Effects of Long-Term Nitrogen Fertilization on Nitrous Oxide Emission and Yield in Acidic Tea (Camellia sinensis L.) Plantation Soils
Abstract
1. Introduction
2. Materials and Methods
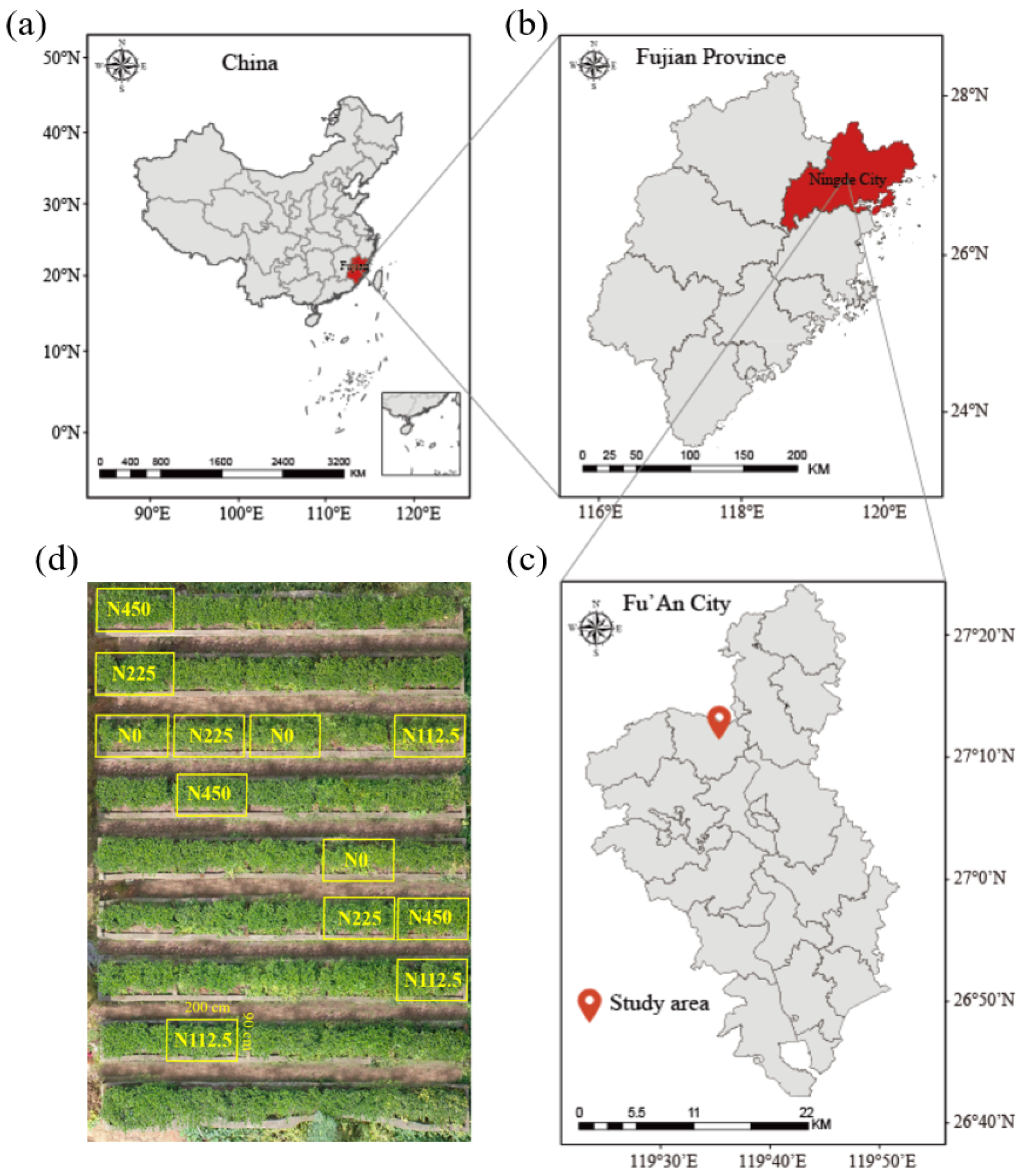
2.1. Site Description
2.2. Experiment Design
2.3. Tea Shoot Sampling and Yield Calculation
2.4. Measurement of N2O Emissions and Calculation of Indicators
2.5. Measurement of Environmental Factors
2.6. Statistical Analysis
3. Results
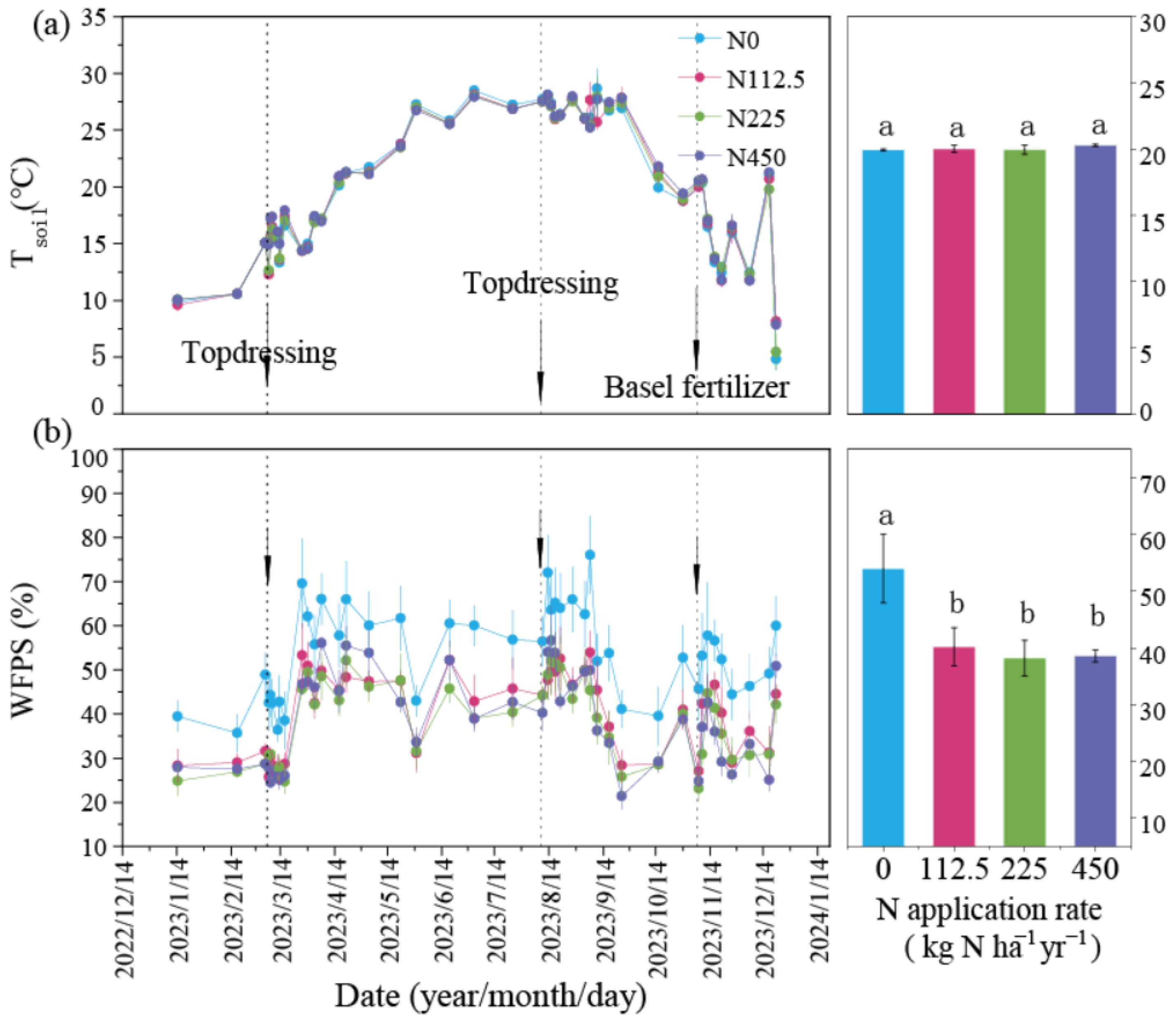
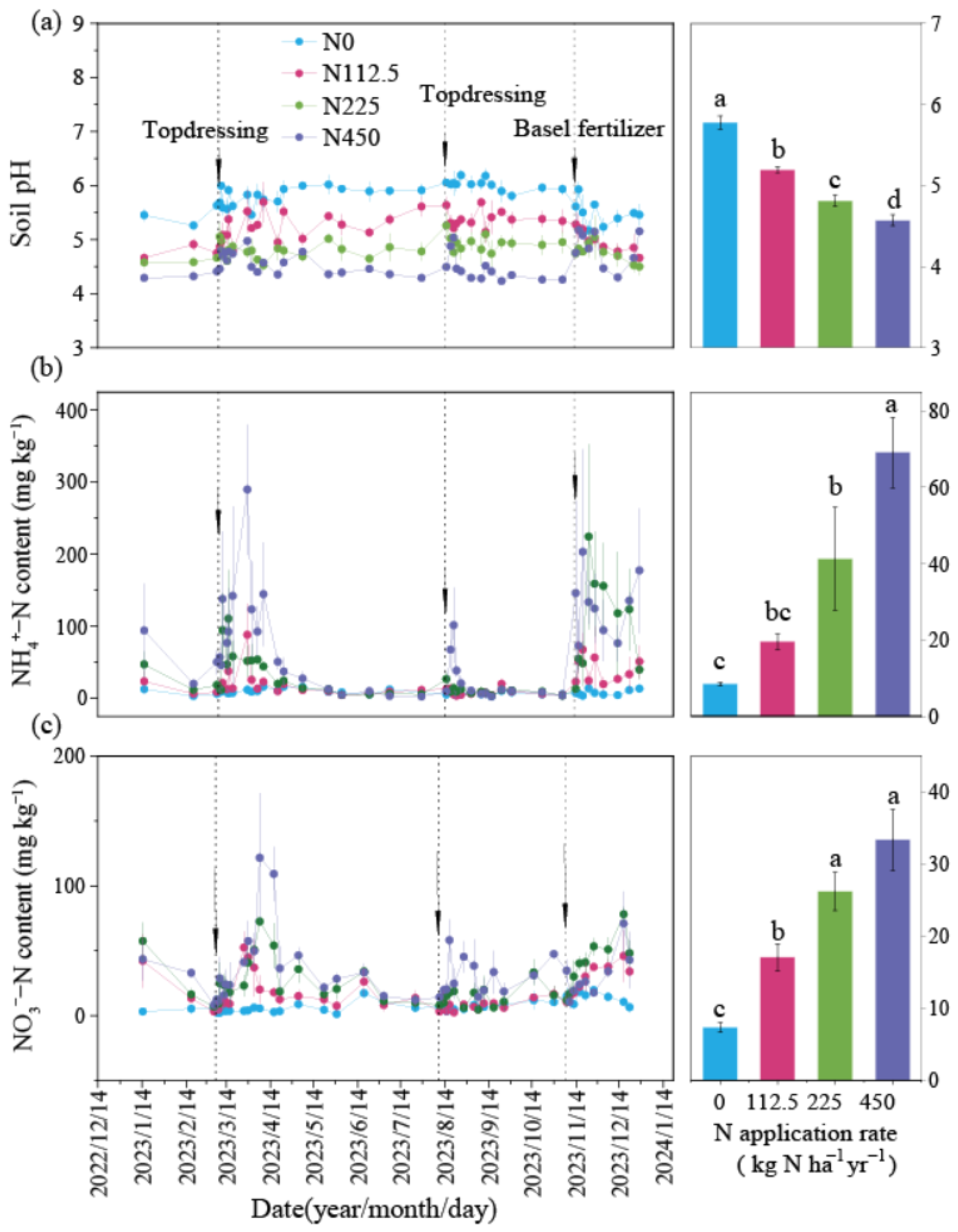
3.1. Changes in Tsoil, WFPS, Soil pH, and Inorganic N Under Different N Rates
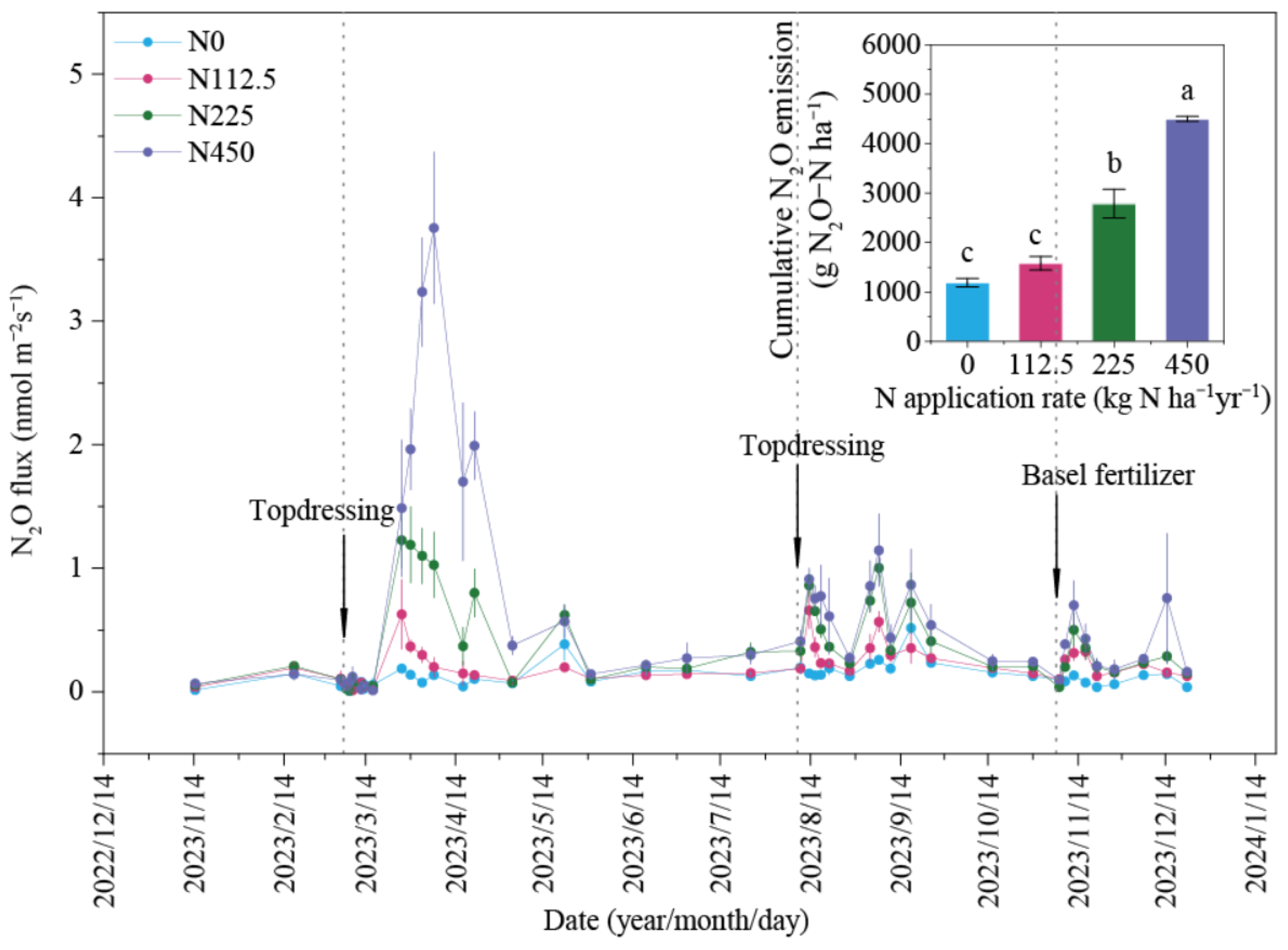
3.2. Changes in N2O Flux and Cumulative N2O Emissions Under Different N Rates
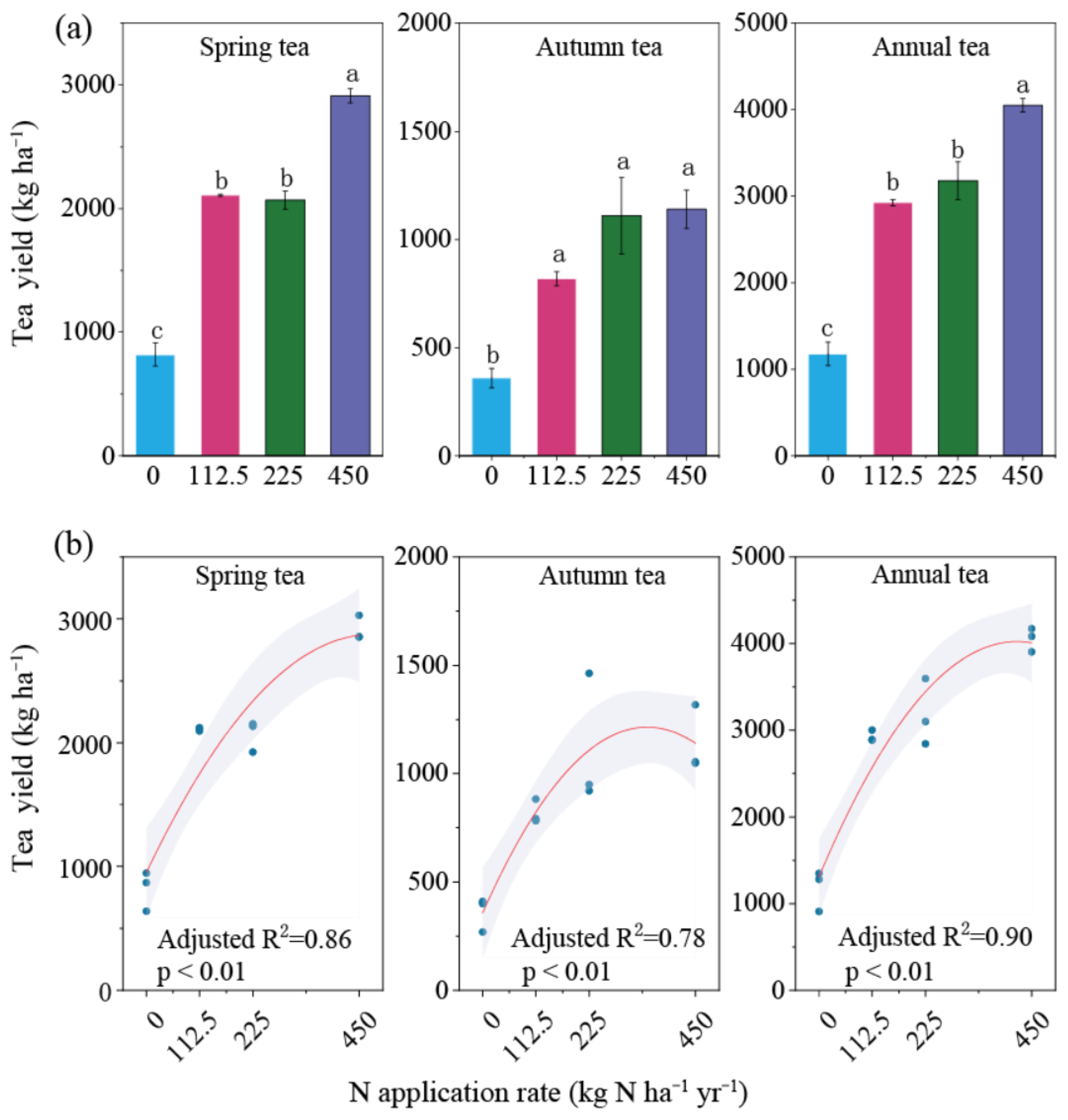
3.3. Tea Yield, YSNE, and N2O EF Under Different N Rates
3.4. Driving Factors for Influencing N2O Flux in Tea Plantations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; p. 714. [Google Scholar]
- Huang, X.H.; Zheng, Y.T.; Li, P.F.; Cui, J.X.; Sui, P.; Chen, Y.Q.; Gao, W.S. Organic management increases beneficial microorganisms and promotes the stability of microecological networks in tea plantation soil. Front. Microbiol. 2023, 14, 1237842. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Ma, L.F.; Shi, Y.Z. Potassium management in tea plantations: Its uptake by field plants, status in soils, and efficacy on yields and quality of teas in China. J. Plant Nutr. Soil Sci. 2013, 176, 450–459. [Google Scholar] [CrossRef]
- Tokuda, S.; Hayatsu, M. Nitrous oxide production from strongly acid tea field soils. Soil Sci. Plant Nutr. 2000, 46, 835–844. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited Greenhouse. Gas Meas. Manage 2011, 1, 17–26. [Google Scholar]
- Han, W.Y.; Xu, J.M.; Wei, K.; Shi, Y.X.; Ma, L.F. Estimation of N2O emission from tea garden soils, their adjacent vegetable garden and forest soils in eastern China. Environ. Earth Sci. 2013, 70, 2495–2500. [Google Scholar] [CrossRef]
- Yao, Z.; Wei, Y.; Liu, C.; Zheng, X.; Xie, B. Organically fertilized tea plantation stimulates N2O emissions and lowers no fluxes in subtropical China. Biogeosciences 2015, 12, 5915–5928. [Google Scholar] [CrossRef]
- Hou, M.; Ohkama-Ohtsu, N.; Suzuki, S.; Tanaka, H.; Schmidhalter, U.; Bellingrath-Kimura, S.D. Nitrous oxide emission from tea soil under different fertilizer managements in Japan. Catena 2015, 135, 304–312. [Google Scholar] [CrossRef]
- Zaw, A.O.; Shigeto, S.; Hiroko, A.; Thuzar, W.K.; Akira, S.; Akinori, Y.; Tomohito, S.; Yuhei, H.; Jorge, P.F. Effect of dolomite and biochar addition on N2O and CO2 emissions from acidic tea field soil. PLoS ONE 2018, 13, e0192235. [Google Scholar]
- Wang, Y.; Yao, Z.S.; Pan, Z.L.; Wang, R.; Yan, G.X.; Liu, C.Y.; Su, Y.Y.; Zheng, X.H.; Klaus, B.B. Tea-planted soils as global hotspots for N2O emissions from croplands. Environ. Res. Lett. 2020, 15, 104018. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan ; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Gu, J.X.; Nie, H.H.; Guo, H.J.; Xu, H.H.; Gunnathorn, T. Nitrous oxide emissions from fruit orchards: A review. Atmos. Env. 2019, 201, 166–172. [Google Scholar] [CrossRef]
- Ding, W.C.; Xu, X.P.; He, P.; Zhang, J.; Zhou, W. Estimating regional N application rates for rice in China based on target yield, indigenous N supply, and N loss. Environ. Pollut. 2020, 263 Pt B, 114408. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Naokawa, M.; Honda, S.; Nakajima, Y. Lime-nitrogen application affects nitrification, denitrification, and N2O emission in an acidic tea soil. Biol. Fertil. Soils 2014, 50, 53–62. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Z.; Zheng, X.; Liu, C.; Shi, J. Effects of fertilization and stand age on N2O and NO emissions from tea plantations: A site-scale study in a subtropical region using a modified biogeochemical model. Atmos. Chem. Phys. 2020, 20, 6903–6919. [Google Scholar] [CrossRef]
- Agusto, L.E.; Qin, G.M.; Thibodeau, B.; Tang, J.W.; Zhang, J.F.; Zhou, J.G.; Wu, J.T.; Zhang, L.L.; Thapa, P.; Wang, F.M.; et al. Fiddling with the blue carbon: Fiddler crab burrows enhance CO2 and CH4 efflux in saltmarsh. Ecol. Indic. 2022, 144, 109538. [Google Scholar] [CrossRef]
- Bao, Q.; Ju, X.; Gao, B.; Qu, Z.; Christie, P.; Lu, Y. Response of nitrous oxide and corresponding bacteria to managements in an agricultural soil. Soil Sci. Soc. Am. J. 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zou, C.Q.; Gao, X.P.; Guan, X.L.; Zhang, W.S.; Zhang, Y.Q.; Shi, X.J.; Chen, X.P. Nitrous oxide emissions in chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef]
- Ma, L.F.; Yang, X.D.; Shi, Y.Z.; Yi, X.Y.; Ji, L.F.; Cheng, Y.; Ruan, J.Y. Response of tea yield, quality and soil bacterial characteristics to long-term nitrogen fertilization in an eleven-year field experiment. Appl. Soil Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J.Y. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Env. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Hayatsu, M.; Kosuge, N. Autotrophic nitrification in acid tea soils. Soil Sci. Plant Nutr. 1993, 39, 209–217. [Google Scholar] [CrossRef][Green Version]
- Aulakh, M.S.; Rennie, D.A.; Paul, E.A. Acetylene and n-serve effects upon N2O emissions from NH4+ and NO3− treated soils under aerobic and anaerobic conditions. Soil Biol. Biochem. 1984, 16, 351–356. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cheng, S.L.; Fang, H.J.; Yu, G.R.; Xu, M.J.; Dang, X.S.; Li, L.S.; Wang, L. Simulated Nitrogen Deposition Reduces CH4 Uptake and Increases N2O Emission from a Subtropical Plantation Forest Soil in Southern China. PLoS ONE 2014, 9, e93571. [Google Scholar] [CrossRef] [PubMed]
- Dobbie, K.E.; Smith, K.A. The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur. J. Soil Sci. 2010, 52, 667–673. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Change Biol 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Machon, A.; Horváth, L.; Weidinger, T.; Grosz, B.; Pintér, K.; Tuba, Z.; Führer, E. Estimation of net nitrogen flux between the atmosphere and a semi-natural grassland ecosystem in Hungary. Eur. J. Soil Sci. 2010, 61, 631–639. [Google Scholar] [CrossRef]
- Granli, T.; Bockman, O.C. Nitrous oxide from agriculture. Nor. J. Agric. Sci. 1994, 12, 128. [Google Scholar]
- Wang, B.; Wang, S.; Li, G.Y.; Fu, L.B.; Chen, H.; Yin, M.; Chen, J.F. Reducing nitrogen fertilizer usage coupled with organic substitution improves soil quality and boosts tea yield and quality in tea plantations. J. Food Agric. 2024, 25, 1228–1238. [Google Scholar] [CrossRef]
- Wang, F.E.; He, Z.L.; Zhang, X.L.; Iqbal, J.; Shaaban, M.; Hu, R.G.; Lin, S.; Yan, Z.W. Comparative effects of straw and biochar on N2O emissions from acidic soils. J. Soil Sci. Plant Nutr. 2024, 24, 2080–2090. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Yang, X.D.; Tang, S.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Ma, Q.X.; Cai, Y.J.; Ma, L.F.; Ruan, J.Y. Long-term nitrogen addition increases denitrification potential and functional gene abundance and changes denitrifying communities in acidic tea plantation soil. Environ. Res. 2023, 216, 114679. [Google Scholar] [CrossRef]
- Yao, Z.S.; Wang, Y.; Wang, R.; Liu, C.Y.; Zheng, X.H. Nitrous oxide emissions and controlling factors of tea plantations in China. J. Agro-Environ. Sci. 2020, 39, 715–725. (In Chinese) [Google Scholar]
- Duan, P.P.; Wang, D.B.; Xiao, K.C.; Zheng, L.; Chen, H.; Wang, K.L.; De, J. Responses of soil nitrous oxide e mission to nitrogen addition at two topographic positions of a subtropical forest. JGR Biogeosci. 2022, 127, e2021JG006539. [Google Scholar] [CrossRef]


| N Application Rates (kg N ha−1 yr−1) | YSNE (gN2O-N kg−1 tea) | N2O-EF (%) |
|---|---|---|
| 0 | 1.03 ± 0.06 ab | |
| 112.5 | 0.54 ± 0.04 c | 0.35 ± 0.07 b |
| 225 | 0.88 ± 0.1 b | 0.71 ± 0.09 a |
| 450 | 1.11 ± 0.02 a | 0.73 ± 0.01 a |
| ANOVA | ||
| F-value | 16.69 | 10.53 |
| p-value | <0.01 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Chang, Y.; Han, J.; Yang, X.; Wu, Z. Effects of Long-Term Nitrogen Fertilization on Nitrous Oxide Emission and Yield in Acidic Tea (Camellia sinensis L.) Plantation Soils. Agronomy 2025, 15, 7. https://doi.org/10.3390/agronomy15010007
Jiang F, Chang Y, Han J, Yang X, Wu Z. Effects of Long-Term Nitrogen Fertilization on Nitrous Oxide Emission and Yield in Acidic Tea (Camellia sinensis L.) Plantation Soils. Agronomy. 2025; 15(1):7. https://doi.org/10.3390/agronomy15010007
Chicago/Turabian StyleJiang, Fuying, Yunni Chang, Jiabao Han, Xiangde Yang, and Zhidan Wu. 2025. "Effects of Long-Term Nitrogen Fertilization on Nitrous Oxide Emission and Yield in Acidic Tea (Camellia sinensis L.) Plantation Soils" Agronomy 15, no. 1: 7. https://doi.org/10.3390/agronomy15010007
APA StyleJiang, F., Chang, Y., Han, J., Yang, X., & Wu, Z. (2025). Effects of Long-Term Nitrogen Fertilization on Nitrous Oxide Emission and Yield in Acidic Tea (Camellia sinensis L.) Plantation Soils. Agronomy, 15(1), 7. https://doi.org/10.3390/agronomy15010007





