Circular Approach in Development of Microbial Biostimulants Using Winery Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Genomic DNA Extraction and 16S rRNA Sequencing
2.3. Biochemical Characterization of Bacillus sp. 10/R
2.4. Screening of Plant-Growth Promotion Traits
2.4.1. Screening of Enzyme Production on Agar Plates
Pectinase Activity
Cellulase and Xylanase Activity
Protease Activity
2.4.2. ACC Deaminase Activity
2.4.3. Phosphate Solubilization Assay
2.4.4. Indole Acetic Acid Production
2.5. Cultivation Media
2.6. Physicochemical Characterization of WFW
2.7. Inoculum Preparation and Cultivation Media and Conditions
2.8. Biomass Content Determination
2.9. Quantitative Determination of Enzyme Activity in Liquid Culture
2.10. Seed Germination Assay
2.11. Statistical Analyses
3. Results
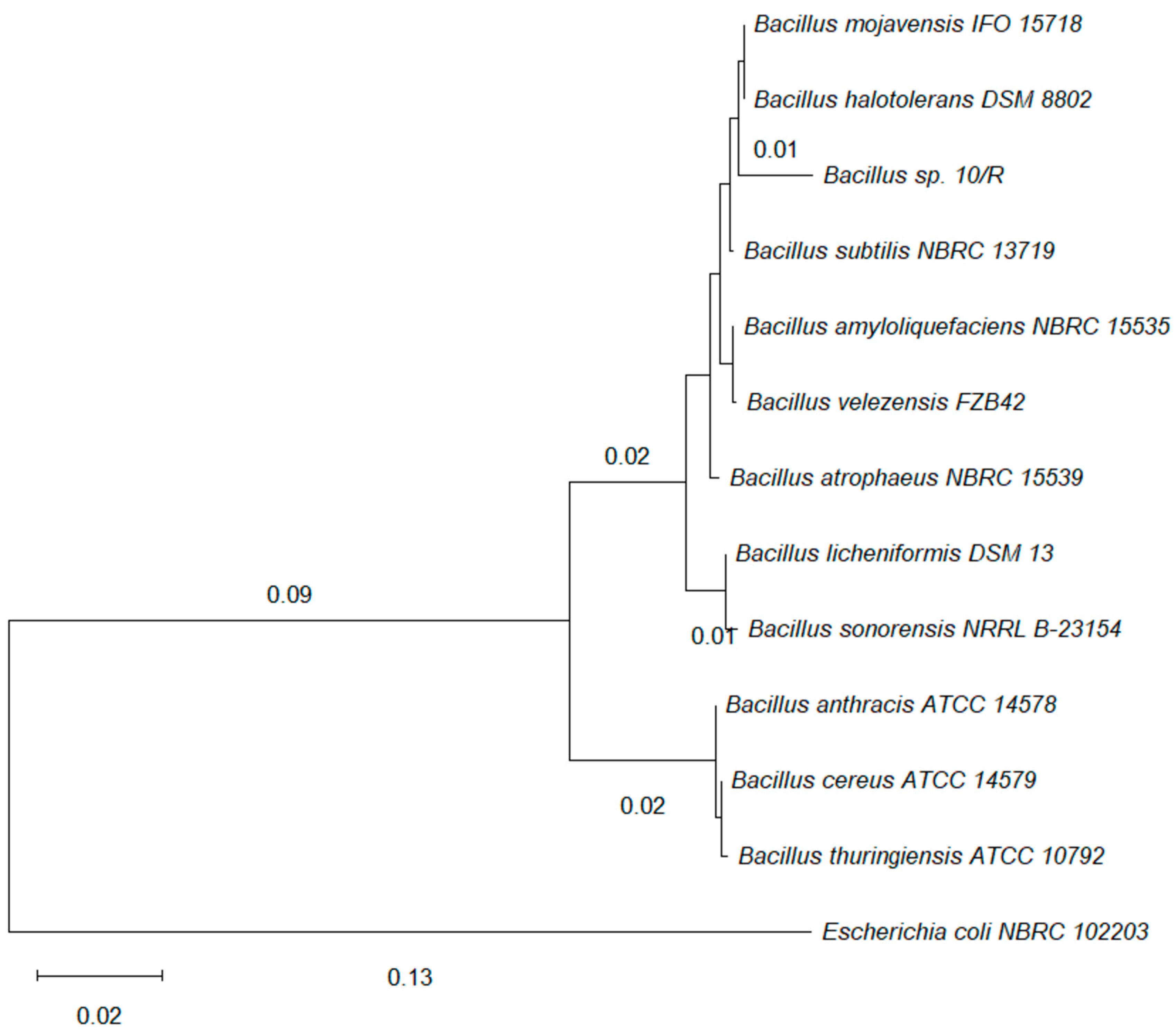
3.1. Identification of the Producing Microorganism
3.2. Biochemical Profiling of the Strain Bacillus sp. 10/R
3.3. Plant Growth Promoting Traits of the Strain Bacillus sp. 10/R
3.4. Basic Nutritional Profile of WFW Medium and Cultivation Broth
3.5. Bacterial Growth in WFW Medium During 96 h of Cultivation
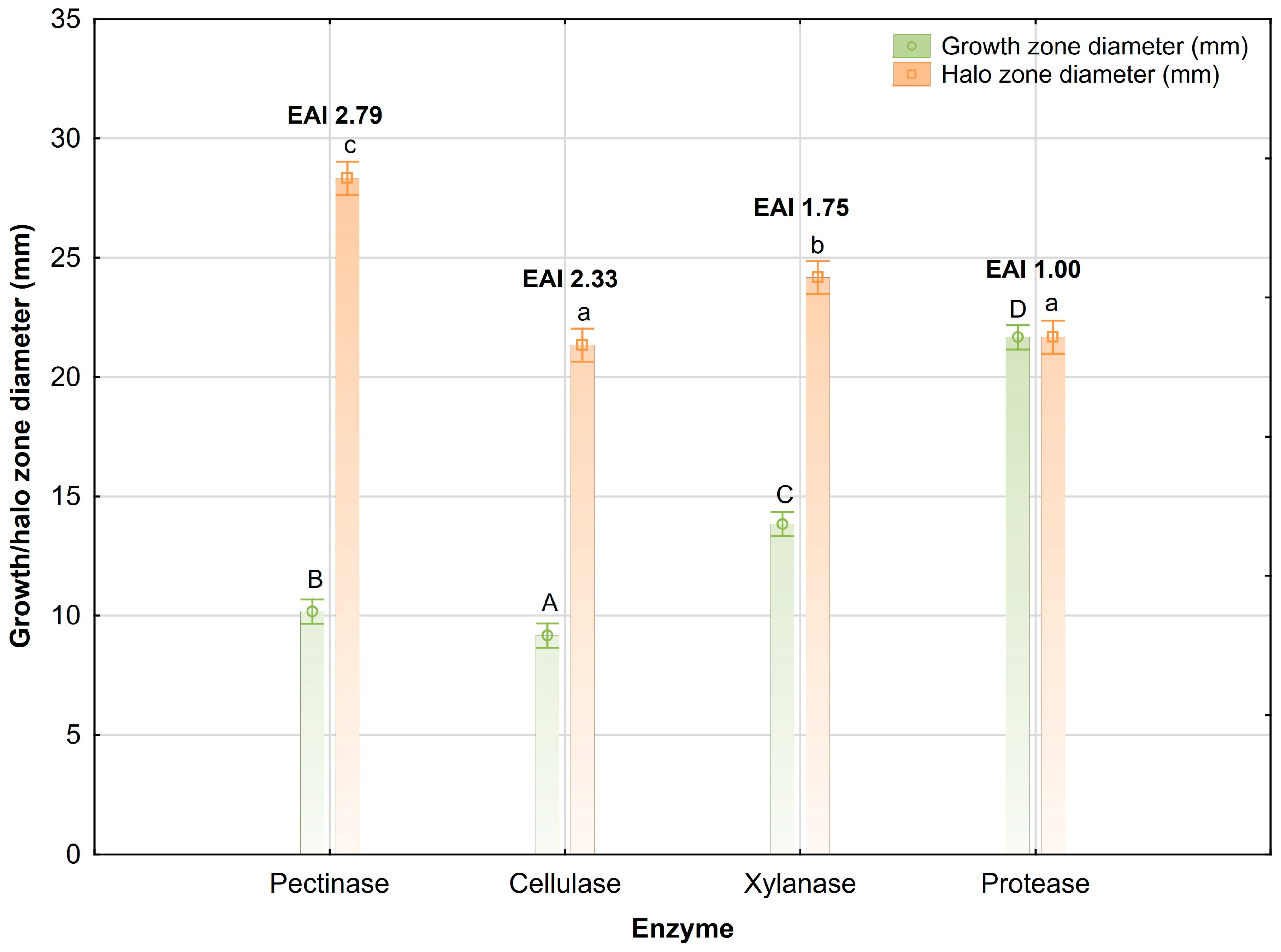
3.6. Enzyme Activity in Liquid WFW-Based Culture
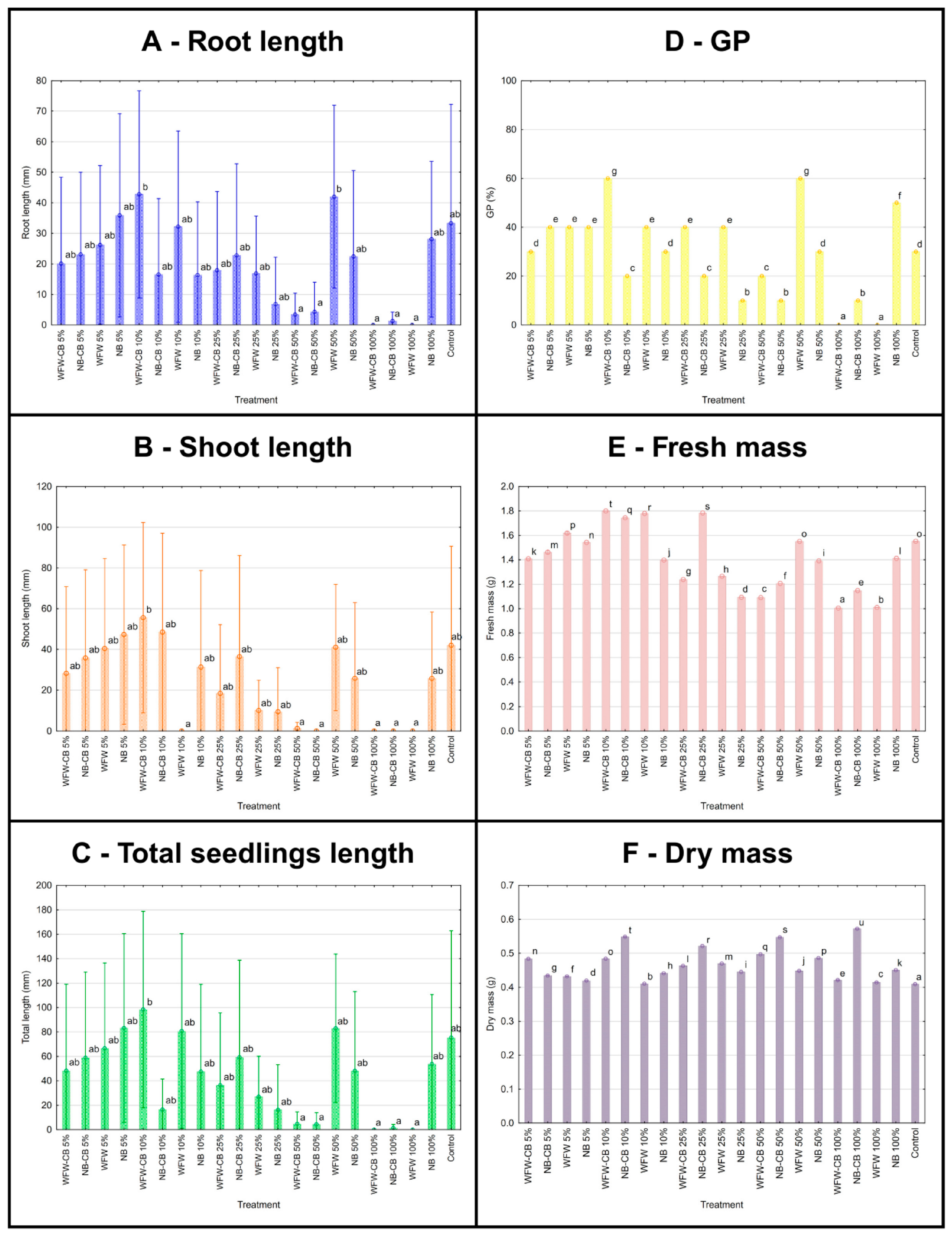
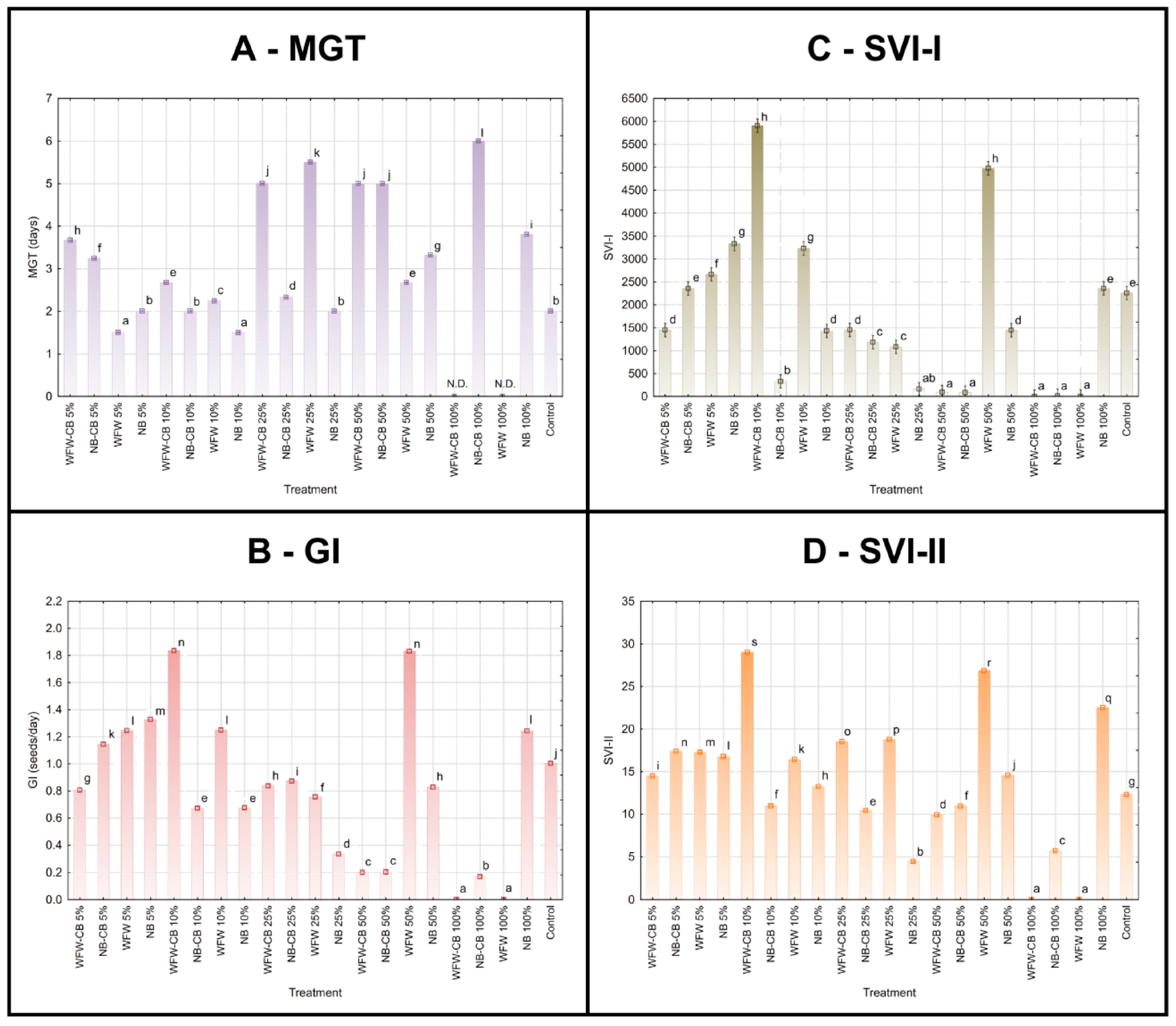
3.7. Seed Germination
4. Discussion
4.1. PGP Traits of B. mojavensis/B. halotolerans Strains
4.2. Biochemical Profile of Bacillus sp. 10/R
4.3. Enzymatic Activity of Bacillus sp. 10/R
4.4. Direct and Indirect PGP Mechanisms Exhibited by Bacillus sp. 10/R
4.5. Winery Flotation Wastewater as a Substrate for Bacillus sp. 10/R Growth
4.6. Seed Treatment of Barley Using Circular Biostimulant Based on Bacillus sp. 10/R and Winery Flotation Wastewater
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGP | plant-growth promotion |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| WFW | winery flotation wastewater |
| IAA | indole-3-acetic acid |
| NB | nutrient broth |
| EAI | enzymatic activity index |
| CB | cultivation broth |
References
- Elumalai, P.; Gao, X.; Parthipan, P.; Luo, J.; Cui, J. Agrochemical pollution: A serious threat to environmental health. Curr. Opin. Environ. Sci. Health 2025, 43, 100597. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Asghar, W.; Kondo, S.; Iguchi, R.; Mahmood, A.; Kataoka, R. Agricultural utilization of unused resources: Liquid food waste material as a new source of plant growth-promoting microbes. Agronomy 2020, 10, 954. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Kumar, V.V. Biofertilizers and Biopesticides in Sustainable Agriculture. In Role of Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; Volume 1, pp. 377–398. [Google Scholar] [CrossRef]
- Ansabayeva, A.; Makhambetov, M.; Rebouh, N.Y.; Abdelkader, M.; Saudy, H.S.; Hassan, K.M.; Nasser, M.A.; Ali, M.A.A.; Ebrahim, M. Plant growth-promoting microbes for resilient farming systems: Mitigating environmental stressors and boosting crops productivity—A review. Horticulturae 2025, 11, 260. [Google Scholar] [CrossRef]
- Etesami, H. The dual nature of plant growth-promoting bacteria: Benefits, risks, and pathways to sustainable deployment. Curr. Res. Microb. Sci. 2025, 9, 100421. [Google Scholar] [CrossRef]
- Vlajkov, V.; Pajčin, I.; Vučetić, S.; Anđelić, S.; Loc, M.; Grahovac, M.; Grahovac, J. Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. Plants 2023, 12, 1024. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Alina, S.O.; Constantinscu, F.; Petruta, C.C. Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Rom. Biotechnol. Lett. 2015, 20, 10737–10750. [Google Scholar]
- Azeem, M.; Javed, S.; Zahoor, A.F. Bacillus species as potential plant growth promoting Rhizobacteria for drought stress resilience. Russ. J. Plant Physiol. 2023, 70, 59. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant Growth-Promoting Bacteria as an Emerging Tool to Manage Bacterial Rice Pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef]
- Dmitrović, S.; Pajčin, I.; Vlajkov, V.; Grahovac, M.; Jokić, A.; Grahovac, J. Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents. Bioengineering 2022, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Slatni, T.; Zorrig, W.; Razzegui, A.; Hernández, J.A.; Barba-Espín, G.; Hamed, K.B.; Díaz-Vivancos, P. Halophilic Bacillus improve barley growth on calcareous soil via enhanced photosynthetic performance and metabolomic re-programing. J. Plant Physiol. 2025, 309, 154495. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Arkhipova, T.; Sharipova, G.; Ivanov, R.; Nuzhnaya, T.; Kudoyarova, G.; Veselov, D. The effect of plant growth-promoting bacteria Bacillus subtilis IB-22 on the hydraulic conductivity and abundance of PIP2 aquaporins in the roots of an abscisic acid-deficient barley mutant. Int. J. Mol. Sci. 2024, 25, 10706. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, O.; Grover, S.; Yimer, B.; Vinje, M.; Mahalingam, R. Bacterial seed endophytes promote barley growth and inhibits Fusarium graminearum in vitro. BMC Res. Notes 2024, 17, 289. [Google Scholar] [CrossRef] [PubMed]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113. [Google Scholar] [CrossRef]
- Yi, H.; Li, M.; Huo, X.; Zeng, G.; Lai, C.; Huang, D.; An, Z.; Qin, L.; Liu, X.; Li, B.; et al. Recent development of advanced biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2020, 40, 99–118. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Statistical Report on World Vitiviniculture. 2024. Available online: https://www.oiv.int/what-we-do/statistics (accessed on 16 July 2025).
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Sousa, A.C.; Dias, C.; Martins, A.R.; Gomes, A.G.; Santos, C.A. Using winery effluents for cultivating microalgae as bio-additives for vineyards. J. Appl. Phycol. 2025, 37, 1619–1632. [Google Scholar] [CrossRef]
- Vlajkov, V.; Grahovac, M.; Budakov, D.; Loc, M.; Pajčin, I.; Milić, D.; Grahovac, J. Distribution, genetic diversity and biocontrol of aflatoxigenic Aspergillus flavus in Serbian maize fields. Toxins 2021, 13, 687. [Google Scholar] [CrossRef]
- Nikodinovic, J.; Barrow, K.D.; Chuck, J.A. High yield preparation of genomic DNA from Streptomyces. Biotechniques 2003, 35, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Raveendran, S.; Parameswaran, B.; Abraham, A.; Athira, R.S.; Mathew, A.K.; Pandey, A. Production of pectinase from Bacillus sonorensis MPTD1. Food Technol. Biotechnol. 2018, 56, 110. [Google Scholar] [CrossRef] [PubMed]
- Amore, A.; Parameswaran, B.; Kumar, R.; Birolo, L.; Vinciguerra, R.; Marcolongo, L.; Faraco, V. Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer’s spent grain saccharification. J. Chem. Technol. Biotechnol. 2015, 90, 573–581. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P.; Prasad, D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 2003, 4, 440–448. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- SFRY. Ordinance on methods of taking samples and performing chemical and physical analyzes for the purpose of quality control of fruit and vegetable products. Off. Gaz. SFRY 1983, 29. [Google Scholar]
- Vračar, O.L. Manual for Quality Control of Fresh and Processed Fruits and Vegetables and Mushrooms and Refreshing Soft Drinks; University of Novi Sad, Faculty of Technology: Novi Sad, Serbia, 2001. [Google Scholar]
- Marcó, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 57, 1019–1026. [Google Scholar] [CrossRef]
- Montana State University. Drop Plate Method. Available online: https://www.cs.montana.edu/webworks/projects/stevesbook/contents/chapters/chapter011/section008/blue/page002.html (accessed on 11 July 2025).
- Vlajkov, V.; Pajčin, I.; Loc, M.; Budakov, D.; Dodić, J.; Grahovac, M.; Grahovac, J. The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering 2022, 9, 797. [Google Scholar] [CrossRef]
- Walia, M.K.; Mohammed, Y.A.; Franck, W.L.; Chen, C. Evaluation of early seedling development of Chickpea and its relation to seed yield. Agrosystems Geosci. Environ. 2020, 3, e20005. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, A.; Srivastava, P.; Choudhary, K.K.; Dikshit, A. Plant growth-promoting microorganisms in sustainable agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using plant growth-promoting microorganisms (PGPMs) to improve plant development under in vitro culture conditions. Planta 2022, 255, 117. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Puopolo, G.; Santoyo, G. Plant growth-promoting microorganisms: New insights and the way forward. Microbiol. Res. 2025, 297, 128168. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.A.; Singh, A.K. Role of bacterial quorum sensing in plant growth promotion. World. J. Microbiol. Biotechnol. 2025, 41, 18. [Google Scholar] [CrossRef]
- Dhawi, F. The role of plant growth-promoting microorganisms (PGPMs) and their feasibility in hydroponics and vertical farming. Metabolites 2023, 13, 247. [Google Scholar] [CrossRef]
- Naamala, J.; Smith, D.L. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy 2020, 10, 1179. [Google Scholar] [CrossRef]
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As Plant Growth-Promoting Bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Prajakta, B.M.; Suvarna, P.P.; Raghvendra, S.P.; Alok, R.R. Potential biocontrol and superlative plant growth promoting activity of indigenous Bacillus mojavensis PB-35(R11) of soybean (Glycine max) rhizosphere. SN Appl. Sci. 2019, 1, 1143. [Google Scholar] [CrossRef]
- Ghazala, I.; Chiab, N.; Saidi, M.N.; Gargouri-Bouzid, R. The plant growth-promoting bacteria strain Bacillus mojavensis I4 enhanced salt stress tolerance in durum wheat. Curr. Microbiol. 2023, 80, 178. [Google Scholar] [CrossRef] [PubMed]
- Sdiri Ghidawi, J.; Ghazala, I.; Haddar, A.; Bouazizi, O.; Gargouri-Bouzid, R.; Nouri-Ellouz, O. Effect of the Plant Growth-Promoting Bacteria Strain Bacillus Mojavensis I4 on Potato Growth, Physiology, Tuber Yield, and Quality Under Salt Stress Conditions. Potato Res. 2025, 68, 2205–2227. [Google Scholar] [CrossRef]
- Danish, M.; Shahid, M.; Zeyad, M.T.; Bukhari, N.A.; Al-Khattaf, F.S.; Hatamleh, A.A.; Ali, S. Bacillus mojavensis, a metal-tolerant plant growth-promoting bacterium, improves growth, photosynthetic attributes, gas exchange parameters, and Alkalo-Polyphenol Contents in Silver Nanoparticle (Ag-NP)-Treated Withania somnifera L. (Ashwagandha). ACS Omega 2022, 7, 13878–13893. [Google Scholar] [CrossRef]
- Kapadia, C.; Patel, N.; Rana, A.; Vaidya, H.; Alfarraj, S.; Ansari, M.J.; Gafur, A.; Poczai, P.; Sayyed, R.Z. Evaluation of Plant Growth-Promoting and Salinity Ameliorating Potential of Halophilic Bacteria Isolated from Saline Soil. Front. Plant Sci. 2022, 13, 946217. [Google Scholar] [CrossRef]
- Oliva, G.; Di Stasio, L.; Vigliotta, G.; Guarino, F.; Cicatelli, A.; Castiglione, S. Exploring the Potential of Four Novel Halotolerant Bacterial Strains as Plant-Growth-Promoting Rhizobacteria (PGPR) under Saline Conditions. Appl. Sci. 2023, 13, 4320. [Google Scholar] [CrossRef]
- Barros, F.F.; Simiqueli, A.P.; de Andrade, C.J.; Pastore, G.M. Production of Enzymes from Agroindustrial Wastes by Biosurfactant-Producing Strains of Bacillus subtilis. Biotechnol. Res. Int. 2013, 2013, 103960. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kognou, A.L.M.; Zhang, J.; Qin, W. Different Facets of Lignocellulosic Biomass Including Pectin and Its Perspectives. Waste Biomass Valorization 2021, 12, 4805–4823. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Kabir, M.S.; Tasmim, T. Isolation of pectinase producing bacteria from the rhizosphere of Andrographis paniculata nees and 16S rRNA gene sequence comparison of some potential strains. Adv. Microbiol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Oumer, O.J.; Abate, D. Screening and molecular identification of pectinase producing microbes from coffee pulp. Biomed. Res. Int. 2018, 2018, 2961767. [Google Scholar] [CrossRef]
- Castaldi, S.; Petrillo, C.; Donadio, G.; Piaz, F.D.; Cimmino, A.; Masi, M.; Evidente, A.; Isticato, R. Plant Growth Promotion Function of Bacillus sp. Strains Isolated from Salt-Pan Rhizosphere and Their Biocontrol Potential against Macrophomina phaseolina. Int. J. Mol. Sci. 2021, 22, 3324. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Boudechicha, A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Screening of Cellulolytic Bacteria from Various Ecosystems and Their Cellulases Production under Multi-Stress Conditions. Catalysts 2022, 12, 769. [Google Scholar] [CrossRef]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Saleem, A.R.; Brunetti, C.; Khalid, A.; Della Rocca, G.; Raio, A.; Emiliani, G.; De Carlo, A.; Mahmood, T.; Centritto, M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE 2018, 13, e0191218. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Khan, S.S.; Kour, H.; Kaur, T.; Devi, R.; Rai, A.K.; Yadav, A.N. ACC deaminase producing phytomicrobiomes for amelioration of abiotic stresses in plants for agricultural sustainability. J. Plant Growth Regul. 2024, 43, 963–985. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.; Hussain, A.; Jamil, M. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: A promising approach for improving cotton growth. Folia Microbiol. 2021, 66, 115–125. [Google Scholar] [CrossRef]
- Afzal, A.; Bahader, S.; Ul Hassan, T.; Naz, I.; Din, A.U. Rock phosphate solubilization by plant growth-promoting Bacillus velezensis and its impact on wheat growth and yield. Geomicrobiol. J. 2023, 40, 131–142. [Google Scholar] [CrossRef]
- Cendales, T.C.; González, C.A.R.; Cuásquer, C.P.V.; Alzate, O.A.T.; Rodríguez, A.H. Efecto de Bacillus sobre la germinación y crecimiento de plántulas de tomate (Solanum lycopersicum L.). Acta Biol. Colomb. 2017, 22, 37. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, P.; Kiran, S.; Kumari, S.; Singh, A. Characterization of culture condition dependent, growth responses of phosphate solubilizing bacteria (Bacillus subtilis DR2) on plant growth promotion of Hordeum vulgare. Vegetos 2024, 37, 266–276. [Google Scholar] [CrossRef]
- Chandra, P.; Khobra, R.; Sundha, P.; Sharma, R.K.; Jasrotia, P.; Chandra, A.; Singh, D.P.; Singh, G.P. Plant growth promoting Bacillus-based bio formulations improve wheat rhizosphere biological activity, nutrient uptake and growth of the plant. Acta Physiol. Plant. 2021, 43, 139. [Google Scholar] [CrossRef]
- Danilov, I.; Vlajkov, V.; Šumić, Z.; Milić, A.; Horecki, A.T.; Dujković, T.; Živanović, N.; Simin, N.; Lesjak, M.; Grahovac, J. Valorization of Strawberry Juice Production Wastewater: Possibilities for Polyphenols Recovery and Plant Biostimulant Production. Foods 2024, 13, 3224. [Google Scholar] [CrossRef]
- Goud, M.S.; Sharma, S.K.; Kharbikar, L.L.; Prasanna, R.; Sangwan, S.; Dahuja, A.; Dixit, A. Bacillus species consortium with tryptophan-dependent and-independent pathways mediated production of IAA and its derivatives modulates soil biological properties, growth and yield of wheat. Plant Soil 2025, 508, 71–97. [Google Scholar] [CrossRef]
- Latessa, S.H.; Hanley, L.; Tao, W. Characteristics and practical treatment technologies of winery wastewater: A review for wastewater management at small wineries. J. Environ. Manag. 2023, 342, 118343. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef]
- Ngwenya, N.; Gaszynski, C.; Ikumi, D. A review of winery wastewater treatment: A focus on UASB biotechnology optimisation and recovery strategies. J. Environ. Chem. Eng. 2022, 10, 108172. [Google Scholar] [CrossRef]
- Khan, I.G.; Barate, D.L. Effect of various parameters on activity of pectinase enzyme. Int. J. Adv. Res. 2016, 4, 853–862. [Google Scholar]
- Utami, A.P.; Fahrurrozi, F.; Meryandini, A. Production and immobilization pectinase from Bacillus sp. 2P11 using alginate beads. Biodiversitas 2022, 23, 3960–3966. [Google Scholar] [CrossRef]
- Malik, W.A.; Javed, S. Biochemical Characterization of Cellulase from Bacillus subtilis Strain and its Effect on Digestibility and Structural Modifications of Lignocellulose Rich Biomass. Front. Bioeng. Biotechnol. 2021, 9, 800265. [Google Scholar] [CrossRef]
- Abada, E.A.; Elbaz, R.M.; Sonbol, H.; Korany, S.M. Optimization of cellulase production from Bacillus albus (MN755587) and its involvement in bioethanol production. Pol. J. Environ. Stud. 2021, 30, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.N.; de Andrade Melo, L.F.; Finkler, C.L.L. Optimization of the cultivation conditions of Bacillus licheniformis BCLLNF-01 for cellulase production. Biotechnol. Rep. 2021, 29, e00599. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; You, Y.; Yang, N.; Lu, S.; Xue, J.; Xing, X.; Sha, S.; Zhao, L. Introduction of Cellulolytic Bacterium Bacillus velezensis Z2.6 and Its Cellulase Production Optimization. Microorganisms 2024, 12, 979. [Google Scholar] [CrossRef]
- Widnyana, I.K.; Javandira, C. Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric. Agric. Sci. Procedia 2016, 9, 419–423. [Google Scholar] [CrossRef]
- Song, P.; Zhao, B.; Sun, X.; Li, L.; Wang, Z.; Ma, C.; Zhang, J. Effects of Bacillus subtilis HS5B5 on Maize Seed Germination and Seedling Growth under NaCl Stress Conditions. Agronomy 2023, 13, 1874. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Li, L.; Liu, Y.; Zhang, H.; Wang, J.; Jiang, X. Seed biostimulant Bacillus sp. MGW9 improves the salt tolerance of maize during seed germination. AMB Express 2021, 11, 74. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-Priming of Soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to Improve Seed Germination and the Initial Seedling Growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Buntić, A.; Stajković-Srbinović, O.S.; Knežević, M.M.; Rasulić, N.V.; Kuzmanović, Đ.Ž.; Dimitrijević-Branković, S.; Delić, D.I. The effect of bacterial isolates from rhizosphere soils on wheat and barley seed germination. Zemljište I Biljka 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Teker Yıldız, M.; Acar, O. Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress. Plants 2025, 14, 723. [Google Scholar] [CrossRef]
- Górski, R.; Rosa, R.; Niewiadomska, A.; Wolna-Maruwka, A.; Płaza, A. Innovative Spring Barley Cultivation Technology Based on the Use of Microbial Products Together with Living Mulch in Organic Farming. Agronomy 2023, 13, 1914. [Google Scholar] [CrossRef]
- Mosse, K.P.; Patti, A.F.; Christen, E.W.; Cavagnaro, T.R. Winery wastewater inhibits seed germination and vegetative growth of common crop species. J. Hazard. Mater. 2019, 180, 63–70. [Google Scholar] [CrossRef] [PubMed]

| API 50 CH Test Strip Results | |||||
|---|---|---|---|---|---|
| Glycerol | + | D-mannitol | + | D-raffinose | + |
| Erythritol | − | D-sorbitol | + | Starch (amidon) | + |
| D-arabinose | − | methyl-α-D-mannopyranoside | − | Glycogen | + |
| L-arabinose | + | methyl- α-D-glycopyranoside | + | Xylitol | − |
| D-ribose | + | N-acetylglucosamide | − | Gentiobiose | − |
| D-xylose | + | Amygdalin | + | D-turanose | − |
| L-xylose | − | Arbutin | + | L-lyxose | − |
| D-adonitol | − | Esculin | + | D-tagatose | + |
| Methyl-β-D-xylopyranoside | − | Salicin | + | D-fucose | − |
| D-galactose | − | D-cellobiose | + | L-fucose | − |
| D-glucose | + | D-maltose | + | D-arabitol | − |
| D-fructose | + | D-lactose | − | L-arabitol | − |
| D-mannose | + | D-melibiose | − | Potassium gluconate | + |
| L-sorbose | − | D-saccharose | + | Potassium 2-ketoglyconate | − |
| L-rhamnose | − | D-trehalose | + | Potassium 5-ketoglyconate | − |
| Dulcitol | − | Inulin | + | ||
| Inositol | − | D-melezitose | − | ||
| API 20 E Test Strip Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ONPG | + | ADH | + | LDC | − | ODC | − | CIT | + |
| H2S | − | URE | − | TDA | − | IND | + | VP | + |
| API ZYM Test Strip Results | |||
|---|---|---|---|
| Control | Acid phosphatase | + | |
| Alkaline phosphatase | + | Naphthol-AS-BI-phosphohydrolase | + |
| Esterase (C 4) | + | α-galactosidase | − |
| Esterase Lipase (C 8) | + | β-galactosidase | − |
| Lipase (C 14) | − | β-glucuronidase | − |
| Leucine arylamidase | + | α-glucosidase | + |
| Valine arylamidase | + | β-glucosidase | − |
| Cystine arylamidase | − | N-acetyl-β-glucosaminidase | − |
| Trypsin | − | α-mannosidase | − |
| α-chymotrypsin | − | α-fucosidase | − |
| Parameter | Growth Zone Diameter (mm) | Halo Zone Diameter (mm) | PSI |
|---|---|---|---|
| ACC deaminase production | 54.67 ± 0.58 | / | / |
| Phosphate solubilization | 11.17 ± 0.29 | 12.67 ± 1.15 | 1.13 ± 0.07 |
| Parameter | Before Cultivation | After Cultivation |
|---|---|---|
| Total solids (%) | 7.96 ± 0.06 | 6.99 ± 0.13 |
| Soluble solids (°Bx) | 6.42 ± 0.35 | 6.00 ± 0.00 |
| Total sugar content (%) | 6.61 ± 1.35 | 2.68 ± 0.06 |
| Reducing sugar content (%) | 4.28 ± 0.47 | / |
| Protein content (%) | 0.64 ± 0.06 | 0.29 ± 0.02 |
| Cellulose content (%) | 0.01 ± 0.01 | 0.00 ± 0.00 |
| pH value | 3.54 ± 0.27 | 5.95 ± 0.25 |
| Time of Cultivation (h) | Pectinase Activity (U/mL) | Cellulase Activity (CellG5 U/mL) | Xylanase Activity (XylX6 U/mL) |
|---|---|---|---|
| 12 | 2.19 | 0.00 | 0.00 |
| 24 | 2.19 | 0.01 | 0.00 |
| 36 | 2.19 | 0.00 | 0.00 |
| 48 | 2.20 | 0.08 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujković, T.; Danilov, I.; Vlajkov, V.; Savić, M.; Šumić, Z.; Jokić, A.; Grahovac, J. Circular Approach in Development of Microbial Biostimulants Using Winery Wastewater. Agronomy 2025, 15, 2272. https://doi.org/10.3390/agronomy15102272
Dujković T, Danilov I, Vlajkov V, Savić M, Šumić Z, Jokić A, Grahovac J. Circular Approach in Development of Microbial Biostimulants Using Winery Wastewater. Agronomy. 2025; 15(10):2272. https://doi.org/10.3390/agronomy15102272
Chicago/Turabian StyleDujković, Tatjana, Ivana Danilov, Vanja Vlajkov, Marina Savić, Zdravko Šumić, Aleksandar Jokić, and Jovana Grahovac. 2025. "Circular Approach in Development of Microbial Biostimulants Using Winery Wastewater" Agronomy 15, no. 10: 2272. https://doi.org/10.3390/agronomy15102272
APA StyleDujković, T., Danilov, I., Vlajkov, V., Savić, M., Šumić, Z., Jokić, A., & Grahovac, J. (2025). Circular Approach in Development of Microbial Biostimulants Using Winery Wastewater. Agronomy, 15(10), 2272. https://doi.org/10.3390/agronomy15102272










