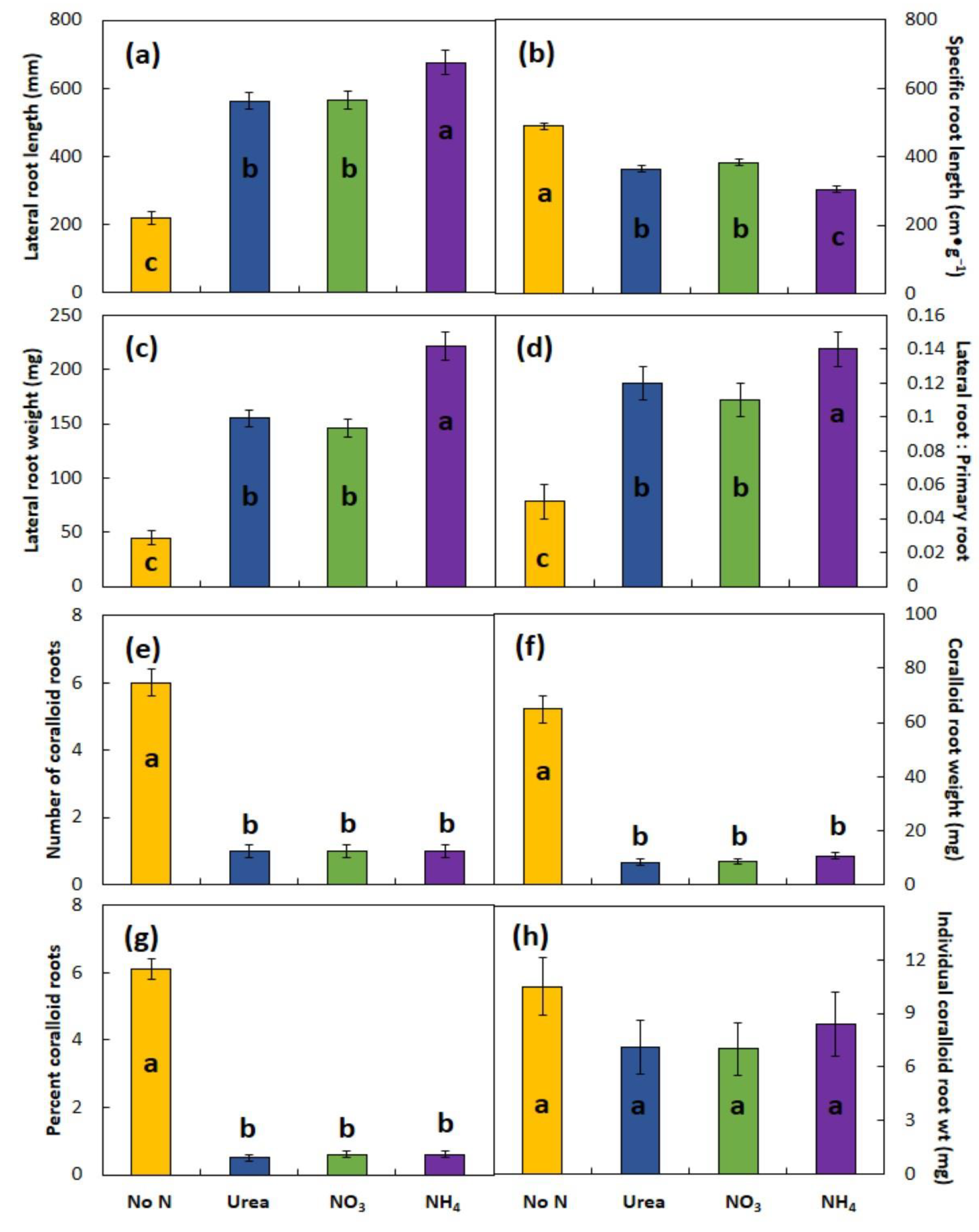
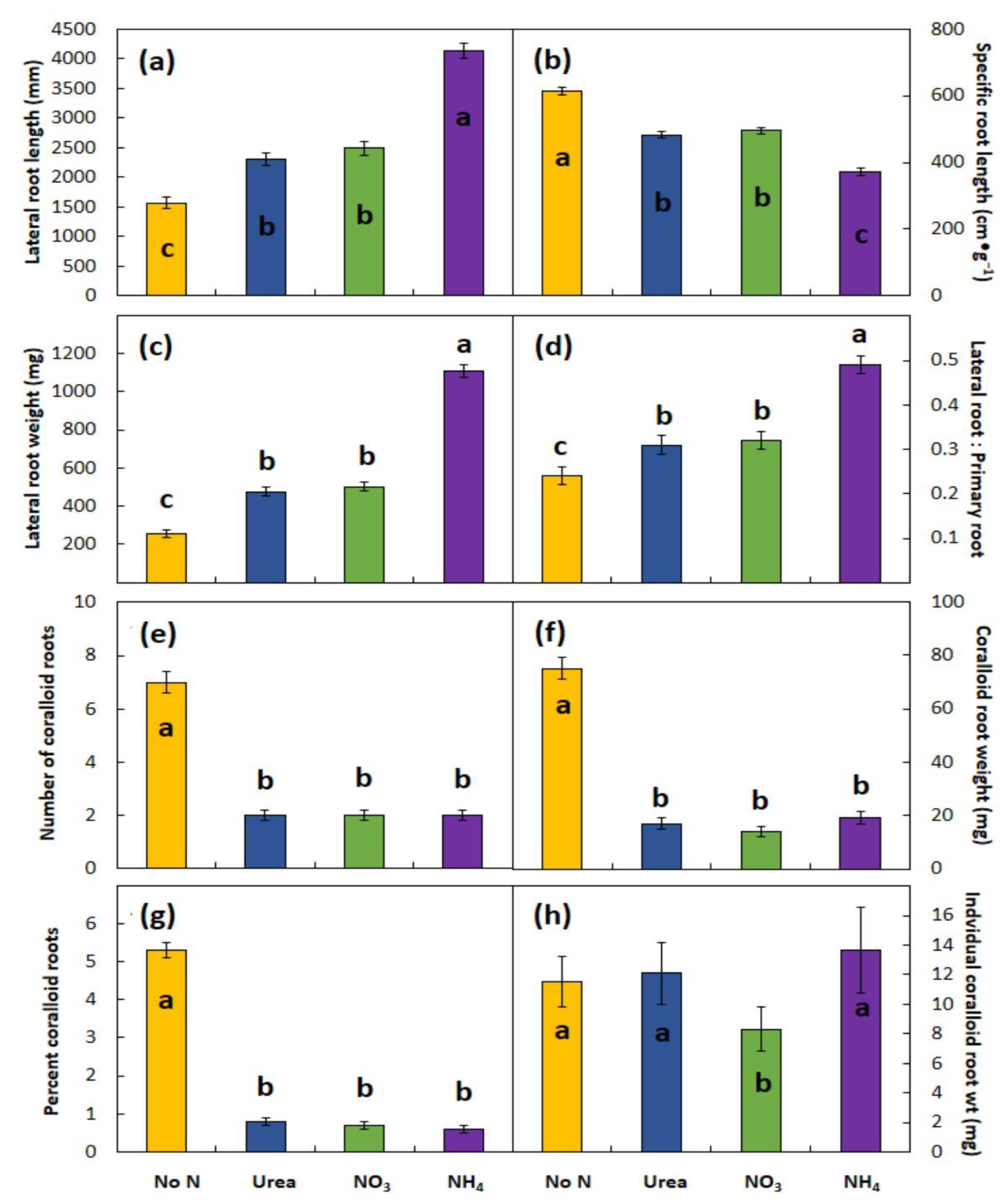
The form of N supplied in fertilizers may exert a profound influence on plant growth and root plasticity, but the responses are not canonical. This study is the first experimental look at the N form using a cycad species as a model, and plants of the two Cycas species with N supplied in the nutrient solutions responded similarly among the three experiments. Supplying N greatly increased LR length and DW, increased PR DW but not PR length, and greatly decreased coralloid root growth when compared with the control plants which received no N in the otherwise complete nutrient solution. The influences of N fertilization on shoot traits were less pronounced.
4.1. Forms of Nitrogen
The literature comparing the form of N supplied in fertilizers and plant growth responses is substantial. Although generalities may emerge, such as ammonium nutrition leading to greater root growth for many species [
16,
17], what is most clear is that a preference for one form over another is species-specific. Species that have evolved in habitats with acidic soils may prefer ammonium, and those in habitats with alkaline soils may prefer nitrate [
34]. Some notable crops with clear preferences include rice and sugarcane with a preference for ammonium; and wheat, maize, and citrus with a preference for nitrate [
35,
36]. In situations where ammonium toxicity occurs, the addition of nitrates may ameliorate the negative outcomes [
37]. In this study with two
Cycas species, the stimulations to root growth caused by adding N was greatest for ammonium and similar for urea and nitrate. Ammonium nutrition led to shorter and thicker LRs and increased the RSR in every experiment when compared with urea or nitrate nutrition. The mechanisms by which ammonium influences root growth of these two cycad species remain unknown. Potential mechanisms include influences on auxin synthesis, inactivation, or translocation; acidification of the rhizosphere; or inhibition of cell division and elongation in the root meristem. The mechanisms that underly how N form influences cycad root growth and morphology need to be studied to more fully refine fertilizer management. The results herein lend support for previously published claims that ammonium may improve cycad plant growth more than other forms of N [
30,
31].
The notable similarity among the N forms and among the experiments involved the root traits that included coralloid root growth. The number of coralloid root structures, mean size of each structure, and the total coralloid root DW per plant were similar among all three N forms.
Although most plant traits behaved similarly among the three experiments, the influence of fertilizer treatment on shoot DW and total plant DW was inconsistent. Shoot DW increased with the three +N treatments, but there were no differences among the N forms, and the arithmetic increase was NS for one of the three experiments. The universal increase in PR DW and LR DW for ammonium compared with urea or nitrate generated an increase in total plant DW for the two
C. edentata experiments but not for the
C. revoluta experiment. The specie-level differences in how RSR changed may have played a part in these dissimilarities. The
C. revoluta seedlings exhibited relatively more root growth as compared to stem and leaf growth than the
C. edentata seedlings. The resulting species-level difference in RSR may have been a mediating factor in the findings that ammonium increased total plant DW for
C. edentata but not for
C. revoluta. This difference between the two species may involve the early seedling growth stage selected for this study. The large cycad gametophyte is a considerable source of non-structural resources that are deployed for initial seedling development [
38,
39]. These resources include mineral nutrients, and the much greater availability of internal resources within the larger
C. edentata gametophytes may have modulated the seedlings growth responses to the externally supplied nutrients.
This study was limited to two arborescent
Cycas species. Extrapolating these findings to cycads as a group would be ill-advised, considering the differences in how ammonium influenced total plant growth when only two species were compared. Indeed, the manner in which plants exploit available N is strongly influenced by genetics [
40], and the 380 described species of cycads are separated into two plant families and 10 genera [
41]. The considerable phylogenetic differences among members of this plant group indicate there may be differences in responses to the N form among the species. Differences in the ecotype of origin may also differentiate cycad species more so than taxonomy. In the Philippines alone,
Cycas saxatilis K.D. Hill & A. Lindstr. is restricted to limestone outcrops,
Cycas wadei Merr. is restricted to impoverished soils, and
Cycas zambalensis Madulid & Agoo is restricted to metal-rich ultramafic soils [
42,
43,
44]. These three endemic species from a single country may exhibit disparities in root response to the N form because of their highly contrasting soils of origin.
4.2. Ammonium and Nitrate in Combination
Recent studies have shown that synergistic effects may occur for some species when ammonium and nitrate are supplied at the same time [
36]. The two forms of inorganic N provide a complementary effect under these conditions. However, the response variable under consideration may determine which ratio of ammonium to nitrate is considered preferable. For example, Wang et al. [
45] reported increased yield of maize and assimilation of N with a 50:50 mixture of ammonium and nitrate, but the quality, defined as starch content, was increased most with 100% ammonium. Uncovering the optimum combination of various forms of N in fertilizers for cycad culture is a field of study that is long overdue.
The sole mixture experiment in this study employed a single mixture of 50:50 for determining the influence of ammonium and nitrate in combination. This initial look at using mixtures of the N form indicated no synergism occurred with the 50:50 mixture, whereby the plants receiving both N forms increased in growth. Moreover, the results were not additive because the response variables did correspond to the mean of nitrate alone and ammonium alone. Instead, the root responses to an ammonium–nitrate mixture at this 1:1 ratio were in line with the 100% ammonium treatment for every response variable. More studies are urgently needed to determine if any synergisms occur for combinations of ammonium, nitrate, and urea in fertilizer formulations in cycad culture.
4.3. Caveats
Nitrification inhibitors are chemicals that inhibit the transformation of ammonium to nitrate, and they are used commercially to prolong the availability of ammonium for crop uptake thereby reducing leaching losses [
46]. The controlled hydroponic solutions were unlikely to contain consequential numbers of nitritation and nitratation microorganisms [
47], but comparisons of the N form in mineral soil substrates may benefit from nitrification inhibitors. Moreover, the weekly replacement of the solutions minimized the time for considerable oxidation of ammonium to occur. Future cycad fertilizer studies may increase interpretation efficiencies by adding nitrification inhibitors for treatments containing ammonium.
The preference of ammonium for increasing
Cycas root growth was unambiguous in this hydroponics study. However, the clear preference of one form of N may be context-dependent and vary depending on environment. For example, the invasive plant
Wedelia trilobata (L.) Hitchc. exhibited clear preference for nitrate in some conditions but ammonium in other conditions [
48,
49]. Similarly, the invasive plant
Solidago canadensis L. preferred nitrate in some environments but preferred ammonium in other environments [
50]. More studies are clearly needed to determine how influential the N form is on cycad plant growth under varied growing conditions, including in situ versus ex situ conditions or container nursery versus field conditions.
Considerable coralloid root growth occurred for the no-N plants but was minimal for the +N plants in this study. A discussion of the relationship between coralloid root growth and availability of rhizosphere N is warranted. The diversification of plant-associated microbiomes confers adaptive traits on host plants in some environments [
51]. Indeed, many plant-associated microbiomes enhance the nutrient acquisition and stress resilience of the host plant [
52]. For cycads, the microbiome that is directly associated with coralloid roots is highly biodiverse [
53,
54,
55,
56,
57,
58,
59,
60,
61,
62]. The cyanobionts within these coralloid root structures provide newly fixed N to the host plant [
21], and the greater access to this newly fixed N afforded to the no-N plants may have been responsible for the muted differences in total plant growth among the four treatments. The stimulation of coralloid root growth in N-deficient plants may compensate by way of symbiotic N fixation to support shoot growth. Fixed N originating from non-cyanobacteria coralloid root endosymbionts may also contribute to cycad N needs [
61]. Nutrient-deficient soils may also increase plant reliance on mycorrhizal fungi [
63]. The relationship between mycorrhiza and cycad roots is well-established [
64,
65,
66], and cycad fertilizer management research may be improved by combining mycorrhiza and coralloid root symbionts as mutualists which respond to nutrient availability and cycad plant growth. Impoverished soils can stimulate the root endosphere microbiome in other plant species [
67], so the phenomenon is not restricted to cycads. More studies which include isotope analysis or acetylene reduction protocols are urgently needed to more fully understand how N-fixation is directly involved in management of cycad nutritional needs. For example, soil N concentration determined the percentage of N that was derived from N-fixation for the cycad
Encephalartos natalensis R.A. Dyer & I. Verd. [
61]. The direct influence of fertilizer N form on cycad coralloid endosymbiont behaviors has not been addressed to date.
The availability of microorganisms that come into contact with the cycad rhizosphere may play a direct role in coralloid root development and other root responses to N form. The hydroponics containers and solutions in this study were not aseptic, but they were relatively clean compared to biodiverse field soil. Therefore, fertilizer studies carried out in nursery container conditions and field soil conditions which contain greater microorganism diversity are needed to more fully understand how microorganism biodiversity interacts with the available N form during fertilizer applications. Indeed, the combination of cycad root microbiome management and choice of the N form in fertilizer formulations may enable the harnessing of a more efficient nutrient management approach for sustainable cycad conservation.
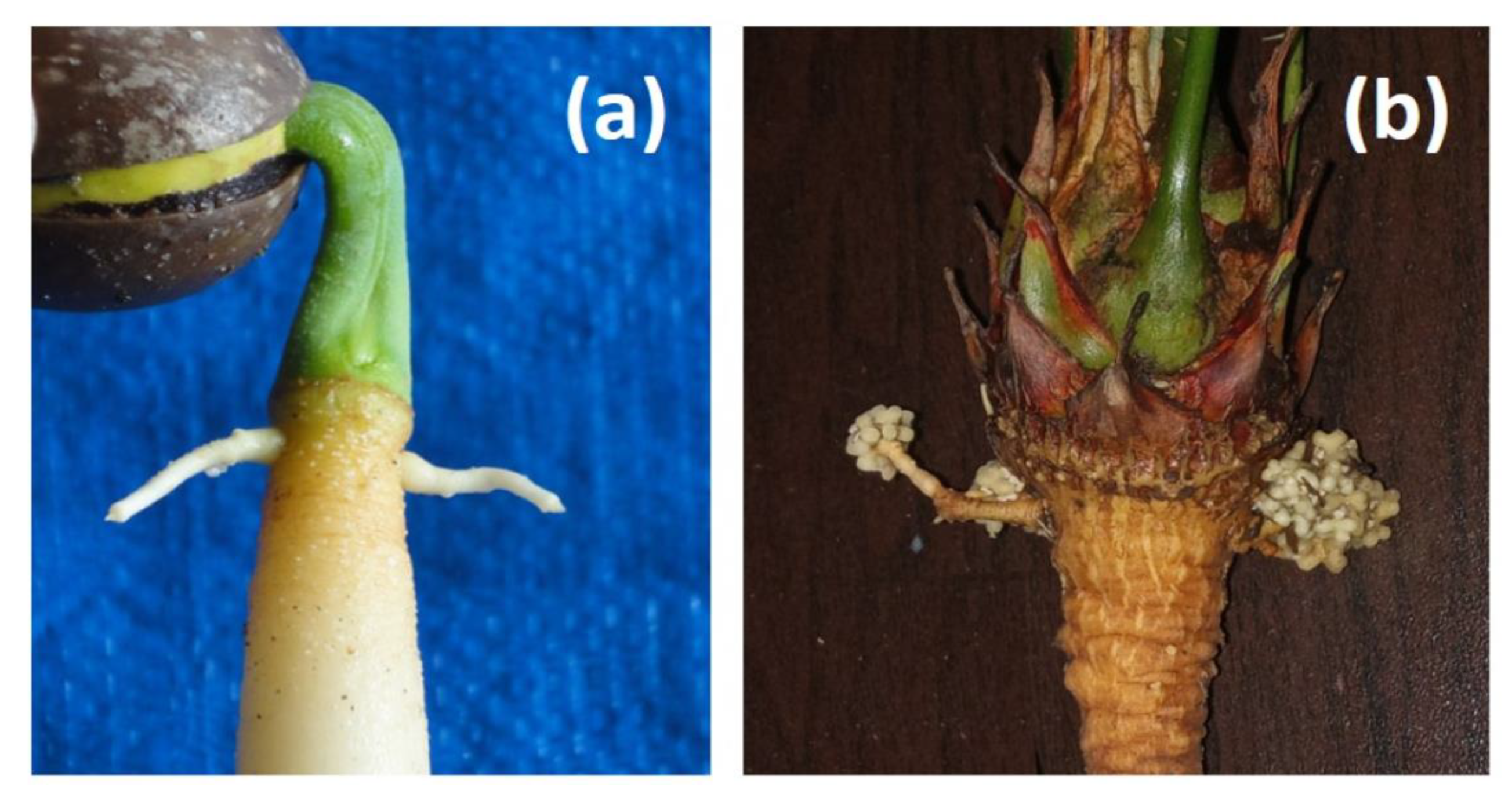
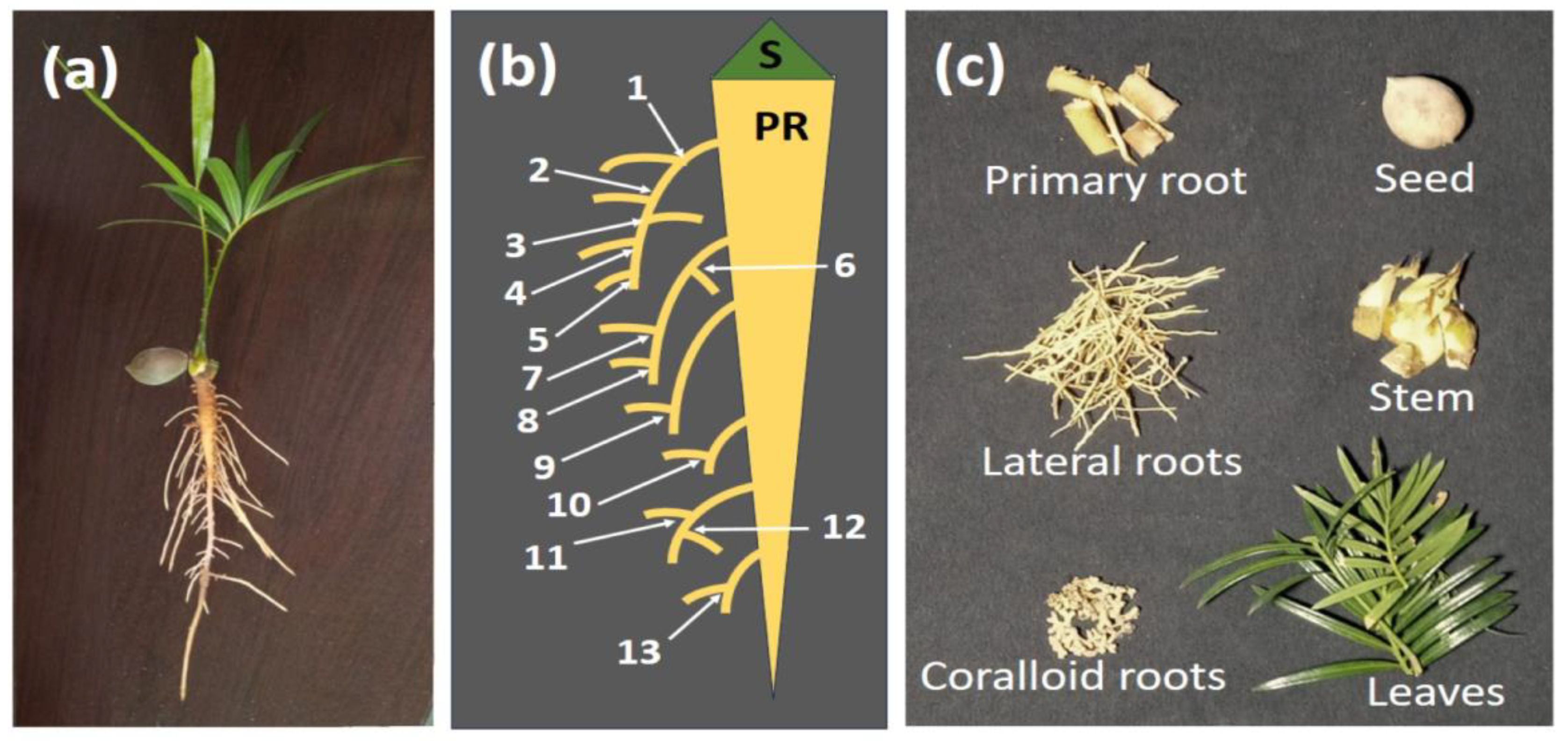
Under the clean, controlled conditions of this nutrient solution study, the coralloid root structures were restricted to a pair of lateral roots which emerged from germinating
Cycas seedlings at the top of the primary root (see
Appendix C Figure A1). Horticulturists may need to refrain from damaging these two frail crown roots during transplanting operations of young seedlings as a means of improving early coralloid root initiation and growth.
Plant growth responses to the form of N supplied in fertilizers may be influenced by temperature [
68]. The fine root system is among the plant components that respond to climate change [
69]. This study was restricted to a single tropical locality with minimal seasonal or diel variation in temperature. More studies from subtropical climates are needed to determine if general cycad growth responses to the N form are influenced by temperature gradients. Additionally, this area of research is ideal for developing an understanding of how climate change may influence changes in cycad plant growth into the future. The role of temperature on retrospective evaluations of cycad biology has also not been adequately developed. The cycad fossil record is traced to the Paleozoic era, with fossils discovered in the Antarctic, Alaska, and Greenland [
21]. Yet today the living cycad community is restricted to latitudes relatively close to the equator. The relative role of temperature in this constriction of cycad distribution throughout the antecedent eons is not currently understood.
Plant responses to the N form may also be influenced by rhizosphere pH [
70,
71]. The
Cycas roots in this study were maintained at a slightly acidic pH and the solutions were changed weekly to ensure minimal variation in pH. The influence of acidity and alkalinity on root response to the N form is not understood for cycads, and this research may continue in two directions. First, a range in rhizosphere pH occurs among the locations where cycad enthusiasts grow cycad germplasm. An understanding of the influence of pH on cycad root responses to the form of N in fertilizer formulations is needed to refine efficient nutrient management in each location. Second, the ecological conditions under which a plant species evolved may shed light on whether ammonium or nitrate is preferred [
34]. The soil conditions within the native range of many cycad species are homogeneous in regard to pH. As more data accumulate in this field of research, the plant responses may enable correlations of each cycad ecotype with preference for ammonium versus nitrate.
The form of N can influence the mobilization of seed reserves during germination and early seedling growth [
3]. I did not record any differences in DW loss for the seeds in this study despite differences in seedling growth, indicating the seedlings did not exhibit differences in exploitation of gametophyte resources. However, this study was relatively short, and longer studies are warranted including cycad species with a range in seed size to determine if the N form may interact with the growing seedling’s ability to acquire stored gametophyte resources to support growth.
Bulk density and mechanical impedance of the rooting substrate may exert a strong influence on plant root growth [
72]. The
Cycas roots in this hydroponics study were floating in nutrient solutions which offered minimal physical impedance to root growth and development. The manner in which the form of N supplied to cycad plants influences root development may differ in traditional media used in container culture or in mineral field soils. More studies are needed to determine if root growth under ammonium fertilization is greater than under urea or nitrate fertilization for nursery and field culture conditions.
4.4. Applied Horticulture Studies Desirable
Continuation of this line of research could be achieved outside of the academic community. Specialty crops such as cycads generally have specialist commercial growers who have developed a working knowledge of horticultural needs, and this practical knowledge underpins their commercial success. Botanic gardens and private cycad collectors could readily advance this research without the support of an academic partner. First, these growers are going to buy fertilizer anyway, so adding trials comparing N forms can occur without asking them to add a new input that requires expenditures. Adding these trials can be performed simply by modifying how the readily available fertilizer is supplied. Second, these successful growers are trusted by other growers because they are not from university ivory towers that many growers place members of academia. Third, if there are enough replications, the results can be believed even if the experimental units are not placed in a perfectly arranged experimental design and subjected to statistical tests then published in peer-reviewed journals. Fourth, cycad growth can be quantified with confidence using non-destructive response variables. The most reliable response variable for quantifying plant growth is the increase in DW of the tissues [
73]. This plant trait reflects the cumulative conversion of abiotic resources into biomass, and combines collective additions of carbon from photosynthesis, construction respiration while developing new plant tissues, maintenance respiration losses, and tissue loss due to senescence of aging plant modules. Growers cannot be expected to sacrifice their stock to obtain research answers such as tissue DW. But the growth of a cycad plant in the seedling and juvenile stages can be accurately quantified by characteristics such as stem diameter, number of leaves per plant, and leaf size expressed by the combined length of the petiole and rachis. A fertilizer trial with data restricted to these types of non-destructive response variables would provide reliable interpretations.
Scientists in academia cannot expect commercial growers to access chemicals used for academic research, such as the chemicals needed to create Hoagland’s solution. But the traditional list of fertilizers available at most agrochemical outlets includes all of the ingredients needed to conduct a study comparing the three sources of N. Urea is available in all supply sources as the least expensive N fertilizer. Nitrate-only sources of N are procured without difficulty as NaNO3 or Ca(NO3)2. Ammonium-only sources of N include commercial fertilizers such as (NH4)2SO4 or NH4Cl. Supplying other macronutrients without complicating the N treatments is also easily accomplished with commercially available fertilizer formulations. For example, calcium and phosphorus can be supplied with triple super phosphate (Ca(H2PO4)2·H2O), potassium can be supplied with muriate of potash (KCl), and magnesium and sulfur are often supplied as Epsom Salts (MgSO4). Finally, various formulations of micronutrients are widely available.