Functions of RNA N6-Methyladenosine Demethylases in Plant Development and Stress Responses
Abstract
1. Introduction
2. RNA m6A Demethylase
2.1. RNA m6A Demethylase in Plants
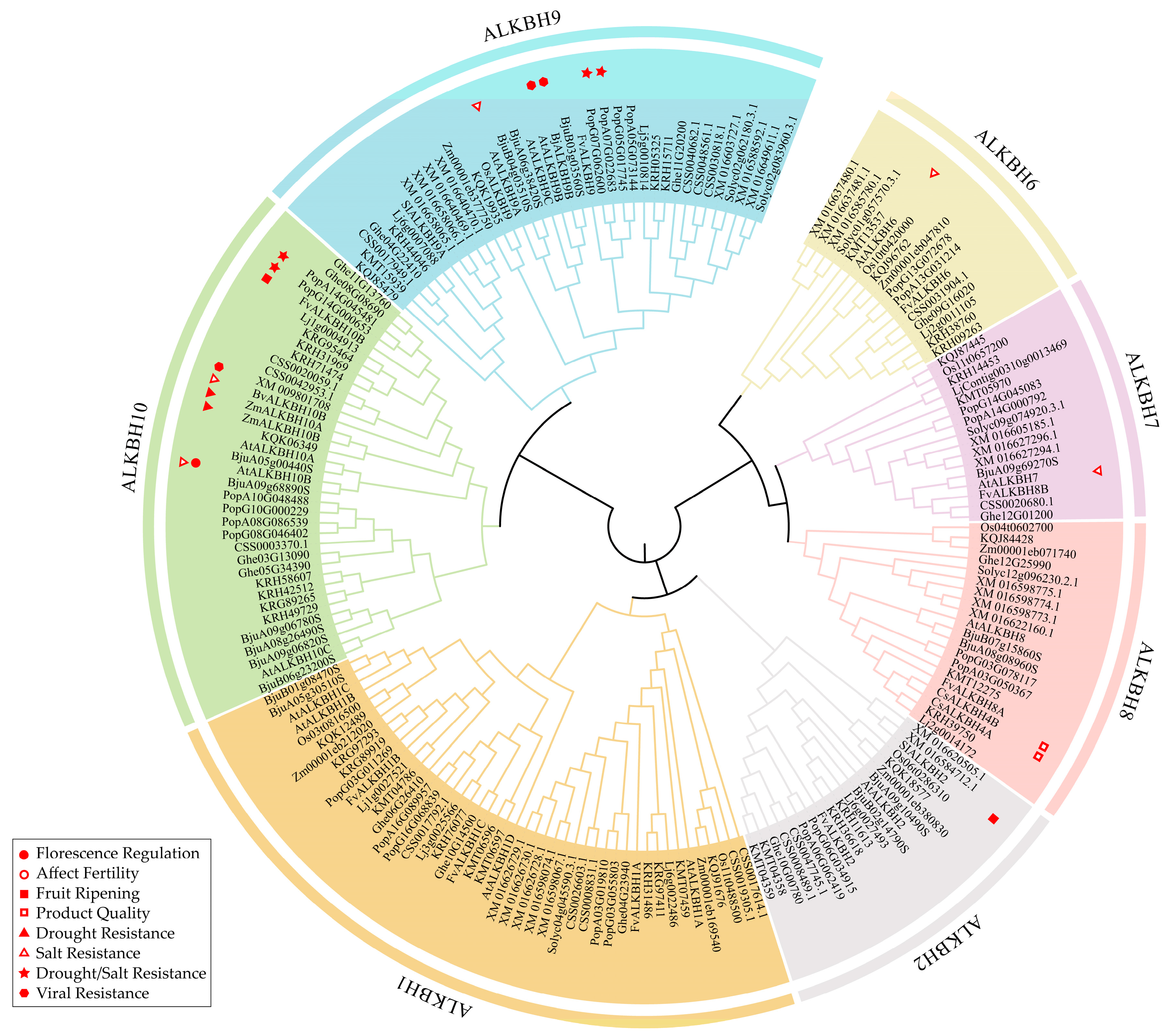
2.2. Phylogenetic Analysis and Functional Annotation of the ALKBH Family in Plants
2.3. Identification Method of m6A Demethylase
2.3.1. Determination of Enzyme Activity In Vitro
2.3.2. High-Throughput Sequencing Technology
2.3.3. Gene Knockout and Overexpress
3. The Role of RNA m6A Demethylase in Plant Growth and Development
3.1. Florescence Regulation
3.2. Affect Fertility
3.3. Fruit Ripening
3.4. Improve Product Quality
4. RNA m6A Demethylase Is Involved in Plant Stress Response
4.1. Participate in Plant Drought Resistance
4.2. Participation in Plant Salt Resistance
4.3. Regulate Plant Viral Invasion
5. Discussion and Prospectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chmielowska-Bąk, J.; Arasimowicz-Jelonek, M.; Deckert, J. In search of the mRNA modification landscape in plants. BMC Plant Biol. 2019, 19, 421. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Jia, G. Detection, regulation, and functions of RNA N6-methyladenosine modification in plants. Plant Commun. 2023, 4, 100546. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-Z.; MacQueen, A.; Zheng, G.; Duan, H.; Dore, L.C.; Lu, Z.; Liu, J.; Chen, K.; Jia, G.; Bergelson, J.; et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 2014, 5, 5630. [Google Scholar] [CrossRef]
- Edens, B.M.; Vissers, C.; Su, J.; Arumugam, S.; Xu, Z.; Shi, H.; Miller, N.; Rojas Ringeling, F.; Ming, G.; He, C.; et al. FMRP modulates neural differentiation through m6A-dependent mRNA nuclear export. Cell Rep. 2019, 28, 845–854.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 2017, 169, 824–835.e14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhao, H.; Zhang, S.; Zong, Z.; Li, C.; Ming, L.; Xie, W.; Yu, H. Feedback regulation of m6A modification creates local auxin maxima essential for rice microsporogenesis. Dev. Cell 2025, 60, 1454–1466.e4. [Google Scholar] [CrossRef]
- Shen, L.; Liang, Z.; Gu, X.; Chen, Y.; Teo, Z.W.N.; Hou, X.; Cai, W.M.; Dedon, P.C.; Liu, L.; Yu, H. N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev. Cell 2016, 38, 186–200. [Google Scholar] [CrossRef]
- Zhang, M.; Bodi, Z.; Mackinnon, K.; Zhong, S.; Archer, N.; Mongan, N.P.; Simpson, G.G.; Fray, R.G. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat. Commun. 2022, 13, 1127. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Wong, C.E.; Fan, S.; Zhang, Y.; Zhang, S.; Liang, Z.; Yu, H.; Shen, L. FIONA1-mediated m6A modification regulates the floral transition in Arabidopsis. Adv. Sci. 2022, 9, 2103628. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, S.; Qin, G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dou, X.; Chen, C.; Chen, C.; Liu, C.; Xu, M.M.; Zhao, S.; Shen, B.; Gao, Y.; Han, D.; et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 2020, 367, 580–586. [Google Scholar] [CrossRef]
- Wei, J.; Yu, X.; Yang, L.; Liu, X.; Gao, B.; Huang, B.; Dou, X.; Liu, J.; Zou, Z.; Cui, X.-L.; et al. FTO mediates LINE1 m6A demethylation and chromatin regulation in mESCs and mouse development. Science 2022, 376, 968–973. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, S.; Yu, L.; Xiao, Y.; Zhang, S.; Wang, X.; Xu, Y.; Yu, H.; Li, Y.; Yang, J.; et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat. Biotechnol. 2021, 39, 1581–1588. [Google Scholar] [CrossRef]
- Duan, H.-C.; Wei, L.-H.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.; Chen, P.R.; He, C.; Jia, G. ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Liu, P.; Liu, H.; Zhang, H.; Guo, S.; Liu, X.; Chen, X.; Chen, M. Genome-wide identification of the Soybean AlkB homologue gene family and functional characterization of GmALKBH10Bs as RNA m6A demethylases and expression patterns under abiotic stress. Plants 2024, 13, 2491. [Google Scholar] [CrossRef]
- Yue, H.; Nie, X.; Yan, Z.; Weining, S. N6-methyladenosine regulatory machinery in plants: Composition, function and evolution. Plant Biotechnol. J. 2019, 17, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, Y.; Liao, C.; Xie, Q.; Chen, G.; Hu, Z.; Wu, T. The AlkB homolog SlALKBH10B negatively affects drought and salt tolerance in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 25, 173. [Google Scholar] [CrossRef]
- Huong, T.T.; Ngoc, L.N.T.; Kang, H. Functional characterization of a putative RNA demethylase ALKBH6 in Arabidopsis growth and abiotic stress responses. Int. J. Mol. Sci. 2020, 21, 6707. [Google Scholar] [CrossRef]
- Huong, T.T.; Yang, Z.; Ngoc, L.N.T.; Kang, H. ALKBH8B, a putative RNA demethylase, plays a role in the response of Arabidopsis to salt stress and abscisic acid. J. Plant Biol. 2022, 65, 319–330. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Li, J.; Cheng, D.; Dai, C. Genome-wide sequence identification and expression analysis of N6-methyladenosine demethylase in sugar beet (Beta vulgaris L.) under salt stress. Peer J. 2022, 10, e12719. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lei, D.; Yang, J.; Chen, S.; Wang, X.; Huang, X.; Zhang, S.; Cai, Z.; Zhu, S.; Wan, J.; et al. OsALKBH9-mediated m6A demethylation regulates tapetal PCD and pollen exine accumulation in rice. Plant Biotechnol. J. 2024, 22, 2410–2423. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, G.; Tang, R.; Liu, J.; Wang, Y.; Liang, Z.; Tian, S.; Qin, G. Redox modification of m6A demethylase SlALKBH2 in tomato regulates fruit ripening. Nat. Plants 2025, 11, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Shu, P.; Hu, N.; Chen, Y.; Wu, Y.; Deng, H.; Du, X.; Zhang, X.; Wang, R.; Li, H.; et al. Dynamic m6A mRNA methylation reveals the involvement of AcALKBH10 in ripening-related quality regulation in kiwifruit. New Phytol. 2024, 243, 2265–2278. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, T.; Qi, Y.; Song, J.; Han, Z.; Ma, C. Evolution of the RNA N6-methyladenosine methylome mediated by genomic duplication. Plant Physiol. 2020, 182, 345–360. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Cao, S.; Tian, Y.; Han, K.; Sun, Y.; Li, J.; Yang, Q.; Ji, Q.; Sederoff, R.; et al. Genome-wide identification of the AlkB homologs gene family, PagALKBH9B and PagALKBH10B regulated salt stress response in Populus. Front. Plant Sci. 2022, 13, 994154. [Google Scholar] [CrossRef]
- Li, B.; Zhang, M.; Sun, W.; Yue, D.; Ma, Y.; Zhang, B.; Duan, L.; Wang, M.; Lindsey, K.; Nie, X.; et al. N6-methyladenosine RNA modification regulates cotton drought response in a Ca2+ and ABA-dependent manner. Plant Biotechnol. J. 2023, 21, 1270–1285. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Zhou, C.; Tian, C.; Shi, B.; Xu, K.; Huang, L.; Sun, Y.; Lin, Y.; Lai, Z.; et al. RNA methylome reveals the m6A-mediated regulation of flavor metabolites in tea leaves under solar-withering. Genom. Proteom. Bioinform. 2023, 21, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, C.; Li, S.; Yuan, M.; Mu, W.; Yang, J.; Ma, Y.; Guan, C.; Ma, C. Changes in m6A RNA methylation are associated with male sterility in wolfberry. BMC Plant Biol. 2023, 23, 456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lv, Z.; Diao, S.; Liu, H.; Duan, A.; He, C.; Zhang, J. Unique features of the m6A methylome and its response to drought stress in sea buckthorn (Hippophae rhamnoides Linn.). RNA Biol. 2021, 18, 794–803. [Google Scholar] [CrossRef]
- Sha, T.; Li, Z.; Xu, S.; Su, T.; Shopan, J.; Jin, X.; Deng, Y.; Lyu, X.; Hu, Z.; Zhang, M.; et al. eIF2Bβ confers resistance to turnip mosaic virus by recruiting ALKBH9B to modify viral RNA methylation. Plant Biotechnol. J. 2024, 22, 3205–3217. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Ge, R.; Ye, C.; Peng, Y.; Dai, Q.; Zhao, Y.; Liu, S.; Wang, P.; Hu, L.; He, C. m6A-SAC-Seq for quantitative whole transcriptome m6A profiling. Nat. Protoc. 2023, 18, 626–657. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Duan, X.; Zhou, L.; Gao, G.; Liu, J.; Wang, Y.; Shao, X.; Qin, G. The FvABF3-FvALKBH10B-FvSEP3 cascade regulates fruit ripening in strawberry. Nat. Commun. 2024, 15, 10912. [Google Scholar] [CrossRef]
- Han, R.; Shoaib, Y.; Cai, J.; Kang, H. ALKBH10B-mediated m6A demethylation is crucial for drought tolerance by affecting mRNA stability in Arabidopsis. Environ. Exp. Bot. 2023, 209, 105306. [Google Scholar] [CrossRef]
- Yang, D.; Xu, H.; Liu, Y.; Li, M.; Ali, M.; Xu, X.; Lu, G. RNA N6-methyladenosine responds to low-temperature stress in tomato anthers. Front. Plant Sci. 2021, 12, 687826. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, Z.; Zhang, Y.; Li, X.; Wang, S.; Wang, G.; Li, H.; Wang, B.; Zhang, M.; Li, W.; et al. Recessive epistasis of a Synonymous mutation confers cucumber domestication through epitranscriptomic regulation. Cell 2025, 188, 4517–4529.e15. [Google Scholar] [CrossRef]
- Kramer, M.C.; Janssen, K.A.; Palos, K.; Nelson, A.D.L.; Vandivier, L.E.; Garcia, B.A.; Lyons, E.; Beilstein, M.A.; Gregory, B.D. N6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis. Plant Direct 2020, 4, e00239. [Google Scholar] [CrossRef]
- Ma, S.; Gong, Q.; Bohnert, H.J. Dissecting salt stress pathways. Exp. Bot. 2006, 57, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, J.; Yu, L.; Zhao, X.; Ran, L.; Hu, D.; Song, B. N6- methyl-adenosine level in Nicotiana tabacum is associated with tobacco mosaic virus. Virol. J. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, L.Q.; Zhao, Y.L.; Yang, C.G.; Roundtree, I.A.; Zhang, Z.; Ren, J.; Xie, W.; He, C.; Luo, G.Z. Single-base mapping of m6A by an antibody-independent method. Sci. Adv. 2019, 5, eaax0250. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ma, J.; Li, P.; Wu, Y.; Yu, H. Recent advances in the plant epitranscriptome. Genome Biol. 2023, 24, 43. [Google Scholar] [CrossRef]
| Biological Process | Plant Species | m6A Demethylase | Regulate Role | Reference | |
|---|---|---|---|---|---|
| Development | Florescence | Arabidopsis thaliana | AtALKBH10B | Promote flowering | [18] |
| Fertility | Oryza sativa | OsALKBH9 | Regulate male reproductive function | [25] | |
| Fruit Ripening | Solanum lycopersicum | SlALKBH2 | Promote fruit ripening | [12] | |
| Solanum lycopersicum | AcALKBH10 | Promote fruit ripening | [27] | ||
| Fragaria vesca | FvALKBH10B | Promote fruit ripening | [37] | ||
| Product Quality | Solanum lycopersicum | AcALKBH10 | Improve Product Quality | [27] | |
| Fragaria vesca | FvALKBH10B | Improve Product Quality | [37] | ||
| Camellia sinensis | CsALKBH4 | Improve Product Quality | [31] | ||
| Abiotic Stress | Drought | Gossypium hirsutum | GhALKBH10B | Improve drought resistance | [30] |
| Zea mays | ZmALKBH10A ZmALKBH10B | Improve drought resistance | [28] | ||
| Hippophae rhamnoides | HrALKBH10B HrALKBH10C HrALKBH10D | Improve drought resistance | [33] | ||
| Poplars | PagALKBH9B PagALKBH10B | Reduce drought resistance | [29] | ||
| Solanum lycopersicum | SlALKBH10B | Reduce drought resistance | [21] | ||
| Salt | Arabidopsis thaliana | AtALKBH10B | Improve salt resistance | [38] | |
| AtALKBH6 | Reduce salt resistance | [22] | |||
| AtALKBH8B | Improve salt resistance | [23] | |||
| Beta vulgaris | BvALKBH10B | Improve salt resistance | [24] | ||
| Poplars | PagALKBH9B PagALKBH10B | Improve salt resistance | [29] | ||
| Solanum lycopersicum | SlALKBH10B | Reduce salt resistance | [21] | ||
| Biotic Stress | Virus | Arabidopsis thaliana | AtALKBH9B | Reduce virus resistance | [11] |
| Brassica juncea | BjALKBH9B | Improve virus resistance | [34] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Cao, Y.; Yu, W.; Lyu, S.; Fan, Y.; Li, H. Functions of RNA N6-Methyladenosine Demethylases in Plant Development and Stress Responses. Agronomy 2025, 15, 2269. https://doi.org/10.3390/agronomy15102269
Su R, Cao Y, Yu W, Lyu S, Fan Y, Li H. Functions of RNA N6-Methyladenosine Demethylases in Plant Development and Stress Responses. Agronomy. 2025; 15(10):2269. https://doi.org/10.3390/agronomy15102269
Chicago/Turabian StyleSu, Ran, Ying Cao, Wenjie Yu, Shanhua Lyu, Yinglun Fan, and Haiyun Li. 2025. "Functions of RNA N6-Methyladenosine Demethylases in Plant Development and Stress Responses" Agronomy 15, no. 10: 2269. https://doi.org/10.3390/agronomy15102269
APA StyleSu, R., Cao, Y., Yu, W., Lyu, S., Fan, Y., & Li, H. (2025). Functions of RNA N6-Methyladenosine Demethylases in Plant Development and Stress Responses. Agronomy, 15(10), 2269. https://doi.org/10.3390/agronomy15102269






