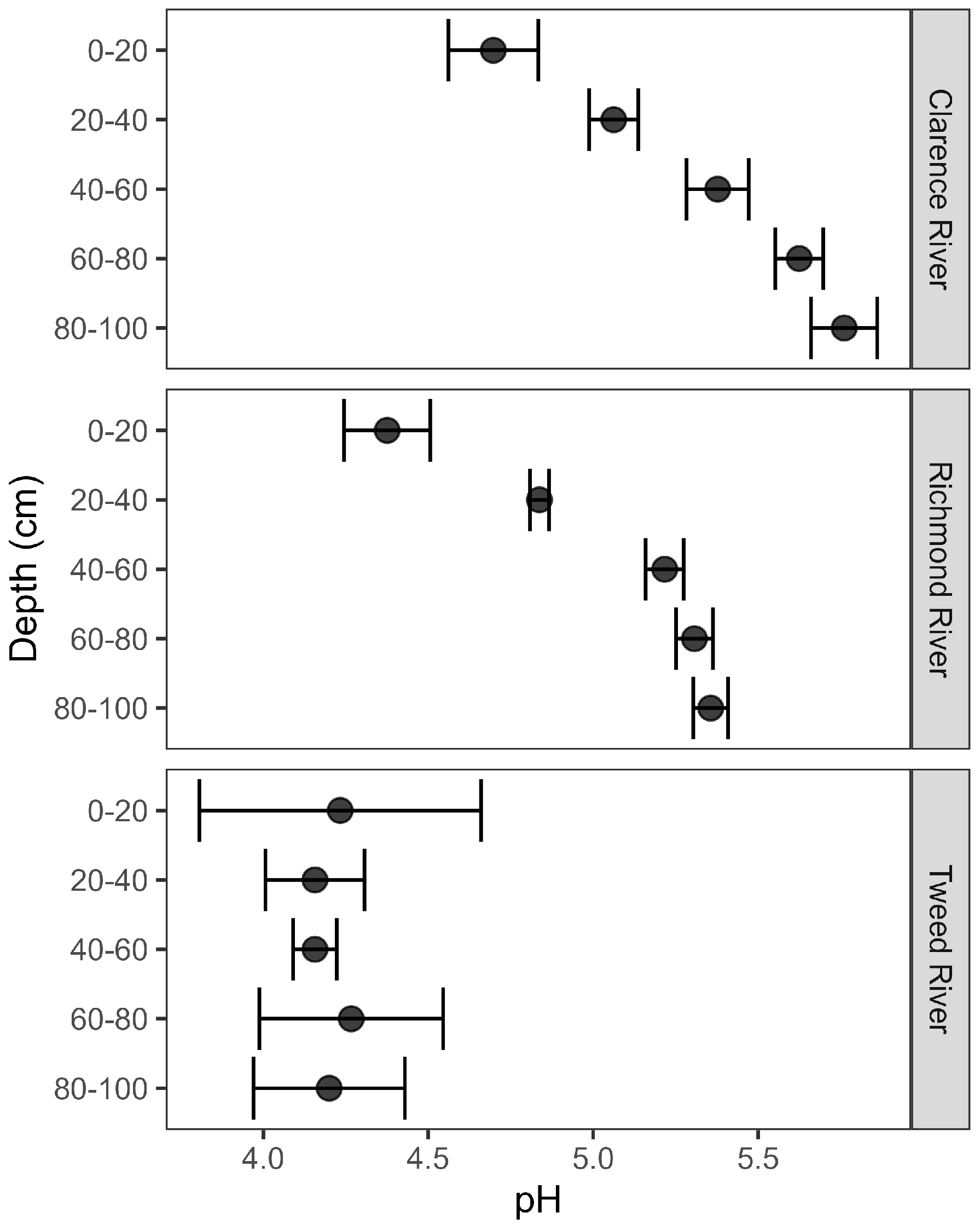
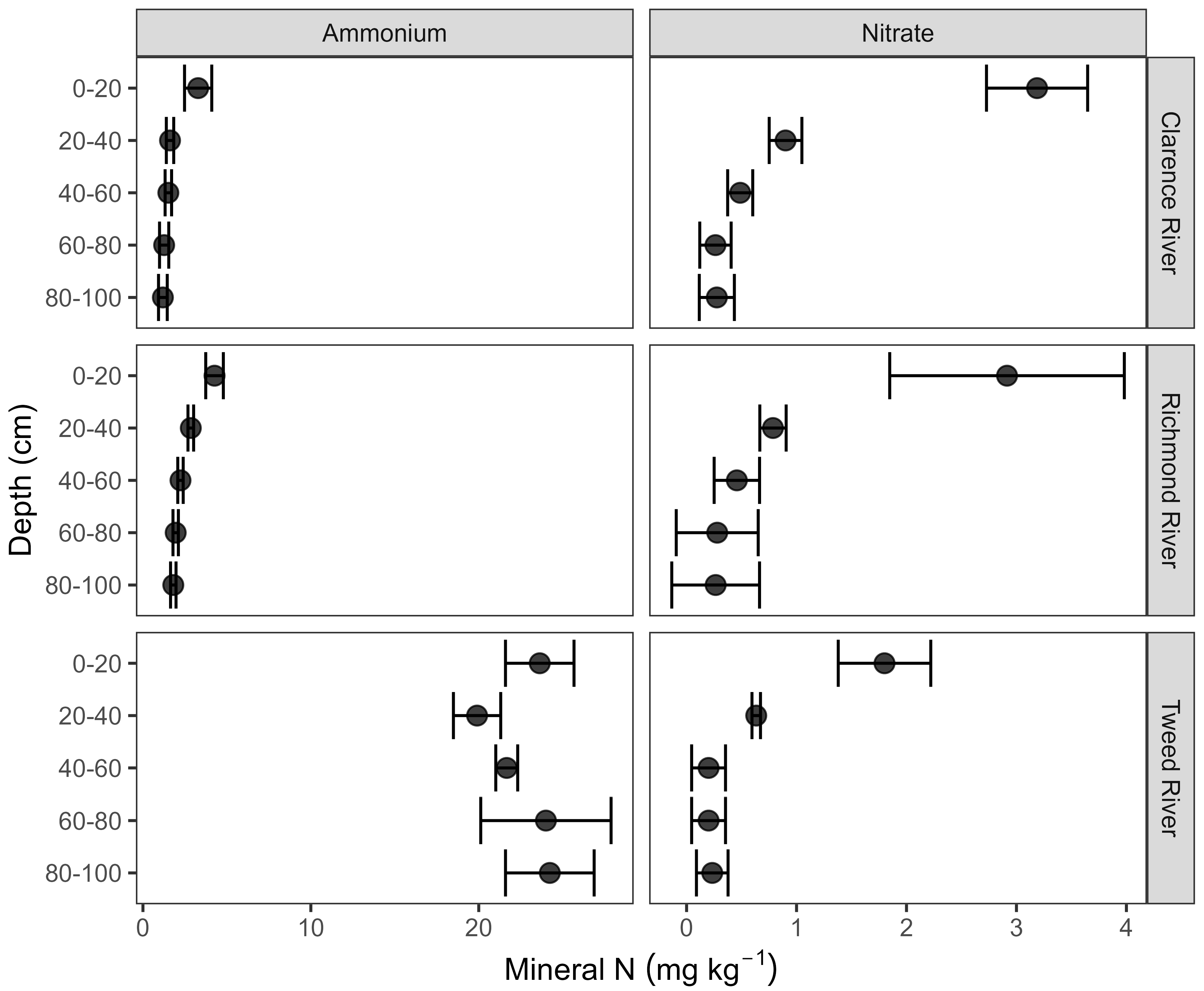
3.1. Soil pH and Concentrations of Total C, Total N and Mineral N Throughout Soil Profiles Across the Clarence, Richmond and Tweed River Catchments
3.2. Mineral N to a Depth of 1 m, Root Depth and Plant-Available Mineral N
3.3. Potentially Mineralisable N to a Depth of 40 cm and Total Available N
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keating, B.A.; Verburg, K.; Huth, N.I.; Robertson, M.J. Nitrogen management in intensive agriculture: Sugarcane in Australia. In Intensive Sugarcane Production: Meeting the Challenges Beyond 2000; Keating, B.A., Wilson, J.J., Eds.; CAB International: Wallingford, UK, 1997; pp. 221–242. [Google Scholar]
- Skocaj, D.M.; Everingham, Y.L.; Schroeder, B.L. Nitrogen management guidelines for sugarcane production in Australia: Can these be modified for wet tropical conditions using seasonal climate forecasting? Springer Sci. Rev. 2013, 1, 51–71. [Google Scholar] [CrossRef]
- Nachimuthu, G.; Bell, M.J.; Halpin, N. Nitrogen losses in terrestrial hydrological pathways in sugarcane cropping systems of Australia. J. Soil Wat. Conserv. 2017, 72, 32–35. [Google Scholar] [CrossRef]
- Bell, M.J. A Review of Nitrogen Use Efficiency in Sugar Cane; Sugar Research Australia Ltd.: Brisbane, Australia, 2014; 344p. [Google Scholar]
- Meier, E.A.; Thorburn, P.J. Long term sugarcane crop residue retention offers limited potential to reduce nitrogen fertilizer rates in Australian wet tropical environments. Front. Plant Sci. 2016, 7, 1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Park, G.; Reeves, S.; Zahmel, M.; Heenan, M.; Salter, B. Nitrous oxide emission and fertiliser nitrogen efficiency in a tropical sugarcane cropping system applied with different formulations of urea. Soil Res. 2016, 54, 572–584. [Google Scholar] [CrossRef]
- Rose, T.J.; Morris, S.G.; Quin, P.; Kearney, L.; Kimber, S.; Van Zwieten, L. The nitrification inhibitor DMPP applied to subtropical rice has an inconsistent effect on nitrous oxide emissions. Soil Res. 2017, 55, 547–552. [Google Scholar] [CrossRef]
- Rose, T.J.; Quin, P.; Morris, S.G.; Kearney, L.J.; Kimber, S.; Rose, M.T.; Van Zwieten, L. No evidence for higher agronomic N use efficiency or lower nitrous oxide emissions from enhanced efficiency fertilisers in aerobic subtropical rice. Field Crops Res. 2018, 225, 47–54. [Google Scholar] [CrossRef]
- Rose, T.J.; Kearney, L.J.; Zeng, J.; Van Zwieten, L.; Rose, M.T. DMPP-urea restricts nitrification in the first month without improving agronomic N use efficiency. Nutr. Cycl. Agroecosyst. 2023, 126, 115–125. [Google Scholar] [CrossRef]
- Rose, T.J.; Rust, J.; Van Zwieten, L.; Rose, M.T. Polymer coated urea does not improve nitrogen use efficiency above urea in ratoon sugarcane crops in the wet subtropics. Nutr. Cycl. Agroecosyst. 2024, 130, 255–267. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Palmer, J.; Meier, E.A.; Verburg, K.; Skocaj, D.M. Prioritizing crop management to increase nitrogen use efficiency in Australian sugarcane crops. Front. Plant Sci. 2017, 8, 1504. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Webster, A.J.; Biggs, I.M. An improved way to determine nitrogen fertiliser requirements of sugarcane crops to meet global environmental challenges. Plant Soil 2011, 339, 51–67. [Google Scholar] [CrossRef]
- Schroeder, B.L.; Wood, A.W.; Moody, P.W.; Bell, M.J.; Garside, A.L. Nitrogen fertiliser guidelines in perspective. Proc. Aust. Soc. Sugar Cane Technol. 2005, 27, 291–304. [Google Scholar]
- Bock, B.R.; Kelley, K.R.; Meisinger, J.J. Predicting N fertilizer needs in humid regions: Summary and future directions. In Predicting N Fertilizer Needs for Corn in Humid Regions; Bock, B.R., Kelley, K.R., Eds.; Bulletin Y-226, Muscle Shoals, A1; National Fertilizer and Environmental Research Center, Tennessee Valley Authority: Muscle Shoals, AL, USA, 1992; p. Ch. 9. [Google Scholar]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods–Australasia; CSIRO Publishing: Clayton, Australia, 2011. [Google Scholar]
- Allen, D.E.; Bloesch, P.M.; Orton, T.G.; Schroeder, B.L.; Skokaj, D.M.; Wang, W.; Masters, B.; Moody, P.M. Nitrogen mineralisation in sugarcane soils in Queensland, Australia: I. evaluation of soil tests for predicting nitrogen mineralisation. Soil Res. 2019, 57, 738–754. [Google Scholar] [CrossRef]
- Hope, R.M. Rmisc: Rmisc: Ryan Miscellaneous. R package Version 1.5. 2013. Available online: https://CRAN.R-project.org/package=Rmisc (accessed on 22 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 May 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Australian Sugar Milling Council. Sugar Cane Statistics. 2025. Available online: https://sugarmanufacturers.org/annual-industry-statistics/ (accessed on 24 September 2025).
- Wood, A.W.; Muchow, R.C.; Robertson, M.J. Growth of sugarcane under high input conditions in tropical Australia. III. Accumulation, partitioning and use of nitrogen. Field Crops Res. 1996, 48, 223–233. [Google Scholar] [CrossRef]
- Robinson, N.; Brackin, R.; Vinall, K.; Soper, F.; Holst, H.; Gamage, H.; Paungfoo-Lonhienne, C.; Rennenberg, H.; Lakshmanan, P.; Schmidt, S. Nitrate paradigm does not hold up for sugarcane. PLoS ONE 2011, 6, e19045. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Trivelin, P.C.O. “Preferential” ammonium uptake by sugarcane does not increase 15N recovery of fertilizer sources. Plant Soil 2018, 429, 253–269. [Google Scholar] [CrossRef]
- Battie Laclau, P.; Laclau, J.P. Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Res. 2009, 114, 351–360. [Google Scholar] [CrossRef]
- Chopart, J.L.; Azevedo, M.C.B.; Le Mezo, L.; Marion, D. Sugarcane root system depth in three different countries. Proc. Int. Soc. Sugar Cane Technol. 2010, 27, 1–8. [Google Scholar]
- Smith, D.M.; Inman-Bamber, N.G.; Thorburn, P.J. Growth and function of the sugarcane root system. Field Crops Res. 2005, 92, 169–183. [Google Scholar] [CrossRef]
- Chopart, J.L.; Rodrigues, S.; de Azevedo, M.C.; Medina, C.D. Estimating sugarcane root length density through root mapping and orientation modelling. Plant Soil 2008, 313, 101–112. [Google Scholar] [CrossRef]
- Chumphu, S.; Jongrungklang, N.; Songsri, P. Association of physiological responses and root distribution patterns of ratooning ability and yield of the second ratoon cane in sugarcane elite clones. Agronomy 2019, 9, 200. [Google Scholar] [CrossRef]
- Pierre, J.S.; Giblot-Ducray, D.; McKay, A.C.; Hartley, D.M.; Perroux, J.M.; Rae, A.L. DNA based diagnostic for the quantification of sugarcane root DNA in the field. Sci. Rep. 2018, 8, 16720. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.A. Nutrient uptake by plant roots growing in soil. In Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons Inc.: New York, NY, USA, 1995; pp. 85–109. [Google Scholar]
- Glover, J.D. The behaviour of the root-system of sugarcane at and after harvest. Proc. S. Afr. Sug. Technol. Ass. 1968, 42, 133–135. [Google Scholar]
- Claassens, A.; Nock, C.J.; Rose, M.T.; Van Zwieten, L.; Rose, T.J. Colonisation dynamics of arbuscular mycorrhizal fungi and dark septate endophytes in the sugarcane crop cycle. Rhizosphere 2018, 7, 18–26. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium Triggers Lateral Root Branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-Dependent Manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef]
- Pries, C.E.H.; Sulman, B.N.; West, C.; O’Neill, C.; Poppleton, E.; Porras, R.C.; Castanha, C.; Zhu, B.; Wiedemeier, D.B.; Torn, M.S. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 2018, 125, 103–114. [Google Scholar] [CrossRef]


| Field | River Catchment | Sugarcane Harvest | Soil Coring | Days Between Harvest and Coring |
|---|---|---|---|---|
| Maclean 1 | Clarence | 27 September 2016 | 18 October 2016 | 21 |
| Maclean 2 | Clarence | 22 September 2016 | 18 October 2016 | 26 |
| Palmers Island 1 | Clarence | 21 August 2016 | 24 October 2016 | 64 |
| Palmers Island 2 | Clarence | 2 October 2016 | 24 October 2016 | 22 |
| Harwood 1 | Clarence | 5 October 2016 | 24 October 2016 | 19 |
| Harwood 2 | Clarence | 5 October 2016 | 24 October 2016 | 19 |
| Palmers Channel 1 | Clarence | 9 October 2016 | 24 October 2016 | 15 |
| Palmers Channel 2 | Clarence | 1 September 2016 | 24 October 2016 | 15 |
| Pimlico 1 | Richmond | 20 September 2016 | 28 September 2016 | 8 |
| Pimlico 2 | Richmond | 25 July 2016 | 28 September 2016 | 65 |
| Pimlico 3 | Richmond | 20 September 2016 | 28 September 2016 | 8 |
| Coraki | Richmond | 25 September 2016 | 10 October 2016 | 15 |
| Empire Vale 1 | Richmond | 16 November 2016 | 17 November 2016 | 1 |
| Empire Vale 2 | Richmond | 14 November 2016 | 17 November 2016 | 3 |
| South Ballina 1 | Richmond | 25 October 2016 | 8 November 2016 | 14 |
| South Ballina 2 | Richmond | 25 October 2016 | 8 November 2016 | 14 |
| Tatham 1 | Richmond | 30 September 2016 | 13 October 2016 | 13 |
| Tatham 2 | Richmond | 28 September 2016 | 13 October 2016 | 15 |
| Tatham 3 | Richmond | 28 September 2016 | 13 October 2016 | 15 |
| Teven 1 | Richmond | 13 October 2016 | 8 November 2016 | 36 |
| Teven 2 | Richmond | 10 October 2016 | 8 November 2016 | 39 |
| Woodburn | Richmond | 29 September 2016 | 7 October 2016 | 8 |
| Stott’s Creek | Tweed | 28 November 2016 | 14 December 2016 | 16 |
| Tyalgah | Tweed | 27 November 2016 | 21 December 2016 | 24 |
| Murwillumbah | Tweed | 21 November 2016 | 21 December 2016 | 23 |
| Field | NH4+-N (kg ha−1) | NO3−-N (kg ha−1) | Total Mineral-N (kg ha−1) | Available Mineral N (kg ha−1) | 14 d PMN to 40 cm (kg ha−1) | Total Available N (kg ha−1) |
|---|---|---|---|---|---|---|
| Maclean 1 | 34 ± 2.9 | 7.8 ± 0.7 | 42 ± 3.0 | 42 | 64 | 106 |
| Maclean 2 | 27 ± 4.4 | 24 ± 2.6 | 51 ± 2.1 | 51 | 111 | 166 |
| Palmers Island 1 | 19 ± 1.2 | 5.7 ± 1.5 | 25 ± 2.1 | 25 | 104 | 129 |
| Palmers Island 2 | 14 ± 0.7 | 11 ± 3.6 | 25 ± 3.2 | 25 | 108 | 133 |
| Harwood 1 | 15 ± 0.5 | 14 ± 2.4 | 29 ± 2.4 | 29 | 65 | 94 |
| Harwood 2 | 19 ± 1.9 | 8.9 ± 2.1 | 28 ± 3.9 | 28 | 96 | 124 |
| Palmers Channel 1 | 23 ± 6.1 | 7.3 ± 2.2 | 30 ± 5.4 | 30 | 59 | 89 |
| Palmers Channel 2 | 11 ± 1.2 | 7.0 ± 1.9 | 18 ± 2.0 | 16 | 76 | 92 |
| Pimlico 1 | 48 ± 6.2 | 3.9 ± 0.1 | 52 ± 6.2 | 52 | 107 | 159 |
| Pimlico 2 | 27 ± 1.6 | 6.2 ± 1.7 | 33 ± 3.1 | 33 | 51 | 84 |
| Pimlico 3 | 13 ± 2.3 | 53 ± 16.5 | 65 ± 17 | 63 | 78 | 141 |
| Coraki | 32 ± 3.3 | 14 ± 1.6 | 47 ± 3.2 | 47 | 193 | 240 |
| Empire Vale 1 | 21 ± 4.1 | 2.1 ± 0.0 | 24 ± 4.1 | 24 | 43 | 67 |
| Empire Vale 2 | 11 ± 0.1 | 1.8 ± 0.1 | 13 ± 0.2 | 8.1 | 36 | 44 |
| South Ballina 1 | 27 ± 6.7 | 2.9 ± 0.4 | 29 ± 6.8 | 20 | 77 | 97 |
| South Ballina 2 | 14 ± 0.2 | 3.9 ± 0.6 | 18 ± 0.7 | 13 | 57 | 70 |
| Tatham 1 | 47 ± 2.7 | 7.2 ± 1.9 | 54 ± 2.1 | 54 | 195 | 249 |
| Tatham 2 | 36 ± 1.3 | 5.4 ± 0.7 | 42 ± 1.0 | 42 | 78 | 120 |
| Tatham 3 | 29 ± 0.3 | 17 ± 4.1 | 47 ± 3.8 | 47 | 153 | 200 |
| Teven 1 | 39 ± 20 | 5.3 ± 0.6 | 44 ± 20 | 44 | 41 | 85 |
| Teven 2 | 22 ± 1.2 | 5.6 ± 2.1 | 27 ± 2.1 | 27 | 60 | 87 |
| Woodburn | 42 ± 3.0 | 3.2 ± 0.7 | 45 ± 2.9 | 45 | 194 | 139 |
| Stott’s Creek | 163 ± 13 | 2.3 ± 0.5 | 165 ± 13 | 165 | 29 | 194 |
| Tyalgah | 236 ± 56 | 6.8 ± 0.6 | 242 ± 56 | 242 | 106 | 346 |
| Murwillumbah | 166 ± 5.7 | 4.0 ± 0.1 | 170 ± 5.6 | 170 | 25 | 195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, T.J.; Rust, J.; Rose, M.T.; Van Zwieten, L. Potential Contributions of Residual Soil Nitrogen to Subsequent Ratoon Sugarcane Crops in the Wet Subtropics. Agronomy 2025, 15, 2299. https://doi.org/10.3390/agronomy15102299
Rose TJ, Rust J, Rose MT, Van Zwieten L. Potential Contributions of Residual Soil Nitrogen to Subsequent Ratoon Sugarcane Crops in the Wet Subtropics. Agronomy. 2025; 15(10):2299. https://doi.org/10.3390/agronomy15102299
Chicago/Turabian StyleRose, Terry James, Joshua Rust, Michael Timothy Rose, and Lukas Van Zwieten. 2025. "Potential Contributions of Residual Soil Nitrogen to Subsequent Ratoon Sugarcane Crops in the Wet Subtropics" Agronomy 15, no. 10: 2299. https://doi.org/10.3390/agronomy15102299
APA StyleRose, T. J., Rust, J., Rose, M. T., & Van Zwieten, L. (2025). Potential Contributions of Residual Soil Nitrogen to Subsequent Ratoon Sugarcane Crops in the Wet Subtropics. Agronomy, 15(10), 2299. https://doi.org/10.3390/agronomy15102299







