Integrated Transcriptomic and Metabolomic Analyses of Seed-Filling Disorders in Soybeans Under Different Ecological Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Physiological Indicators
2.3. Transcriptome Sequencing
2.4. Metabolome Determination and Analysis
2.5. Analysis of Gene Expression by RT-qPCR
2.6. Statistical Analyses
3. Results
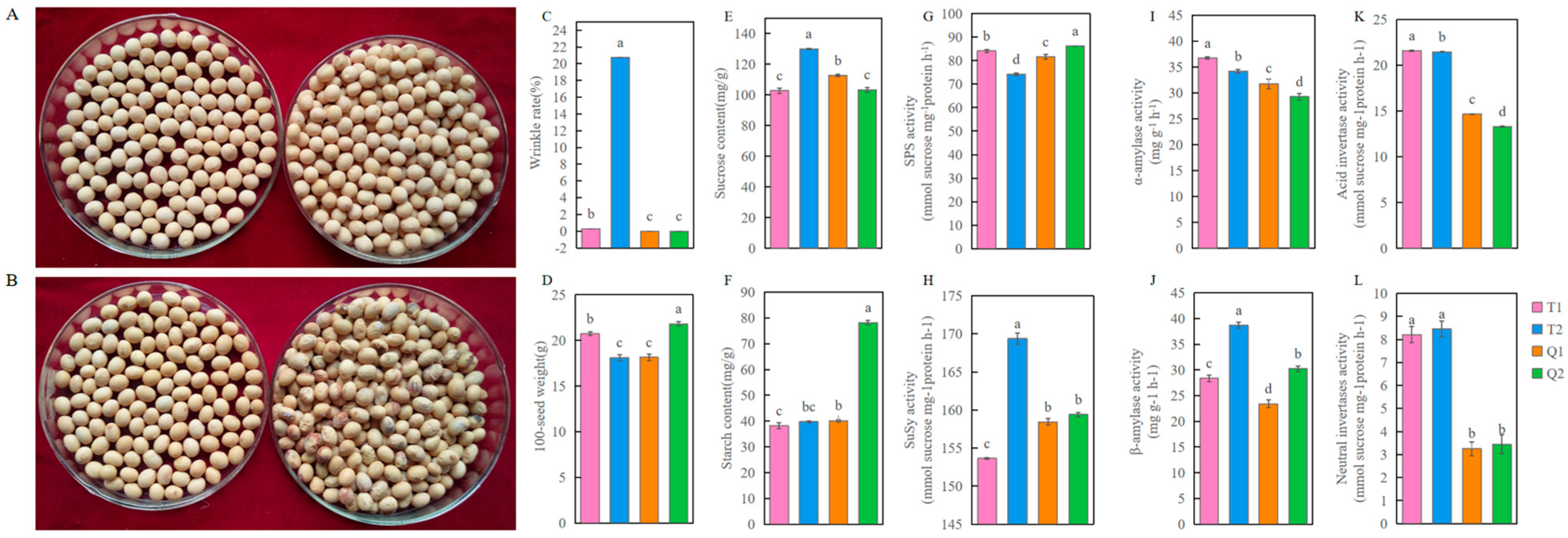
3.1. Analysis of Physiological Parameters
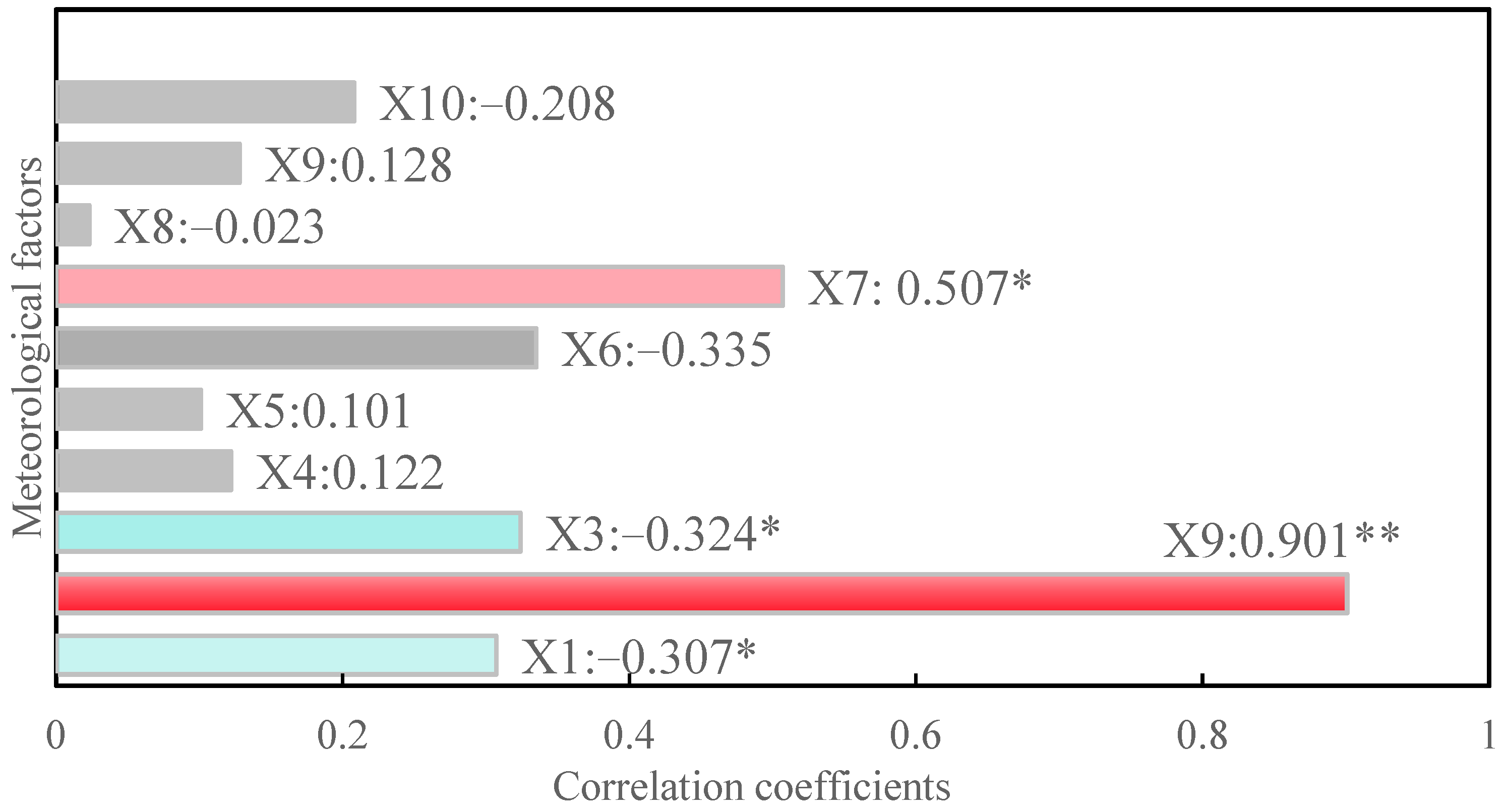
3.2. Correlation Analysis Between Ecological Factors and Soybean Wrinkled-Seed
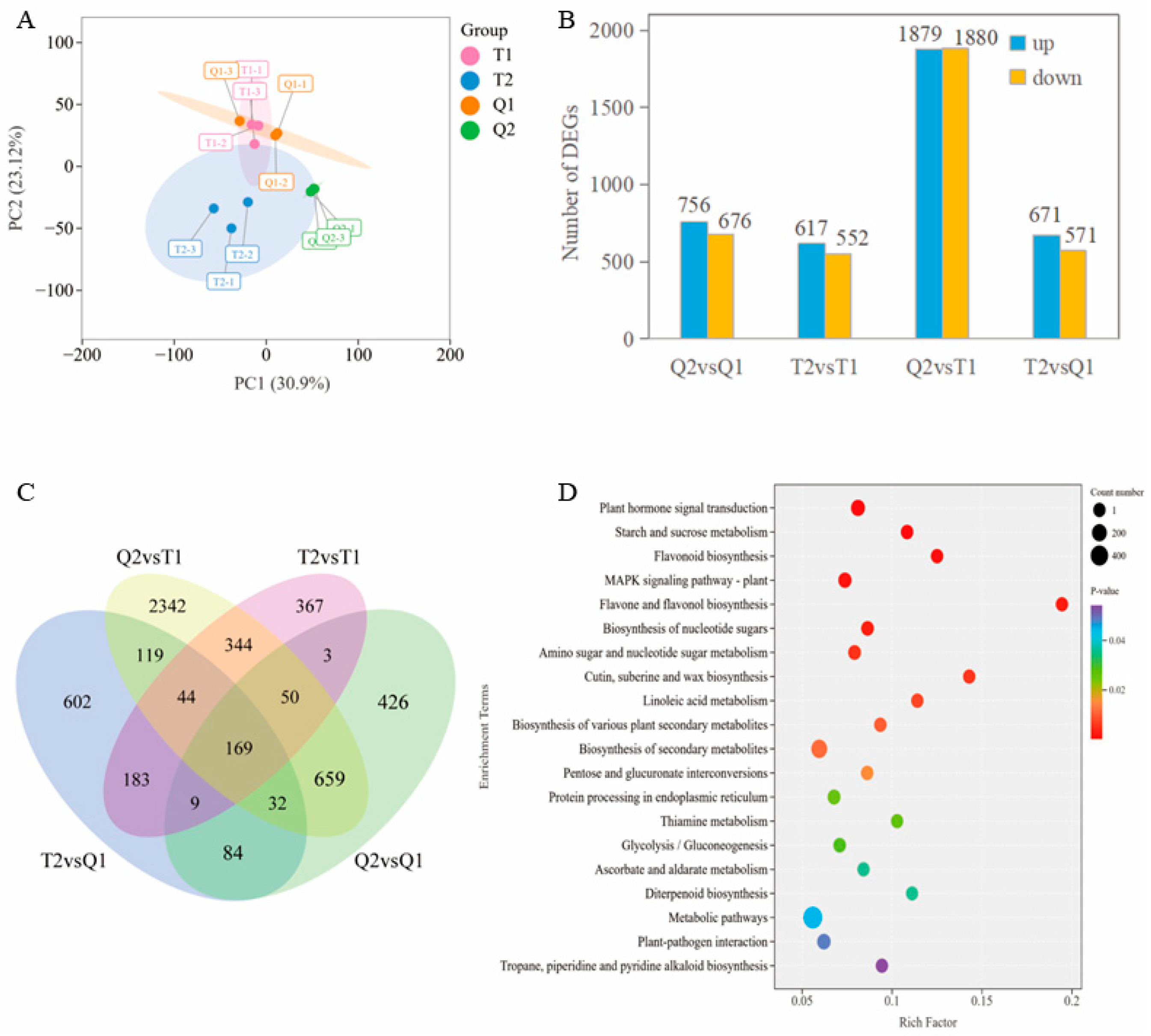
3.3. Transcriptome Analysis
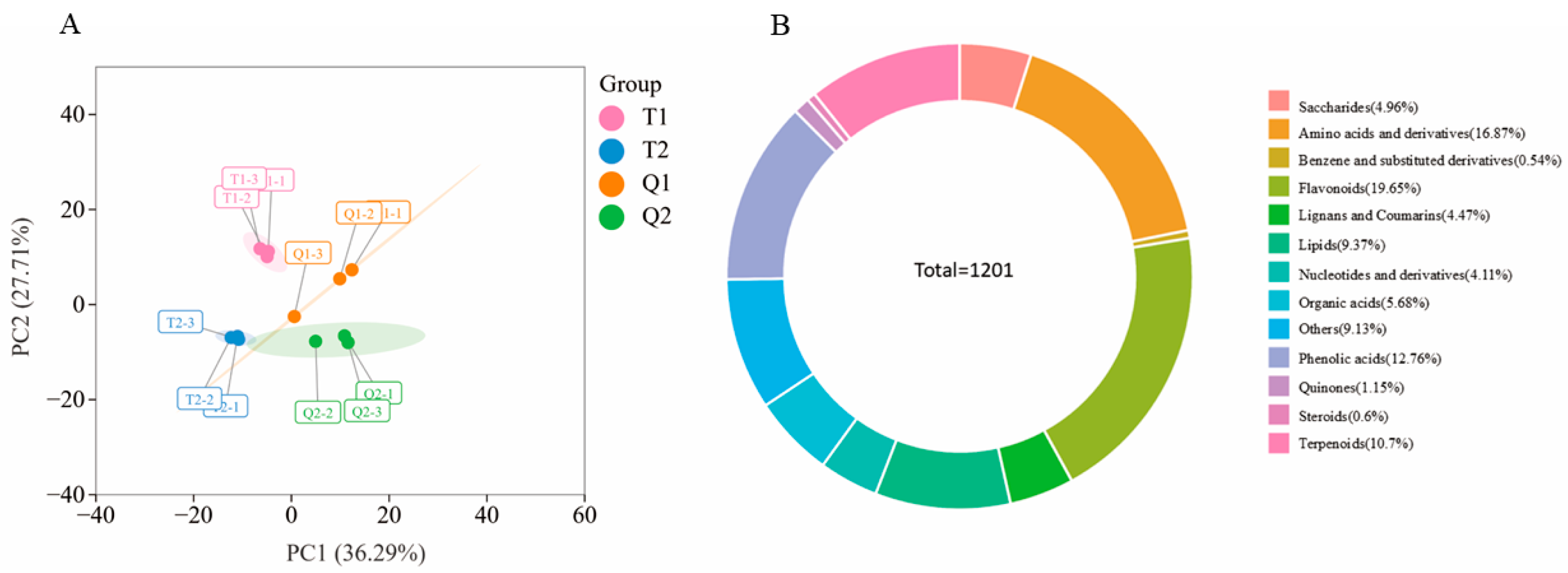
3.4. Metabolome Analysis
3.5. Combined Transcriptome and Metabolome Analysis
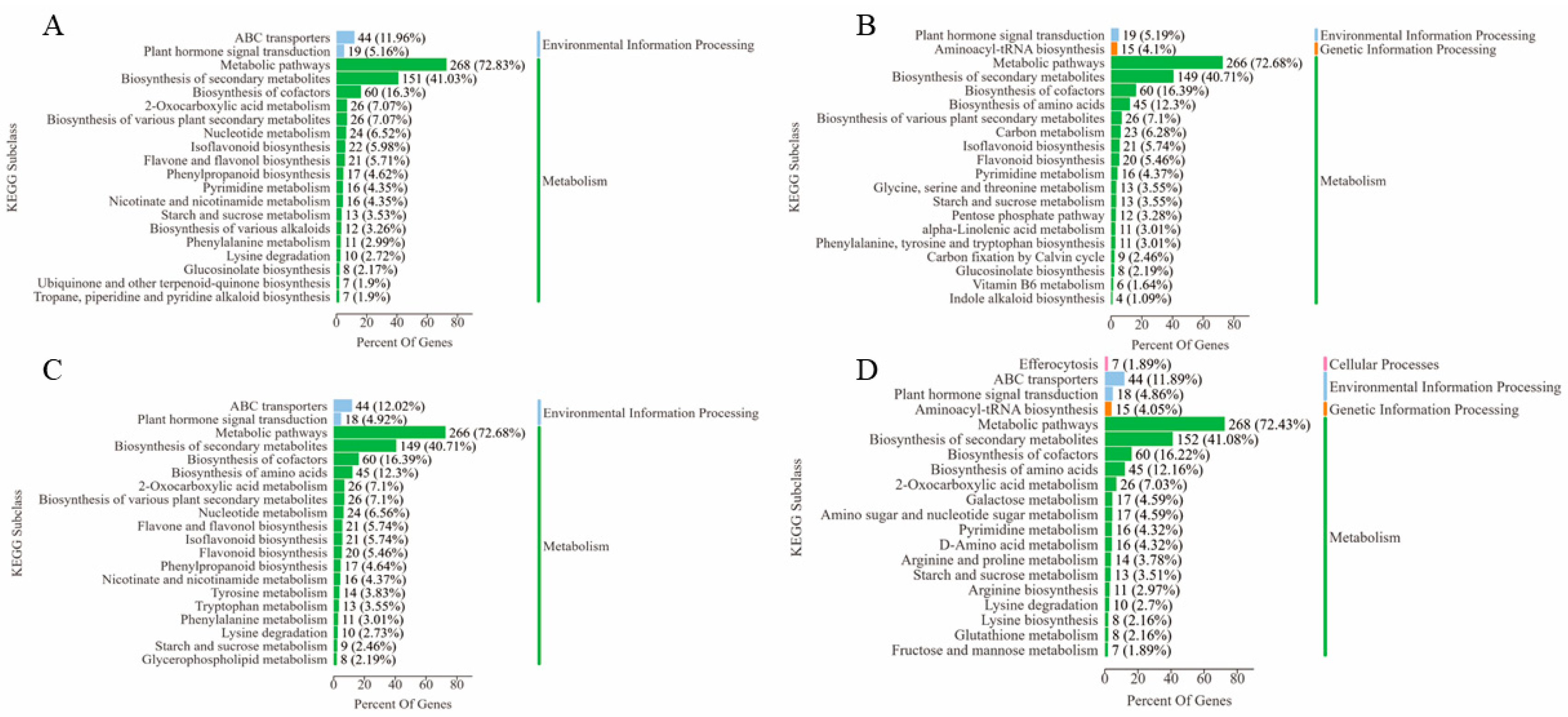
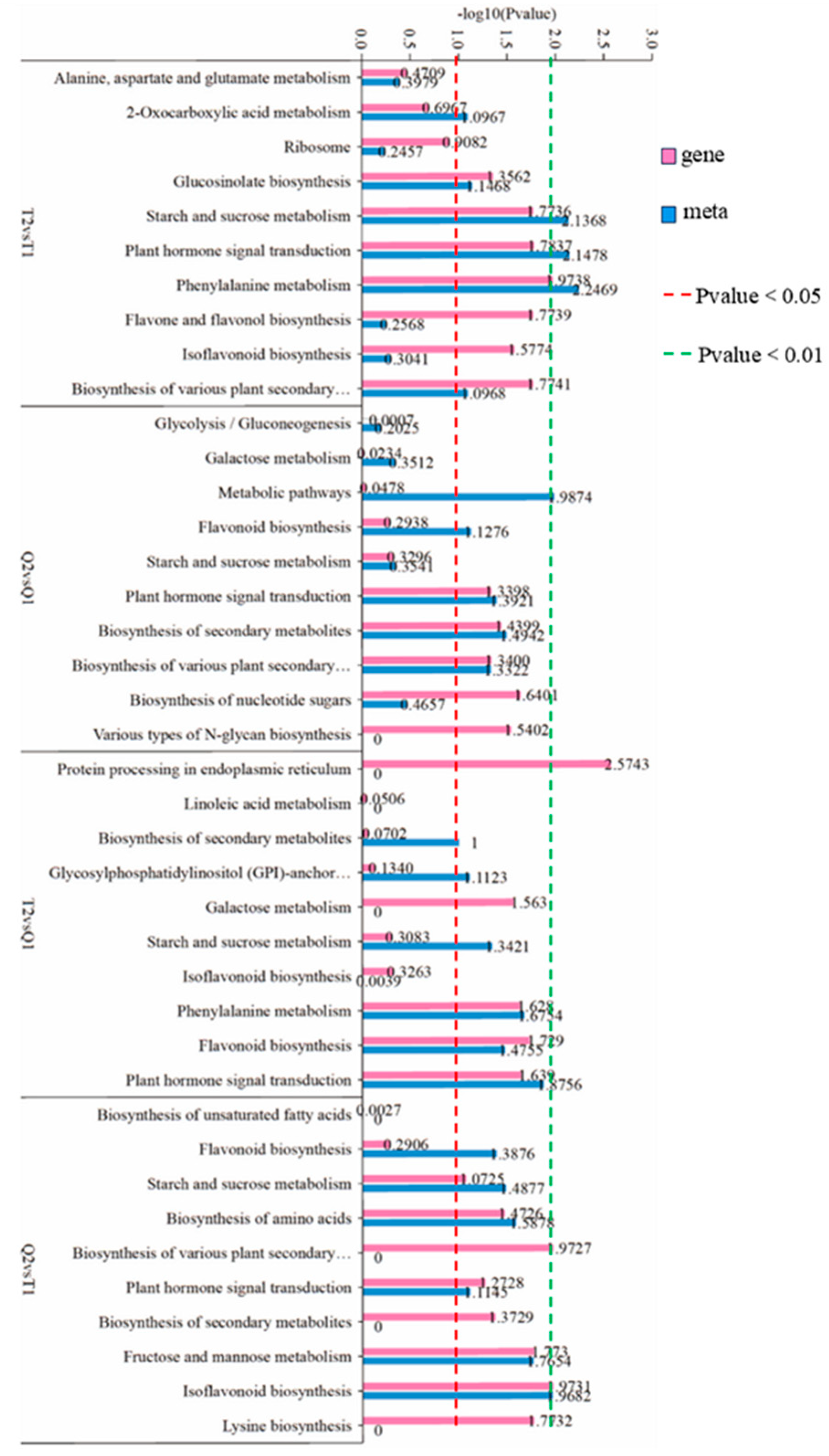
3.5.1. KEGG Enrichment Analysis
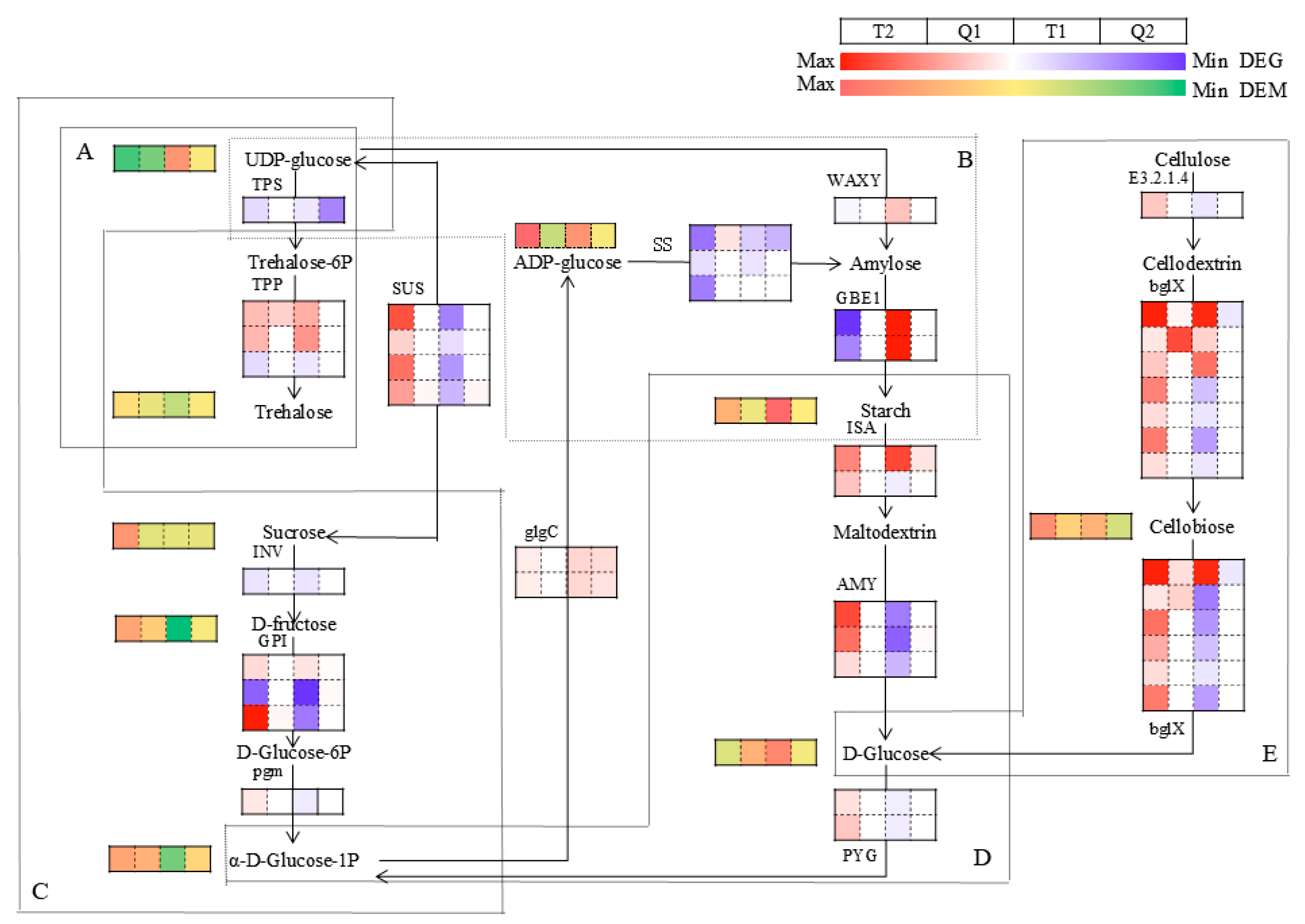
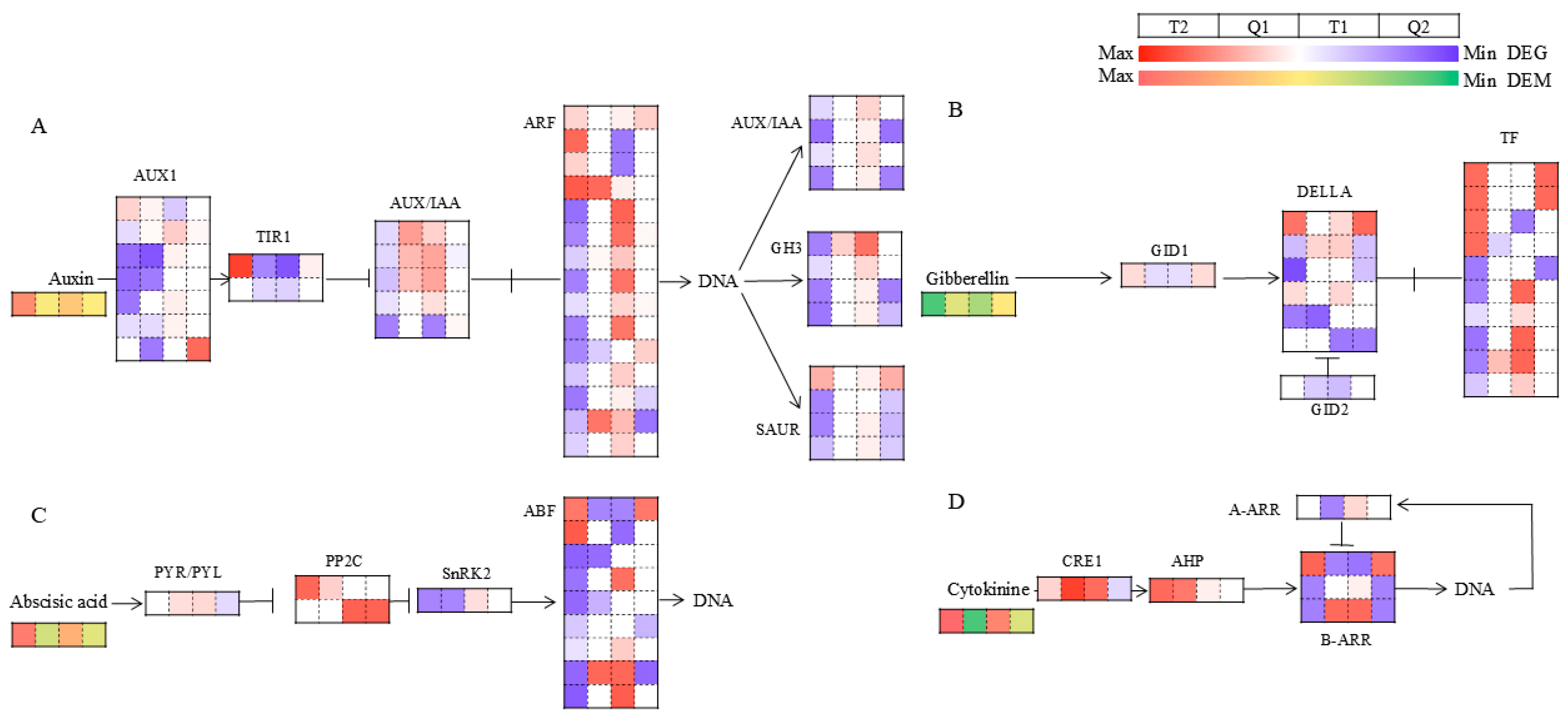
3.5.2. Analysis of Key Metabolic Pathways
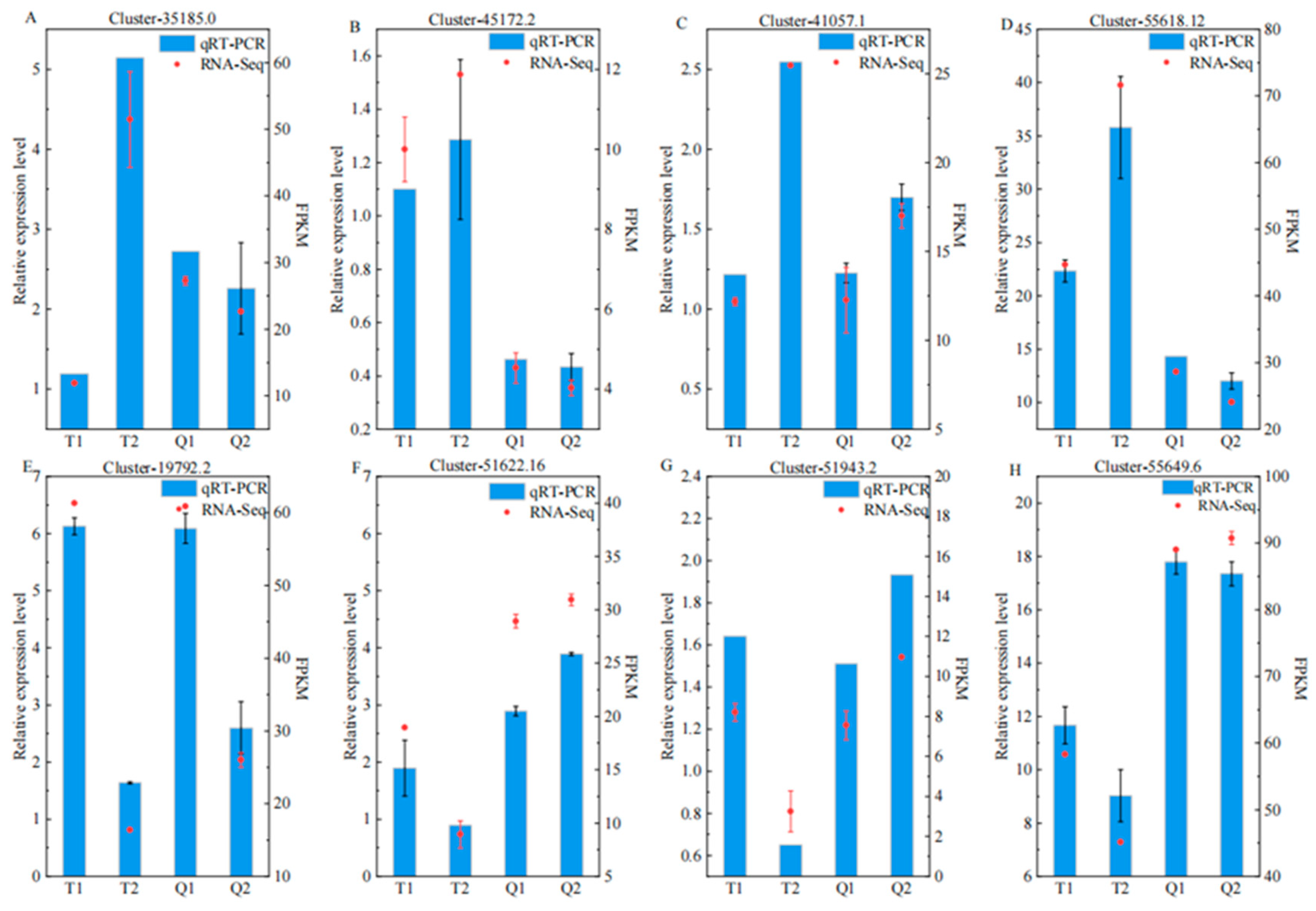
3.6. RT-qPCR Validation of Selected Differentially Expressed Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Singh, T.R.; Hada, A.; Jolly, M.; Ganapathi, A.; Sachdev, A. Probing phosphorus efficient low phytic acid content soybean genotypes with phosphorus starvation in hydroponics growth system. Appl. Biochem. Biotechnol. 2015, 177, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zhang, B.; Hu, Y. Development Trend, Policy Evolution and Trend Prospect of China’s Soybean Industry. Agric. Outlook 2022, 18, 35–41. [Google Scholar]
- Peng, Y.; Hu, S. Research on Seed Wrinkling of Northern Soybean Germplasm after Introduction to the South. Soybean Sci. 1997, 16, 343–347. [Google Scholar]
- Du, Y.; Pan, T.; Tian, Y.; Liu, S.; Liu, X.; Jiang, L.; Zhang, W.; Wang, Y.; Wan, J. Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant fse4. Chin. J. Rice Sci. 2019, 33, 499–512. [Google Scholar]
- Cui, G. Research on Formation Mechanism and Genetic Analysis of Wrinkled Seed of Mutant in Peanut (Arachis hypogaea L.). Master’s Thesis, Shandong Agricultural University, Taian, China, 2010. [Google Scholar]
- Zhang, X. Study on the Grain of Wheat. Acta Agron. Sin. 1982, 2, 87–93. [Google Scholar]
- Bhattacharyya, M.K.; Smith, A.M.; Elis, T.H. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch branching enzyme. Cell 1990, 60, 115. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Martin, C.; Smith, A. The importance of starch biosynthesis in the wrinkled seed shape character of peas studied by Mendel. Plant Mol. Biol. 1993, 22, 525–531. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Song, W.; Dai, J.; Lai, J. Molecular Mapping and Candidate Genes Prediction of Maize Endosperm Mutant Wrk1. J. Maize Sci. 2013, 21, 27–31. [Google Scholar]
- Chen, H.; Zhu, D.; Lin, X.; Zhang, Y. Effects of seed plumpness on germinating rate, seedling rate and growth of hybrid rice. Fujian J. Agric. Sci. 2004, 19, 65–67. [Google Scholar]
- Sun, X.; Gu, J.; Liu, Q.; Hu, A. Effect of seed fullness and water content on germination of rice seeds. Jiangsu Agric. Sci. 2014, 42, 96–97. [Google Scholar]
- Zhao, X.; Peng, B.; Zhang, J.; Yan, H.; Zhang, C.; Zhao, L. Influence of Out-crossing Rate on Seed Quality in Sterile Soybean Lines and Regulating Ways. Soybean Sci. 2017, 36, 487–493. [Google Scholar]
- Li, Z.; Wang, Z.; Yang, T. Studies of Shriveled Seeds of Maie-Sterile Lines and their Hybrids with T. timopheevi Cytoplasm in Wheat. J. Northwest AF Univ. 1987, 2, 1–9. [Google Scholar]
- Liu, Z.; Rao, S.; Pu, Z. Effects of nuclear and cytoplasmic factors on seed quality of hybrid wheat with T. timopheevi cytoplasm. Southwest China J. Agric. Sci. 1999, 12, 26–31. [Google Scholar]
- Fan, L.; Yan, Q.; Xu, Y.; Ruan, S. Studies on seed treatments for improving field seedling emergence of sh2sweet corn (Zea mays L). J. Zhejiang Univ. 1997, 23, 100–104. [Google Scholar]
- Cernac, A.; Benning, C. WRINKLED 1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004, 40, 575–585. [Google Scholar] [CrossRef]
- Focks, N.; Benning, C. A Novel, Low-Seed-Oil Mutant of Arabidopsis with a Deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 1998, 118, 91–101. [Google Scholar]
- Hedley, C.L.; Smith, C.M.; Ambrose, M.J. An analysis of seed development in Pisum sativum II. The effect of the r-Locus on the growth and development of the seed. Ann. Bot. 1986, 58, 371–379. [Google Scholar] [CrossRef]
- Li, X. Difference of Gene Expression Profiling in the Endosperm of ae/wx and sh1 Mutants and Starch Biosynthesis in Maize. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2008. [Google Scholar]
- Yano, M.; Isono, Y.; Satoh, H. Gene analysis of sugary and shrunken mutants of rice, Oryze sativa L. Jpn. J. Breed. 1984, 34, 43–49. [Google Scholar] [CrossRef]
- Wang, T.; Hedley, C.L. Seed development in peas: Knowing your three “r” s (or four, or five). Seed Sci. Res. 1991, 1, 3–14. [Google Scholar] [CrossRef]
- Jiang, S.; An, H.; Luo, J. Comparative Analysis of Transcriptomes to Identify Genes Associated with Fruit Size in the Early Stage of Fruit Development in Pyrus pyrifolia. Int. J. Mol. Sci. 2018, 19, 2342. [Google Scholar] [CrossRef]
- Guan, H.Y.; Dong, R.; Liu, T.S.; He, C.M.; Wang, J.; Liu, Q.; Xu, Q.; Zhang, M.L.; Wang, L.M. Preliminary Mapping of Molecular in Maize Kernel Shrunken Mutant sh2019. Shandong Agric. Sci. 2024, 56, 8–13. [Google Scholar]
- Sun, Z.L. Functional Study of the ZmBT1 Gene Affecting the Shrunken Phenotype of Maize Kernels. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2024. [Google Scholar]
- Rossini, M.A.; Curin, F.; Otegui, M.E. Ear reproductive development components associated with kernel set in maize: Breeding effects under contrasting environments. Field Crops Res. 2023, 304, 109150. [Google Scholar] [CrossRef]
- Han, T.F.; Jiang, B.J. Identification of GmLUX2 paves the way for north-to-south adaption of soybeans. Sci. Sin. Vitae 2021, 51, 472–475. [Google Scholar] [CrossRef]
- Luan, R.W. Effects of Heat Stress on Grain Development and Yield Formation of Setaria italica and Regulation of Exogenous Substances. Master’s Thesis, Shandong Agricultural University, Taian, China, 2024. [Google Scholar]
- Dwivedi, S.K.; Basu, S.; Kumar, S.; Kumar, G.; Prakash, V.; Kumar, S.; Mishra, J.S.; Bhatt, B.P.; Malviya, N.; Singh, G.P.; et al. Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crops Res. 2017, 206, 106–114. [Google Scholar] [CrossRef]
- Wang, H.Q.; Liu, P.; Zhang, J.W.; Zhao, B.; Ren, B.Z. Endogenous Hormones Inhibit Differentiation of Young Ears in Maize (Zea mays L.) Under Heat Stress. Front. Plant Sci. 2020, 11, 533046. [Google Scholar] [CrossRef]
- Mahajan, G.; Wenham, K.; Chauhan, B.S. Mungbean (Vigna radiata) Growth and Yield Response in Relation to Water Stress and Elevated Day/Night Temperature Conditions. Agronomy 2023, 13, 2546. [Google Scholar] [CrossRef]
- Zhuang, T.X.; Zhao, B.; Syed Tahir, A.U.K.; Gilles, L.; Liu, X.J.; Tian, Y.C.; Zhu, Y.; Cao, W.X.; Cao, Q. Exploring the allometry between ear saturated water accumulation and dry mass for diagnosing winter wheat water status during the reproductive growth. Agric. Water Manag. 2025, 309, 109364. [Google Scholar] [CrossRef]
- Singer, W.M.; Lee, Y.C.; Shea, Z.; Vieira, C.C.; Lee, D.; Li, X.; Cunicelli, M.; Kadam, S.S.; Khan, M.A.W.; Shannon, G.; et al. Soybean genetics, genomics, and breeding for improving nutritional value and reducing antinutritional traits in food and feed. Plant Genome 2023, 16, e20415. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, Y.; Lin, K.; Wu, W.; Duan, L.; Wang, P. Cytokinin promotes anthocyanin biosynthesis via regulating sugar accumulation and MYB113 expression in Eucalyptus. Tree Physiol. 2024, 44, 154. [Google Scholar] [CrossRef]
- Feng, W.; Xue, W.; Zhao, Z.; Shi, Z.; Wang, W.; Bai, Y.; Wang, H.; Qiu, P.; Xue, J.; Chen, B. Nitrogen fertilizer application rate affects the dynamic metabolism of nitrogen and carbohydrates in kernels of waxy maize. Front. Plant Sci. 2024, 15, 1416397. [Google Scholar] [CrossRef]
- Li, Y.; Hu, W.; Zou, J.; He, J.; Zhu, H.; Zhao, W.; Wang, Y.; Chen, B.; Meng, Y.; Wang, S.; et al. Effects of soil drought on cottonseed kernel carbohydrate metabolism and kernel biomass accumulation. Plant Physiol. Biochem. 2023, 195, 170–181. [Google Scholar] [CrossRef]
- Liu, H.; Si, X.; Wang, Z.; Cao, L.; Gao, L.; Zhou, X.; Wang, W.; Wang, K.; Jiao, C.; Zhuang, L.; et al. TaTPP-7A positively feedback regulates grain filling and wheat grain yield through T6P-SnRK1 signalling pathway and sugar-ABA interaction. Plant Biotechnol. J. 2023, 21, 1159–1175. [Google Scholar]
- Feng, Y.L.; Yin, F.; Xu, K.; Jia, X.Z.; Zhou, S.; Ma, C. Role of Sucrose Metabolism and Signal Transduction in Plant Development and Stress Response. J. Nucl. Agric. Sci. 2021, 35, 2044–2055. [Google Scholar]
- Lan, G.Q.; Wu, M.Z.; Zhang, Q.H.; Yuan, B.; Shi, G.X.; Zhu, N.; Zheng, Y.B.Y.; Cao, Q.; Qiao, Q.; Zhang, T.C. Tanscriptomic and Physiological Analyses for the Role of Hormones and Sugar in Axillary Bud Development of Wild Strawberry Stolon. Plants 2024, 13, 2241. [Google Scholar]
- Liu, J.; Ma, Y.; Lv, F.; Chen, J.; Zhou, Z.; Wang, Y. Changes of sucrose metabolism in leaf subtending to cotton boll under cool temperature due to late planting. Field Crops Res. 2013, 144, 200–211. [Google Scholar]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci. Rep. 2018, 8, 15665. [Google Scholar] [CrossRef]
- Hu, W.; Loka, D.A.; Fitzsimons, T.R.; Zhou, Z.; Oosterhuis, D.M. Potassium deficiency limits reproductive success by altering carbohydrate and protein balances in cotton (Gossypium hirsutum L.). Environ. Exp. Bot. 2018, 145, 87–94. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T. Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Sloat, L.L.; Davis, S.J.; Gerber, J.S.; Moore, F.C.; Ray, D.K.; West, P.C.; Mueller, N.D. Climate adaptation by crop migration. Nat. Commun. 2020, 11, 1243. [Google Scholar] [CrossRef] [PubMed]
- Girousse, C.; Inchboard, L.; Deswarte, J.C.; Chenu, K. How does post-flowering heat impact grain growth and its deter-mining processes in wheat? J. Exp. Bot. 2021, 72, 6596–6610. [Google Scholar]
- Yu, J.; Du, T.; Zhang, P.; Ma, Z.; Chen, X.; Cao, J.; Li, H.; Li, T.; Zhu, Y.; Xu, F. Impacts of High Temperatures on the Growth and Development of Rice and Measuresfor Heat Tolerance Regulation: A Review. Agronomy 2024, 14, 2811. [Google Scholar]
- Li, C.Y.; Fu, K.Y.; Zhang, R.Q.; Xu, F.F.; Zhu, X.Y.; Zhu, Y.Q.; Qin, A.X.; Li, C. Effect of High Temperature Post Anthesis on the Development of Starch Granules in Winter Wheat. J. Triticeae Crops 2015, 35, 1395–1402. [Google Scholar]
- Wu, Y.L.; Yu, Z.Y.; Lei, F.Y. Effects of Agronomic and Physiological Characters of Soybean Sterile Lines on the Percentage of Wrinkled Kernels per Plant. J. Inn. Mong. Minzu Univ. 2023, 38, 423–427. [Google Scholar]
- Liu, Z.; Jiang, S.; Jiang, L.; Li, W.; Tang, Y.; He, W.; Wang, M.; Xing, J.; Cui, Y.; Lin, Q.; et al. Transcription factor OsSGL is a regulator of starch synthesis and grain quality in rice. J. Exp. Bot. 2022, 73, 3417–3430. [Google Scholar] [CrossRef]
- Mega, R.; Kim, J.S.; Tanaka, H.; Ishii, T.; Abe, F.; Okamoto, M. Metabolic and transcriptomic profiling during wheat seed development under progressive drought conditions. Sci. Rep. 2023, 13, 15001. [Google Scholar] [CrossRef]
- Zhao, S.; Cao, R.; Sun, L.; Zhuang, D.; Zhong, M.; Zhao, F.; Jiao, G.; Chen, P.; Li, X.; Duan, Y.; et al. An Integrative Analysis of the Transcriptome and Proteome of Rice Grain Chalkiness Formation Under High Temperature. Plants 2024, 13, 3309. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, Y.L.; Chen, H.Z.; Xiang, J.; Zhang, Y.K.; Wang, Z.G.; Zhu, D.F.; Zhang, Y.P. Brassinosteroids Mediate Endogenous Phytohormone Metabolism to Alleviate High Temperature Injury at Panicle Initiation Stage in Rice. Rice Sci. 2023, 30, 70–86. [Google Scholar] [CrossRef]
- Luo, B.; Liu, F.; Wan, Y.; Zhang, K.; Zhao, W. Dynamic Changes of Endogenous Hormones Content and Dry Matter Accumulation of Pods and Kernels in Different Varieties (Lines) of Peanut (Arachis hypogaea L.). Acta Agron. Sin. 2013, 39, 2083–2093. [Google Scholar] [CrossRef]
- Xing, M.; Su, H.; Liu, X.; Yang, L.; Zhang, Y.; Wang, Y.; Fang, Z.; Lv, H. Morphological, transcriptomics and phytohormone analysis shed light on the development of a novel dwarf mutant of cabbage (Brassica oleracea). Plant Sci. 2020, 290, 110283. [Google Scholar] [CrossRef]
- Chandler, W.J. Auxin response factors. Plant Cell Environ. 2016, 39, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G. Auxin and Light Control of Adventitious Rooting in Arabidopsis Require ARGO NAUTE1. Plant Cell 2005, 17, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Jain, M.; Garg, R. Genome-wide analysis and expression profiling suggest diverse roles of GH3 genes during development and abiotic stress responses in legumes. Front. Plant Sci. 2015, 5, 789. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Cui, D.Z.; Huang, C.; Sui, X.X.; Fan, Q.Q.; Chu, X.S. Dynamic Changes of Cell Morphology and Endogenous Hormones during Grain Development of New Wheat Variety Jimai 70. Shandong Agric. Sci. 2021, 5, 117–121. [Google Scholar]
- Yang, T.; Wang, H.; Guo, L.; Wu, X.; Xiao, Q.; Wang, J.; Wang, Q.; Ma, G.; Wang, W.; Wu, Y. ABA-induced phosphorylation of basic leucine zipper 29, Abscisic Acid Insensitive 19, and Opaque2 by SnRK2.2 enhances gene transactivation for endosperm filling in maize. Plant Cell 2022, 34, 1933–1956. [Google Scholar] [CrossRef]
- Sarma, B.; Kashtoh, H.; Lama, T.T.; Bhattacharyya, P.N.; Mohanta, Y.K.; Baek, K.H. Abiotic Stress in Rice: Visiting the Physiological Response and Its Tolerance Mechanisms. Plants 2023, 12, 3948. [Google Scholar] [CrossRef]
- Gilroy, E.; Breen, S. Interplay between phytohormone signalling pathways in plant defence-other than salicylic acid and jasmonic acid. Essays Biochem. 2022, 66, 657–671. [Google Scholar]
- Liu, Y.; Xiao, W.H.; Cai, W.L.; Zhang, W.Y.; Wang, Z.Q.; Xu, Y.J. Advances in Studies on the Roles of Plant Hormones in Grain Filling, Grain Weight and Quality of Rice. China Rice 2023, 29, 9–14. [Google Scholar]
- Wu, C.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Ding, Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ 2019, 7, e7792. [Google Scholar] [CrossRef]
- Cao, T.X.; Wang, S.L.; Asjad, A.L.; Shan, N.; Sun, J.Y.; Chen, X.; Wang, P.T.; Zhu, Q.L.; Xiao, Y.; Luo, S.; et al. Transcriptome and metabolome analysis reveals the potential mechanism of tuber dynamic development in yam (Dioscorea polystachya Turcz.). LWT 2023, 181, 114764. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Q.; Liang, X.; Liu, H.; Wu, C.; Fang, H.; Zhang, X.; Ding, S.; Yu, J.; Shi, K. Glucose-G protein signaling plays a crucial role in tomato resilience to high temperature and elevated CO2. Plant Physiol. 2024, 195, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Bao, H.; Wang, J.; Liu, H.; Liu, J.; Huang, L.; Li, D. Role of jasmonates in plant response to temperature stress. Plant Sci. 2025, 355, 112477. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cai, Z.; Huang, X.; Liao, J.; Huang, L.; Liu, D.; Zhao, Z.; Chen, Y.; Lu, C. Carbohydrate and hormone regulatory networks driving dormancy release of Cardiocrinum giganteum (Wall.) Makino bulbs induced by low temperature. Physiol. Plant. 2025, 177, e70108. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
| Experimental Factors | Sowing-Pod Setting | Grain-Filling Stage | Mature Stage | ||||
|---|---|---|---|---|---|---|---|
| Average Daily Temperature (°C) | Daily Maximum Temperature (°C) | Average Daily Temperature (°C) | Daily Maximum Temperature (°C) | Average Daily Temperature (°C) | Daily Maximum Temperature (°C) | ||
| Location | Jiamusi | 19.35 b | 24.70 b | 20.66 b | 25.66 c | 15.61 b | 21.51 b |
| Shenyang | 22.25 a | 25.79 a | 25.71 a | 30.83 a | 21.82 a | 26.58 a | |
| Cultivar | Henong 76 | 20.79 | 25.23 | 23.17 | 28.23 b | 18.70 | 24.03 |
| Heihe 43 | 20.82 | 25.26 | 23.20 | 29.19 b | 18.73 | 24.04 | |
| Mean squares (ANO-VA) | Location (df = 1) | 23.52 *** | 12.54 *** | 8.92 *** | 24.3 *** | 117.34 *** | 67.11 *** |
| Cultivar (df = 1) | ns | ns | ns | 7.18 ** | ns | ns | |
| Location × Cultivar (df = 1) | ns | ns | ns | 9.55 ** | ns | ns | |
| MS error (df = 8) | 0.104 | 0.203 | 0.112 | 0.289 | 0.148 | 0.198 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zheng, W.; Liang, Z.; Zhang, Z.; Li, J.; Zhang, H.; Wang, H.; Ao, X.; Yao, X.; Xie, F. Integrated Transcriptomic and Metabolomic Analyses of Seed-Filling Disorders in Soybeans Under Different Ecological Conditions. Agronomy 2025, 15, 2266. https://doi.org/10.3390/agronomy15102266
Huang J, Zheng W, Liang Z, Zhang Z, Li J, Zhang H, Wang H, Ao X, Yao X, Xie F. Integrated Transcriptomic and Metabolomic Analyses of Seed-Filling Disorders in Soybeans Under Different Ecological Conditions. Agronomy. 2025; 15(10):2266. https://doi.org/10.3390/agronomy15102266
Chicago/Turabian StyleHuang, Junxia, Wei Zheng, Zicong Liang, Zhenghao Zhang, Jiayi Li, Huijun Zhang, Haiying Wang, Xue Ao, Xingdong Yao, and Futi Xie. 2025. "Integrated Transcriptomic and Metabolomic Analyses of Seed-Filling Disorders in Soybeans Under Different Ecological Conditions" Agronomy 15, no. 10: 2266. https://doi.org/10.3390/agronomy15102266
APA StyleHuang, J., Zheng, W., Liang, Z., Zhang, Z., Li, J., Zhang, H., Wang, H., Ao, X., Yao, X., & Xie, F. (2025). Integrated Transcriptomic and Metabolomic Analyses of Seed-Filling Disorders in Soybeans Under Different Ecological Conditions. Agronomy, 15(10), 2266. https://doi.org/10.3390/agronomy15102266






