Pre-Plant Biofumigation and Integrated Post-Plant Strategies for Management of Nacobbus aberrans and Meloidogyne incognita in Greenhouse Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Pre-Plant Biofumigation (Whole Plot)
2.3. Management Product Treatments (Subplots)
2.4. Nematode Population Density
2.5. Nematode Identification, Gall Index, Root-Damage Percentage, and Tomato Yield
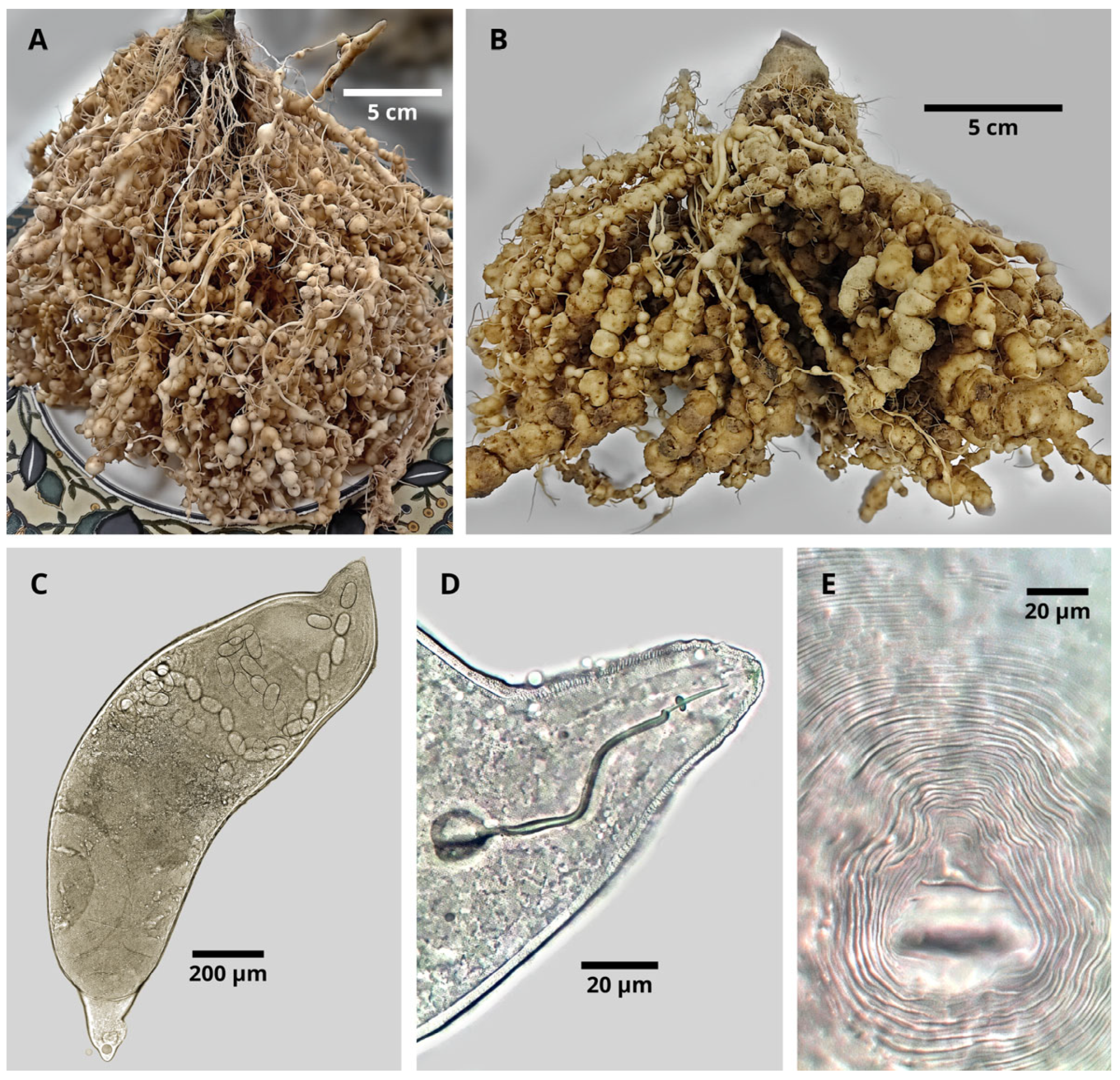
2.5.1. Nematode Identification and Root Assessments
2.5.2. Yield Assessment
2.6. Complementary Greenhouse Bioassay (Context Only)
2.7. Statistical Analysis and Cumulative Indices
3. Results
3.1. Identification of Nacobbus and Meloidogyne
3.2. Interaction Between Biofumigation and Management Product Treatments
3.3. Effects of Biofumigation on Nematode Population, Cumulative Root Damage, and Yield
3.4. Effects of Management Product Treatments on Nacobbus aberrans Population, Cumulative Root Damage, and Yield
4. Discussion
4.1. Effect of Pre-Plant Biofumigation on Nematode Population, Root Damage, and Yield
4.2. Post-Plant Management: Main and Interaction Effects
4.3. Species Composition, Anhydrobiosis, and Putative Soil Suppressiveness
4.4. Practical Implications, Limitations, and Qualitative Consistency Across Trials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUNPC | Area under the nematode population curve |
| AURDC | Area under the root-damage curve |
| DAT | Days after transplanting |
| BT | Before transplanting |
| GI | Gall index |
| WP | Whole plot |
| SP | Subplot |
| J2 | Second-stage juvenile |
| B | Biofumigated (whole plots) |
| NB | Non-biofumigated (whole plots) |
| BA | BioAct® (P. lilacinum, proprietary strain) |
| LS | Lila-Sin® (P. lilacinum, proprietary strain) |
| K | Kastelo® (P. lilacinum, proprietary strain) |
| S | Sterminar® (plant oil blend) |
| M | Majesty® (plant oil blend) |
References
- SIAP (Servicio de Información Agroalimentaria y Pesquera)–SADER (Secretaría de Agricultura y Desarrollo Rural). Producción Agrícola. 2024. Available online: https://www.gob.mx/siap (accessed on 2 March 2025).
- Sikora, R.A.; Fernández, E.; Desaeger, J. Integrated Nematode Management in a World in Transition. Annu. Rev. Phytopathol. 2023, 61, 125–146. [Google Scholar] [CrossRef]
- Kantor, C.; Eisenback, J.D.; Kantor, M. Biosecurity Risks to Human Food Supply Associated with Plant-Parasitic Nematodes. Front. Plant Sci. 2024, 15, 1404335. [Google Scholar] [CrossRef]
- Castro-López, R.; López-Orona, C.A.; Martínez-Gallardo, J.A.; Tirado-Ramírez, M.A.; Gómez, G.; Rubio-Aragón, W.; Edeza-Urias, J.A.; Villa-Medina, M.C. Field Applications of Fluorinated Nematicides for Meloidogyne enterolobii Management on Tomato. J. Nematol. 2024, 56, 20240030. [Google Scholar] [CrossRef]
- Hernández-Santiago, R.; Cid del Prado-Vera, I.; Vargas-Hernández, M.; Rodríguez-Guzmán, M.P.; Gómez-Rodríguez, O.; Ferrera-Cerrato, R. Integrated Management of Nacobbus aberrans (Tylenchida: Pratylenchidae) in Tomato under Organic Production in Greenhouses. Nematropica 2024, 54, 71–86. [Google Scholar]
- Cid del Prado-Vera, I.; Franco-Navarro, F.; Godinez-Vidal, D. Plant Parasitic Nematodes and Management Strategies of Major Crops in Mexico. In Plant Parasitic Nematodes in Sustainable Agriculture of North America; Springer: Cham, Switzerland, 2018; pp. 31–68. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A. Effects of Initial Inoculum Levels of Meloidogyne incognita J2 on development and growth of tomato cv. PT-3 under control conditions. Afr. J. Microbiol. Res. 2015, 9, 1376–1380. [Google Scholar] [CrossRef]
- Brennan, R.J.B.; Glaze-Corcoran, S.; Wick, R.; Hashemi, M. Biofumigation: An Alternative Strategy for the Control of Plant Parasitic Nematodes. J. Integr. Agric. 2020, 19, 1680–1690. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for Fighting Replant Disease—A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Antil, S.; Kumar, R.; Pathak, D.V.; Kumari, A. Recent Advances in Utilizing Bacteria as Biocontrol Agents against Plant Parasitic Nematodes Emphasizing Meloidogyne spp. Biol. Control 2023, 183, 105244. [Google Scholar] [CrossRef]
- Ayaz, M.; Zhao, J.-T.; Zhao, W.; Chi, Y.-K.; Ali, Q.; Ali, F.; Khan, A.R.; Yu, Q.; Yu, J.-W.; Wu, W.-C.; et al. Biocontrol of Plant Parasitic Nematodes by Bacteria and Fungi: A Multi-Omics Approach for the Exploration of Novel Nematicides in Sustainable Agriculture. Front. Microbiol. 2024, 15, 1433716. [Google Scholar] [CrossRef]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for Plant Growth Promotion and Biocontrol against Root-Knot Nematodes Infecting Eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef]
- De Souza Gouveia, A.; Monteiro, T.S.A.; Balbino, H.M.; de Magalhães, F.C.; Soares Ramos, M.A.; Silva Moura, V.A.; Dionizio Luiz, P.H.; de Almeida Oliveira, M.G.; de Freitas, L.G.; de Oliveira Ramos, H.J. Inoculation of Pochonia chlamydosporia Triggers a Defense Response in Tomato Roots, Affecting Parasitism by Meloidogyne javanica. Microbiol. Res. 2023, 266, 127242. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.O.; de Oliveira, C.M.; Ulhoa, C.J.; Côrtes, M.V.D.C.B.; Júnior, M.L.; da Rocha, M.R. Trichoderma harzianum and Trichoderma asperellum Are Potential Biocontrol Agents of Meloidogyne javanica in Banana cv. Grande Naine. Biol. Control 2022, 175, 105054. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the Activity and Biological Control Efficacy of Bacillus subtilis Bs-1 Against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Lax, P.; Passone, M.A.; Becerra, A.G.; Sosa, A.L.; Ciancio, A.; Finetti-Sialer, M.M.; Rosso, L.C. Sustainable Strategies for Management of the “False Root-Knot Nematode” Nacobbus spp. Front. Plant Sci. 2022, 13, 1046315. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Dong, B.; Zhang, H.; Zhang, S.; Qiao, K. Evaluation of Fluopyram for Southern Root-Knot Nematode Management in Tomato Production in China. Crop Prot. 2019, 122, 84–89. [Google Scholar] [CrossRef]
- Laquale, S.; Candido, V.; Avato, P.; Argentieri, M.P.; d’Addabbo, T. Essential Oils as Soil Biofumigant for the Control of the Root-Knot Nematode Meloidogyne incognita on Tomato. Ann. Appl. Biol. 2015, 167, 217–224. [Google Scholar] [CrossRef]
- Rosskopf, E.; Di Gioia, F.; Hong, J.C.; Pisani, C.; Kokalis-Burelle, N. Organic Amendments for Pathogen and Nematode Control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated Control of M. incognita in Tomatoes Using Fluopyram and Purpureocillium lilacinum Strain 251. Crop Prot. 2019, 124, 104874. [Google Scholar] [CrossRef]
- Ploeg, A.T.; Edwards, S.; Loffredo, A.; Becker, J.O. Efficacy of Fluorinated Nematicides for Management of Root-Knot Nematodes in California Processing Tomatoes. J. Nematol. 2024, 56, e2024-1. [Google Scholar] [CrossRef]
- Cobb, N.A. Estimating the Nema Population of the Soil, with Special Reference to the Sugar-Beet and Root-Gall Nemas, Heterodera Schachtii Schmidt and Heterodera Radicicola (Greef) Müller, and with a description of Tylencholaimus aequalis n. sp; Agric. Tech. Circ., No. 1; Bureau of Plant Industry, U.S. Department of Agriculture: Washington, DC, USA, 1918; pp. 1–48.
- Caveness, F.E.; Jensen, H.J. Modification of the Centrifugal-Flotation Technique for the isolation and concentration of nematodes and their eggs from soil and plant tissue. Proc. Helminthol. Soc. Wash. 1955, 22, 87–89. [Google Scholar]
- Jenkins, W.R. A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Manzanilla-López, R.H.; Costilla, M.A.; Doucet, M.; Franco, J.; Inserra, R.N.; Lehman, P.S.; Cid del Prado-Vera, I.; Souza, R.; Evans, K. The Genus Nacobbus Thorne Allen, 1944 (Nematoda: Pratylenchidae): Systematics, Distribution, Biology and Management. Nematropica 2002, 32, 149–227. [Google Scholar]
- Sasser, J.N.; Carter, C.C. An Advanced Treatise on Meloidogyne: Biology and Control; Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA, 1985; Volume I. [Google Scholar]
- Bridge, J.; Page, S.L.J. Estimation of Root-Knot Nematode Infestation Levels on Roots. Trop. Pest Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for Estimating Losses Caused by Diseases in Fungicide Experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Madden, L.; Hughes, G.; van den Bosch, F. The Study of Plant Disease Epidemics; APS Press: St. Paul, MN, USA, 2007. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Mateille, T.; Bailly-Bechet, M.; Marteu, N.; Fazari, A.; Bautheac, P.; Raptopoulo, A.; Van Duong, L.; Tavoillot, J.; Martiny, B.; et al. Evaluating Sorghums as Green Manure against Root-Knot Nematodes. Crop Prot. 2019, 122, 142–150. [Google Scholar] [CrossRef]
- Pérez-Espíndola, A.; Cid del Prado-Vera, I.; Alatorre-Rosas, R.; Suárez-Espinosa, J.; Rodríguez-Guzmán, M.P.; Ferris, M.H. Efecto de la biofumigación y Pochonia chlamydosporia en el Manejo de Nematodos Noduladores en Tomate. Nematropica 2019, 49, 172–180. [Google Scholar]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef]
- García-Velasco, R.; Chavarro-Carrero, E.A. Purpureocillium lilacinum (Hypocreales: Ophiocordycipitaceae) como Biocontrolador de Nacobbus aberrans (Tylenchida: Pratylenchidae) y Meloidogyne incognita (Tylenchida: Meloidogynidae) en Tomate cv. Río Grande. Acta Agríc. Pecu. 2020, 6, E0061021. [Google Scholar] [CrossRef]
- Prasad, L.; Pervez, R.; Gaba, S. Bio-Efficacy of Fungal Bioagents and Its Crude Secondary Metabolites Against Root Knot Nematode Meloidogyne incognita [(Kofoid & White,1919) Chitwood, 1949] Infesting Tomato (Solanum lycopersicum L.). Redia 2021, 104, 37–43. [Google Scholar] [CrossRef]
- Perry, R.N.; Moens, M. (Eds.) Plant Nematology, 2nd ed.; CABI: Wallingford, UK, 2013. [Google Scholar]
- Cid del Prado-Vera, I. Anhydrobiosis in Nacobbus aberrans (Nematoda: Pratylenchidae). In Proceedings of the Abstracts of the Annual Meeting of the Organization of Nematologists of Tropical America (ONTA), San José, Costa Rica, 21–25 July 2019; pp. 8–9. [Google Scholar]
- Schlatter, D.C.; Kinkel, L.L.; Thomashow, L.S.; Weller, D.M.; Paulitz, T.C. Disease suppressive soils: New insights from the soil microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef]
- Timper, P.; Liu, H.; Davis, R.F.; Wu, T. Influence of Crop Production Practices on Pasteuria penetrans and Suppression of Meloidogyne incognita. Biol. Control 2016, 99, 64–71. [Google Scholar] [CrossRef]
| No. | Active Ingredient/Product Type | Trade Name | Dose (ha−1) | Application Schedule |
|---|---|---|---|---|
| 1 | BA (biocontrol fungus) | BioAct® | 0.8 L | BT + every 20 DAT |
| 2 | Fluopyram (nematicide) | Verango® | 1 L (0.5 + 0.5) | BT (0.5 L) + 15 DAT (0.5 L) |
| 3 | T. erecta (botanical) | Nemacem® | 3 L | BT + every 15 DAT |
| 4 | S (mixed plant oils) | Sterminar® | 5 L | BT, 12 DAT, then every 30 DAT |
| 5 | T. viride (biocontrol fungus) | Trichomix | 3 L | BT + every 20 DAT |
| 6 | LS (biocontrol fungus) | Lila-Sin® | 480 g | BT + every 20 DAT |
| 7 | M (mixed plant oils) | Majesty® | 12 → 2 L | BT, 7 d ×3, then 14 d (12 → 9 → 6 → 3 → 2 L) |
| 8 | K (biocontrol fungus) | Kastelo® | 2 L | BT, 14 DAT ×2, then 28 DAT |
| 9 | P. chlamydosporia (biocontrol fungus) | Genexis PH® | 250 g | BT + every 15 DAT |
| 10 | Technological package 1: M + K | – | See 7 and 8 | Same schedules as 7 and 8 |
| 11 | Technological package 2: BA + T. erecta + T. viride + M | – | See 1, 3, 5, and 7 | BT (BA) → 7 DAT (T. viride) → 14 DAT (M) → 21 DAT (T. erecta); cycle repeats every 28 d |
| 12 | Technological package 3: T. erecta + S + LS + P. chlamydosporia | – | See 3, 4, 6, and 9 | BT (S) → 7 DAT (LS) → 14 DAT (P. chlamydosporia) → 21 DAT (T. erecta); cycle repeats every 28 d |
| 13 | Untreated control | – | – | – |
| No. | Management Treatments | AUNPC (Units) | AURDC (Units) | Tomato Yield (t ha−1) | |||
|---|---|---|---|---|---|---|---|
| B | NB | B | NB | B | NB | ||
| 1 | P. lilacinum (BA) | 13,830 a | 16,072 a | 551 ± 26 abc | 615 ± 6 a | 32 a | 30 a |
| 2 | Fluopyram | 6375 a | 27,690 a | 454 ± 17 c | 491 ± 25 bc | 48 a | 78 a |
| 3 | T. erecta | 2325 a | 12,015 a | 536 ± 25 abc | 607 ± 10 a | 32 a | 37 a |
| 4 | S | 15,638 a | 10,027 a | 570 ± 6 ab | 611 ± 17 a | 34 a | 37 a |
| 5 | T. viride | 7725 a | 13,192 a | 559 ± 15 abc | 607 ± 13 a | 31 a | 38 a |
| 6 | LS | 25,342 a | 37,942 a | 566 ± 9 ab | 600 ± 20 a | 32 a | 39 a |
| 7 | M | 20,715 a | 21,007 a | 532 ± 16 abc | 589 ± 25 ab | 37 a | 22 a |
| 8 | K | 12,600 a | 20,032 a | 566 ± 17 ab | 596 ± 9 a | 38 a | 35 a |
| 9 | P. chlamydosporia | 9150 a | 10,155 a | 562 ± 23 abc | 592 ± 52 ab | 45 a | 24 a |
| 10 | Technological package 1 | 4957 a | 15,135 a | 562 ± 13 ab | 577 ± 14 ab | 33 a | 27 a |
| 11 | Technological package 2 | 20,280 a | 4867 a | 540 ± 29 abc | 592 ± 10 a | 36 a | 31 a |
| 12 | Technological package 3 | 8107 a | 23,055 a | 574 ± 15 ab | 577 ± 29 ab | 35 a | 46 a |
| 13 | Control | 14,400 a | 77,430 a | 585 ± 30 ab | 630 ± 0 a | 19 a | 32 a |
| Nematode | Pre-Biofumigation (B Only) | −1 DAT | 60 DAT | 120 DAT | |||
|---|---|---|---|---|---|---|---|
| B | NB | B | NB | B | NB | ||
| N. aberrans | 3166 | 435 | 3627 | 1869 | 7330 | 16,218 | 17,984 |
| M. incognita | 0 | 0 | 0 | 35 | 0 | 105 | 135 |
| Whole-Plot Treatments | AUNPC (Units) | AURDC (Units) | Tomato Yield (t ha−1) |
|---|---|---|---|
| Biofumigation | 12,472 ± 1881 b | 548 ± 6.39 b | 35 a |
| Non-biofumigated | 20,402 ± 2256 a | 590 ± 9.11 a | 37 a |
| No. | Management Treatments | AUNPC (Units) | AURDC (Units) | Tomato Yield (t ha−1) |
|---|---|---|---|---|
| 1 | BA | 14,951 a | 583 ± 17.3 a | 31 ± 4.8 b |
| 2 | Fluopyram | 17,032 a | 472 ± 15.5 b | 63 ± 7.0 a |
| 3 | T. erecta | 7170 a | 572 ± 18.2 a | 34 ± 7.7 b |
| 4 | S | 12,832 a | 591 ± 11.3 a | 36 ± 6.4 b |
| 5 | T. viride | 10,459 a | 583 ± 13.1 a | 34 ± 6.7 b |
| 6 | LS | 31,642 a | 583 ± 12.1 a | 36 ± 5.5 b |
| 7 | M | 20,861 a | 560 ± 17.2 a | 30 ± 7.0 b |
| 8 | K | 16,316 a | 581 ± 10.5 a | 37 ± 5.3 b |
| 9 | P. chlamydosporia | 9652 a | 577 ± 24.8 a | 34 ± 6.6 b |
| 10 | Technological package 1 | 10,046 a | 570 ± 9.4 a | 30 ± 5.6 b |
| 11 | Technological package 2 | 12,574 a | 566 ± 17.4 a | 33 ± 5.3 b |
| 12 | Technological package 3 | 15,581 a | 575 ± 15.2 a | 40 ± 11.4 b |
| 13 | Control | 45,915 a | 607 ± 17.8 a | 26 ± 5.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magallanes-Tapia, M.A.; Cid del Prado-Vera, I.; Ferris, H.; Nava-Díaz, C.; González-Camacho, J.M.; Ochoa-Martínez, D.L. Pre-Plant Biofumigation and Integrated Post-Plant Strategies for Management of Nacobbus aberrans and Meloidogyne incognita in Greenhouse Tomato. Agronomy 2025, 15, 2284. https://doi.org/10.3390/agronomy15102284
Magallanes-Tapia MA, Cid del Prado-Vera I, Ferris H, Nava-Díaz C, González-Camacho JM, Ochoa-Martínez DL. Pre-Plant Biofumigation and Integrated Post-Plant Strategies for Management of Nacobbus aberrans and Meloidogyne incognita in Greenhouse Tomato. Agronomy. 2025; 15(10):2284. https://doi.org/10.3390/agronomy15102284
Chicago/Turabian StyleMagallanes-Tapia, Marco Antonio, Ignacio Cid del Prado-Vera, Howard Ferris, Cristian Nava-Díaz, Juan Manuel González-Camacho, and Daniel Leobardo Ochoa-Martínez. 2025. "Pre-Plant Biofumigation and Integrated Post-Plant Strategies for Management of Nacobbus aberrans and Meloidogyne incognita in Greenhouse Tomato" Agronomy 15, no. 10: 2284. https://doi.org/10.3390/agronomy15102284
APA StyleMagallanes-Tapia, M. A., Cid del Prado-Vera, I., Ferris, H., Nava-Díaz, C., González-Camacho, J. M., & Ochoa-Martínez, D. L. (2025). Pre-Plant Biofumigation and Integrated Post-Plant Strategies for Management of Nacobbus aberrans and Meloidogyne incognita in Greenhouse Tomato. Agronomy, 15(10), 2284. https://doi.org/10.3390/agronomy15102284






