Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Plant and Soil Sampling
2.3.2. Measurement of Plant Growth Indicators
2.3.3. Measurement of Plant Physiological and Ecological Indicators
2.3.4. Determination of Soil Physical and Chemical Properties
2.4. Data Processing and Statistical Analysis
3. Results
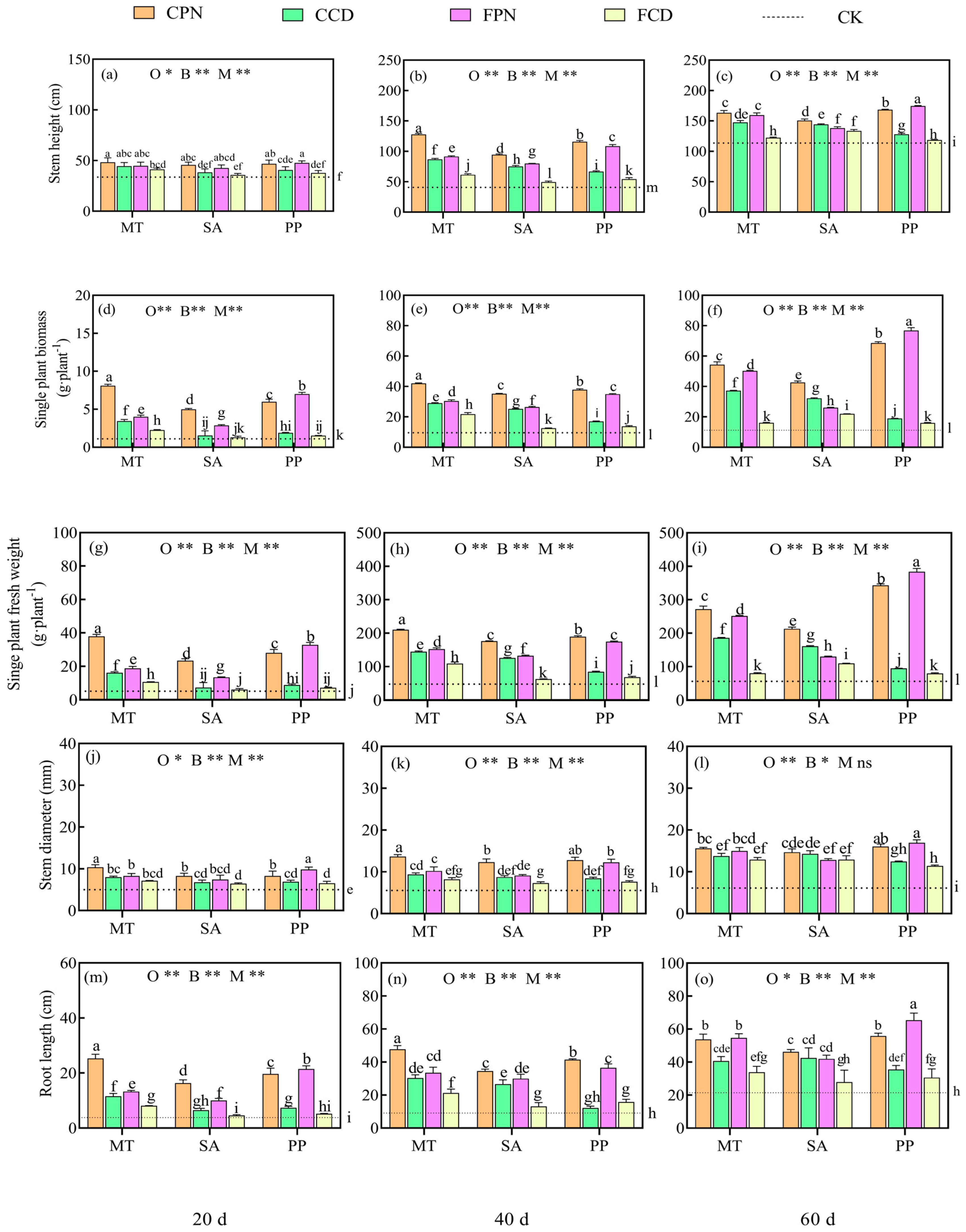
3.1. Impact of Organic Acid Bio-Modifier on Morphological Characteristics of Sweet Sorghum
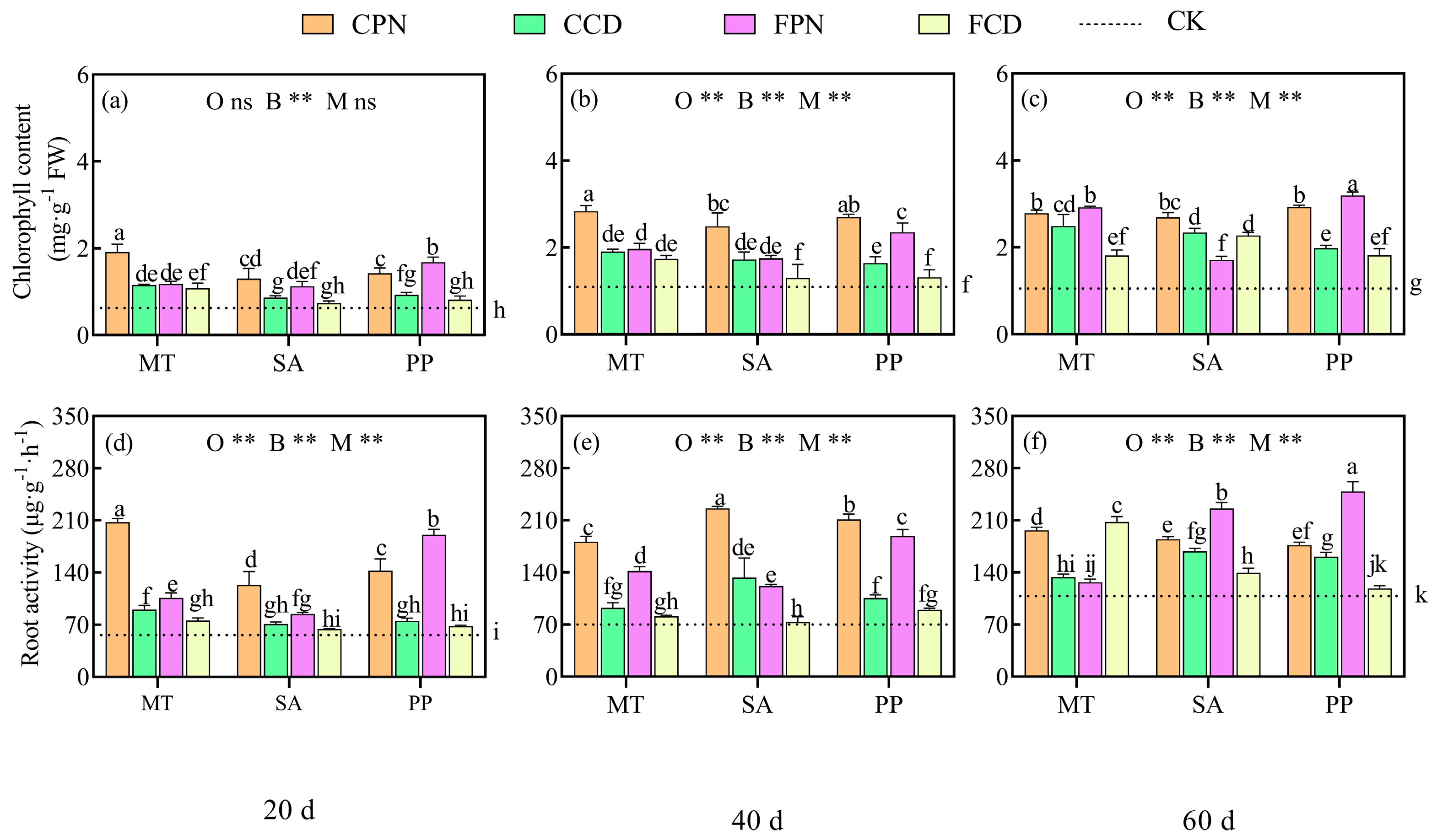
3.2. Impact of Organic Acid Bio-Modifier on Photosynthesis and Root Physiology of Sweet Sorghum
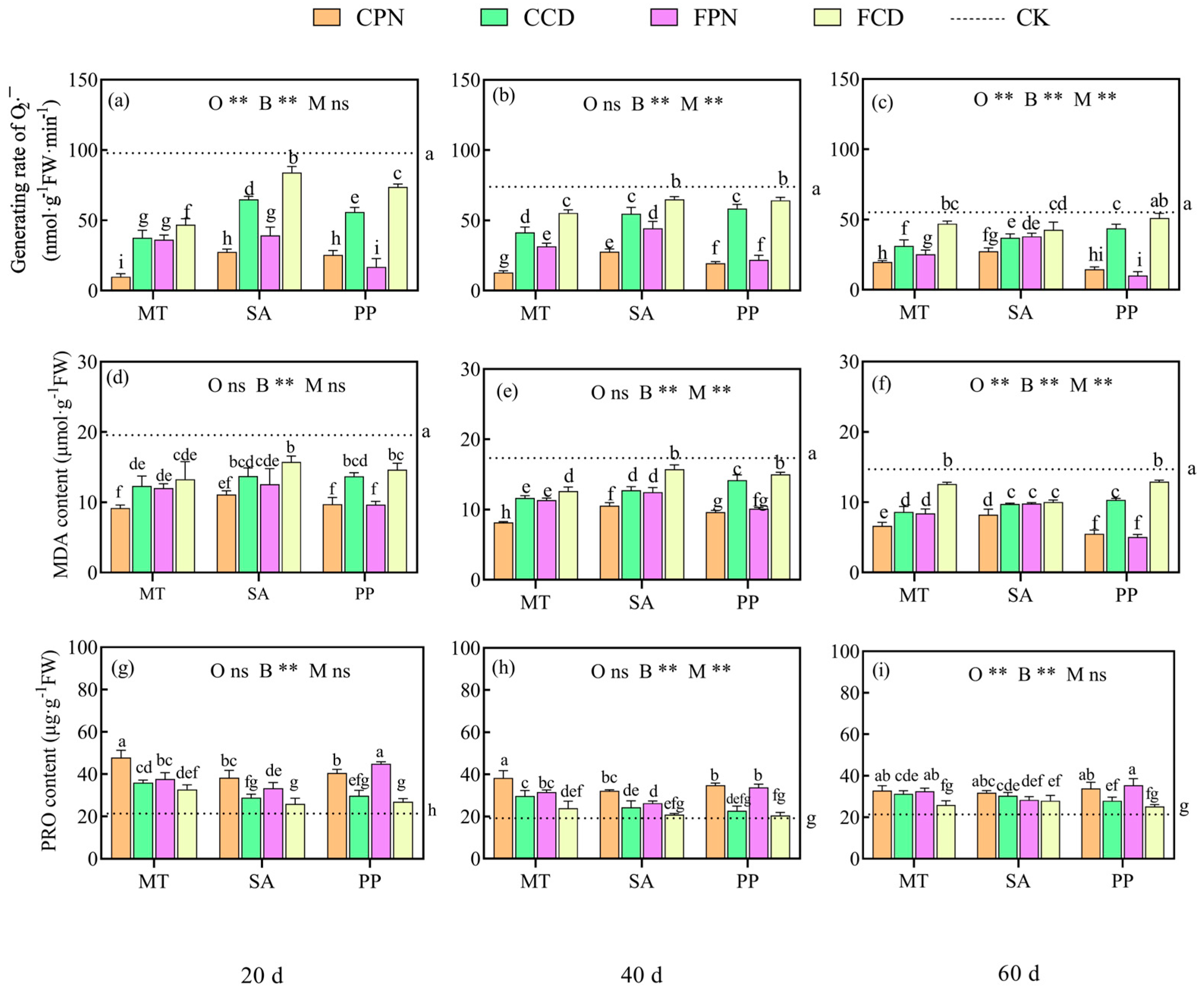
3.3. Impact of Organic Acid Bio-Modifier on Antioxidant Enzyme Systems of Sweet Sorghum
3.4. Impact of Organic Acid Bio-Modifier on Osmoregulation in Sweet Sorghum
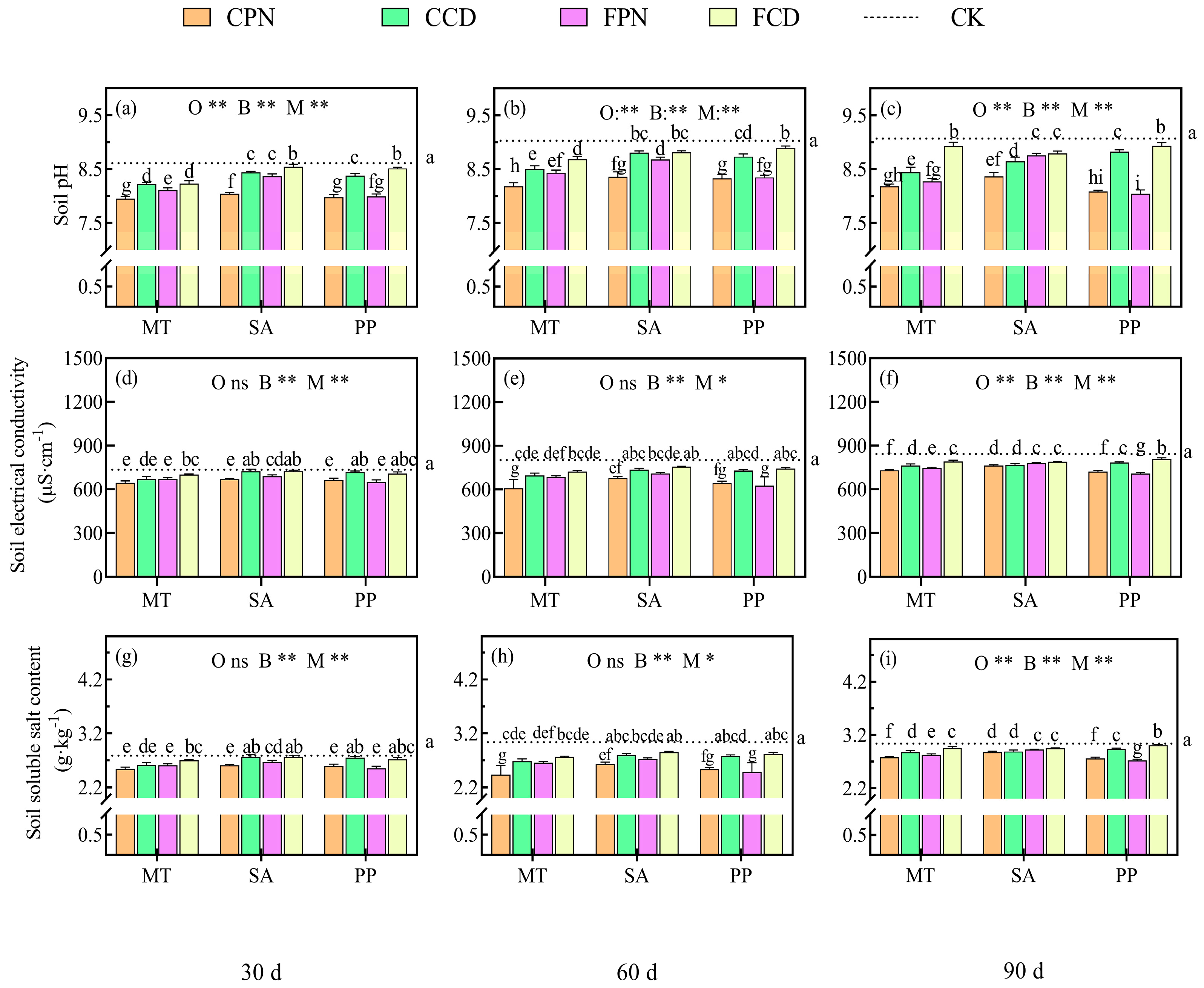
3.5. Impact of Organic Acid Bio-Modifier on Saline and Alkaline Characteristics of Beach Soisl
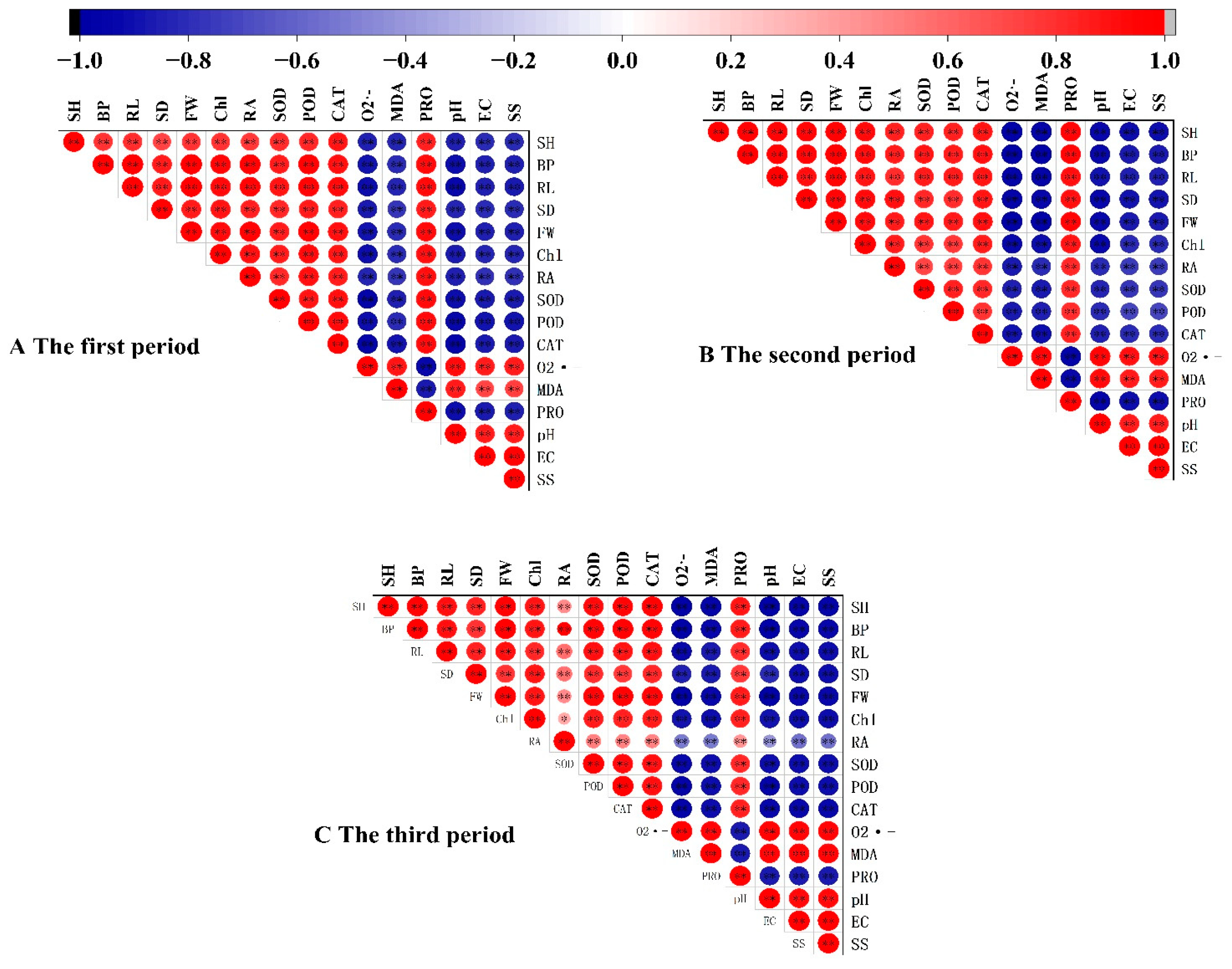
3.6. Correlation Analysis Between Growth Physiology of Sweet Sorghum and Soil Saline–Alkali Indexes in Different Growth Periods
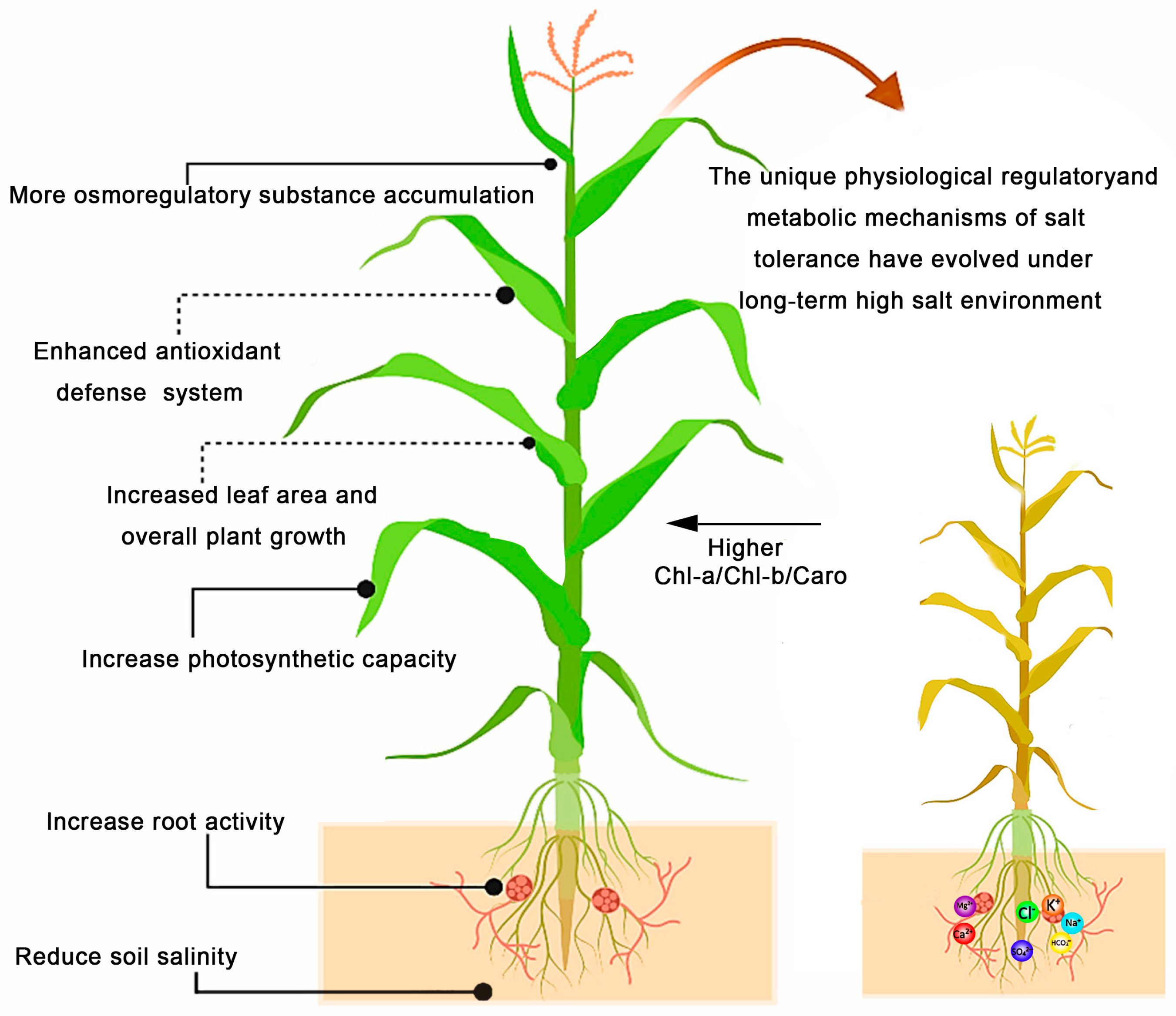
4. Discussion
4.1. Mechanisms of Organic Acid Bio-Modifier Formulated to Regulate the Growth of Sweet Sorghum in Saline Soil
4.2. The Dynamic Change Pattern of Salinity and Alkalinity of Beach Soil by Organic Acid Bio-Amendments
4.3. The Feedback Mechanism of Organic Acid Bio-Amendment Formulation on Plants and Soil Microorganisms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, T.; Gu, X.; Zhu, T.; Pan, X.; Zhu, Y.; Long, X.; Shao, H.; Liu, M.; Rengel, Z. Industrial crop Jerusalem artichoke restored coastal saline soil quality by reducing salt and increasing diversity of bacterial community. Appl. Soil Ecol. 2019, 138, 195–206. [Google Scholar] [CrossRef]
- Yang, H.; Xia, J.; Cui, Q.; Liu, J.; Wei, S.; Feng, L.; Dong, K. Effects of different Tamarix chinensis-grass patterns on the soil quality of coastal saline soil in the Yellow River Delta, China. Sci. Total Environ. 2021, 772, 145501. [Google Scholar] [CrossRef]
- Long, X.; Liu, L.; Shao, T.; Shao, H.; Liu, Z. Developing and sustainably utilize the coastal mudflat areas in China. Sci. Total Environ. 2016, 569–570, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Zhang, M.; Zhu, X.; Wang, M.; Xu, X.; Liu, G. Impact of sea rice planting on enzymatic activity and microbial community of coastal soils: Focus on proteinase. Agronomy 2023, 13, 2089. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Abebe, H.; Tu, Y. Impact of salt and alkali stress on forage biomass yield, nutritive value, and animal growth performance: A comprehensive review. Grasses 2024, 3, 355–368. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Emam, M.M.; Salama, K.H.A.; Morsy, A.A. Sorghum under saline conditions: Responses, tolerance mechanisms, and management strategies. Planta 2021, 254, 24. [Google Scholar] [CrossRef]
- Martin, B.C.; George, S.J.; Price, C.A.; Ryan, M.H.; Tibbett, M. The role of root exuded low molecular weight organic anions in facilitating petroleum hydrocarbon degradation: Current knowledge and future directions. Sci. Total Environ. 2014, 472, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sehrawat, A.; Glick, B.R. The involvement of organic acids in soil fertility, plant health and environment sustainability. Arch. Microbiol. 2022, 204, 720. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, Z.; Liu, X.; Long, X.; Gao, L.; Shen, Y. Biomass composite with exogenous organic acid addition supports the growth of sweet sorghum (Sorghum bicolor ‘Dochna’) by reducing salinity and increasing nutrient levels in coastal saline-alkaline soil. Front. Plant Sci. 2023, 14, 1163195. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, S.; Liu, L.; Liu, J.; Ding, X. Organic fertilization increased soil organic carbon stability and sequestration by improving aggregate stability and iron oxide transformation in saline-alkaline soil. Plant Soil 2022, 474, 233–249. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Zhang, M.; Fei, C.; Ding, X. Phosphorus fractions and adsorption–desorption in aggregates in coastal saline-alkaline paddy soil with organic fertilizer application. J. Soils Sediments 2021, 21, 3084–3097. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Si, B.; Wang, Y.; Chen, X.; Wang, X.; Chen, H.; Wang, H.; Zhang, F.; Bai, Y.; et al. Optimizing biochar application to improve soil physical and hydraulic properties in saline-alkali soils. Sci. Total Environ. 2021, 771, 144802. [Google Scholar] [CrossRef]
- Guan, R.; Li, Y.; Jia, Y.; Jiang, F.; Li, L. Acidified biochar one-off application for saline-alkali soil improvement: A three-year field trial evaluating the persistence of effects. Ind. Crops Prod. 2024, 222, 119972. [Google Scholar] [CrossRef]
- Naveed, M.; Zulekha, R.; Khan, K.S.; Younas, N.; Qadeer, M.F.; Brtnicky, M.; Holatko, J.; Mustafa, A. Unveiling the potential of acidified cow dung in combination with plant growth promoting endophytes on growth, physiology, and yield improvement of maize in salt-affected soil. Arab. J. Geosci. 2023, 16, 551. [Google Scholar] [CrossRef]
- Wang, J.; Riaz, M.; Babar, S.; Xia, H.; Li, Y.; Xia, X.; Wang, X.; Jiang, C. Iron-modified biochar reduces nitrogen loss and improves nitrogen retention in Luvisols by adsorption and microbial regulation. Sci. Total Environ. 2023, 879, 163196. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, J.; Yu, X.; Borjigin, Q.; Qu, J.; Zhang, B.; Zhang, S.; Li, Q.; Guo, J.; Li, D. Evaluation of the microbial community in various saline alkaline-soils driven by soil factors of the Hetao Plain, Inner Mongolia. Sci. Rep. 2024, 14, 28931. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhong, L.; Yang, R.X.; Wang, H.Y.; Liu, X.B.; Xue, W.; Yang, H.; Shen, Y.X.; Li, J.L.; Sun, Z.G. Modifying soil bacterial communities in saline mudflats with organic acids and substrates. Front. Microbiol. 2024, 15, 1392441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Bai, J.; Zhai, Y.; Jia, J.; Zhao, Q.; Wang, W.; Hu, X. Microbial diversity and functions in saline soils: A review from a biogeochemical perspective. J. Adv. Res. 2024, 59, 129–140. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Min, K.; Liu, T.; Li, C.; Xu, J.; Hu, F.; Li, H. The potential role of fertilizer-derived exogenous bacteria on soil bacterial community assemblage and network formation. Chemosphere 2022, 287, 132338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hao, L.; Ji, C.; Zhou, Q.; Song, X.; Liu, Y.; Li, H.; Li, C.; Gao, Q.; Li, J.; et al. The effect of salt-tolerant antagonistic bacteria CZ-6 on the rhizosphere microbial community of winter jujube (Ziziphus jujuba mill. “Dongzao”) in saline-alkali land. BioMed Res. Int. 2021, 2021, 5171086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. The application of Bacillus Megaterium alters soil microbial community composition, bioavailability of soil phosphorus and potassium, and cucumber growth in the plastic shed system of North China. Agric. Ecosyst. Environ. 2021, 307, 107236. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, Z.; Zhou, X. Application of Trichoderma species increases plant salinity resistance: A bibliometric analysis and a meta-analysis. J. Soils Sediments 2023, 23, 2641–2653. [Google Scholar] [CrossRef]
- Li, Y.; Cui, J.; Kang, J.; Zhao, W.; Yang, K.; Fu, J. Trichoderma rhizosphere soil improvement: Regulation of nitrogen fertilizer in saline—Alkali soil in semi—Arid region and its effect on the microbial community structure of maize roots. Agronomy 2024, 14, 2340. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah, K.I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Ni, H.; Wu, Y.; Zong, R.; Ren, S.; Pan, D.; Yu, L.; Li, J.; Qu, Z.; Wang, Q.; Zhao, G.; et al. Combination of Aspergillus niger MJ1 with Pseudomonas stutzeri DSM4166 or mutant Pseudomonas fluorescens CHA0-nif improved crop quality, soil properties, and microbial communities in barrier soil. Front. Microbiol. 2023, 14, 1064358. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Hansen, V.; Bonnichsen, L.; Nunes, I.; Sexlinger, K.; Lopez, S.R.; van der Bom, F.J.T.; Nybroe, O.; Nicolaisen, M.H.; Jensen, L.S. Seed inoculation with Penicillium bilaiae and Bacillus simplex affects the nutrient status of winter wheat. Biol. Fertil. Soils 2020, 56, 97–109. [Google Scholar] [CrossRef]
- Escobar, D.P.; Dos, S.R.; Baron, N.C.; Gil, O.; Rigobelo, E.C. Effect of Aspergillus and Bacillus concentration on cotton growth promotion. Front. Microbiol. 2021, 12, 737385. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, X.; Li, Z.; Wang, X.; Hou, N.; Li, D. Remediation of soda-saline-alkali soil through soil amendments: Microbially mediated carbon and nitrogen cycles and remediation mechanisms. Sci. Total Environ. 2024, 924, 171641. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, M.; Fallah, S.; Kaul, H.; Salehi, A. Effects of cow manure and humic acid on Echinacea purpurea (L.) performance and essential oils accumulation under drought conditions. Ind. Crops Prod. 2024, 222, 119826. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Ekprasert, J.; Cooper, J.; Boonlue, S. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci. Rep. 2021, 11, 6501. [Google Scholar] [CrossRef]
- Zai, X.; Fan, J.; Hao, Z.; Liu, X.; Zhang, W. Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 2021, 11, 5761. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, X.; Song, J.; Li, W.; Wang, H. Application of cotton straw biochar and compound Bacillus biofertilizer decrease the bioavailability of soil cd through impacting soil bacteria. BMC Microbiol. 2022, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Pourakbar, L.; Siavash Moghaddam, S.; Rezaee Danesh, Y.; Popovi’c-Djordjevi’c, J. Effectiveness of fungal bacterial biofertilizers on agrobiochemical attributes of quinoa (Chenopodium quinoa willd.) under salinity stress. Int. J. Environ. Sci. Technol. 2022, 19, 11989–12002. [Google Scholar] [CrossRef]
- Qu, H.; Liu, X.B.; Dong, C.F.; Lu, X.Y.; Shen, Y.X. Field performance and nutritive value of sweet sorghum in eastern China. Field Crops Res. 2014, 157, 84–88. [Google Scholar] [CrossRef]
- Velmurugan, B.; Narra, M.; Rudakiya, D.M.; Madamwar, D. 10—Sweet sorghum: A potential resource for bioenergy production. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 215–242. ISBN 978-0-12-818996-2. [Google Scholar]
- Miller, D. Chapter 10—Plant and field sampling. In Application of Sampling and Detection Methods in Agricultural Plant Biotechnology; Shillito, R., Shan, G., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 177–189. [Google Scholar]
- Lawrence, P.; Roper, W.; Morris, T.; Guillard, K. Guiding soil sampling strategies using classical and spatial statistics: A review. Agron. J. 2020, 112, 493–510. [Google Scholar] [CrossRef]
- Ma, S.; Wang, T.; Ma, S. Effects of drip irrigation on root activity pattern, root-sourced signal characteristics and yield stability of winter wheat. Agric. Water Manag. 2022, 271, 107783. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Determination of Chlorophyll. In Plant-Microbe Interactions: Laboratory Techniques; Senthilkumar, M., Amaresan, N., Sankaranarayanan, A., Eds.; Springer: New York, NY, USA, 2021; pp. 145–146. ISBN 978-1-0716-1080-0. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 317–331. ISBN 978-1-60761-702-0. [Google Scholar]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.; Mohammadi, G.; Jalali-Honarmand, S. Antioxidant Capacity, Photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zou, Z.R.; Liu, Y.; Hu, X.H. Deciphering the protective role of spermidine against saline-alkaline stress at physiological and proteomic levels in tomato. Phytochemistry 2015, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zahir, S.; Zhang, F.; Chen, J.; Zhu, S. Determination of oxidative stress and antioxidant enzyme activity for physiological phenotyping during heavy metal exposure. Methods Mol. Biol. 2021, 2326, 241–249. [Google Scholar] [CrossRef]
- Makkaveev, P.N.; Stunzhas, P.A. Salinity measurements in hyperhaline brines: A case study of the present aral sea. Russ. Acad. Sci. Oceanol. 2017, 57, 892–898. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Sousa, V.F.O.; Santos, A.S.; Sales, W.S.; Silva, A.J.; Gomes, F.A.L.; Dias, T.J.; Goncalves-Neto, A.C.; Faraz, A.; Santos, J.P.O.; Santos, G.L.; et al. Exogenous application of salicylic acid induces salinity tolerance in eggplant seedlings. Braz. J. Biol. 2022, 84, e257739. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.N.; Xu, C.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Ajeethan, N.; Smertenko, A. Response of plant-associated microbiome to plant root colonization by exogenous bacterial endophyte in perennial crops. Front. Microbiol. 2022, 13, 863946. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, Y.; Zhao, Y.; Wang, Y.; Zou, F.; Huang, S.; Yang, X.; Xu, Z.; Hu, H. Physiological and transcriptional evaluation of sweet sorghum seedlings in response to single and combined drought and salinity stress. S. Afr. J. Bot. 2022, 146, 459–471. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; James, R.A.; Weligama, C.; Delhaize, E.; Rattey, A.; Lewis, D.C.; Bovill, W.D.; Mcdonald, G.; Rathjen, T.M.; Wang, E.; et al. Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol. Plant. 2014, 151, 230–242. [Google Scholar] [CrossRef]
- Duputel, M.; Devau, N.; Brossard, M.; Jaillard, B.; Jones, D.L.; Hinsinger, P.; Gérard, F. Citrate adsorption can decrease soluble phosphate concentration in soils: Results of theoretical modeling. Appl. Geochem. 2013, 35, 120–131. [Google Scholar] [CrossRef]
- Ali, N.; Rafiq, R.; Zaib-Un-Nisa; Wijaya, L.; Ahmad, A.; Kaushik, P. Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress. J. King Saud Univ. Sci. 2024, 36, 103012. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Yao, R.; Chen, X. Biochar and fulvic acid to activate soil fertility for achieving agro-ecology benefits in a newly reclaimed coastal wetland of China. Emir. J. Food Agric. 2019, 31, 459–469. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Vrantsi, E.; Lakka, A.; Bozinou, E.; Athanasiadis, V.; Papadaki, E.; Dourtoglou, V.; Lalas, S. Humic and fulvic acids as specific sorbents of herbicides in water. Clean Soil Air Water 2021, 49, 2000467. [Google Scholar] [CrossRef]
- Guan, N.; Li, J.; Shin, H.; Du, G.; Chen, J.; Liu, L. Microbial response to environmental stresses: From fundamental mechanisms to practical applications. Appl. Microbiol. Biotechnol. 2017, 101, 3991–4008. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. Trichoderma harzianum favours the access of arbuscular mycorrhizal fungi to non-host Brassicaceae roots and increases plant productivity. Sci. Rep. 2019, 9, 11650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, X.; Yang, X.; Ran, W.; Shen, Q. Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol. Biochem. 2014, 83, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol. 2016, 18, 2767. [Google Scholar] [CrossRef]
- Kashyap, B.K.; Solanki, M.K.; Pandey, A.K.; Prabha, S.; Kumar, P.; Kumari, B. Bacillus as Plant Growth Promoting Rhizobacteria (PGPR): A Promising Green Agriculture Technology. In Plant Health Under Biotic Stress: Volume 2: Microbial Interactions; Ansari, R.A., Mahmood, I., Eds.; Springer Singapore: Singapore, 2019; pp. 219–236. ISBN 978-981-13-6040-4. [Google Scholar]
- Zhao, T.; Yong, X.; Zhao, Z.; Dolce, V.; Li, Y.; Curcio, R. Research status of Bacillus phytase. 3 Biotech 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chu, Y.; Zhang, W.; Lang, D.; Zhang, X. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch. Environ. Exp. Bot. 2019, 158, 99–106. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Q. Secondary Metabolites of Purpureocillium lilacinum. Molecules 2022, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Jan, F.G.; Hamayun, M.; Jan, G.; Khan, S.A.; Rehman, G.; Ali, S.; Lee, I. An endophytic fungal isolate Paecilomyces lilacinus produces bioactive secondary metabolites and promotes growth of Solanum lycopersicum under heavy metal stress. Agronomy 2023, 13, 883. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, S.; Zhou, S.; Xu, Z.; Liu, X. Phosphate-solubilizing capacity of Paecilomyces lilacinus PSF7 and optimization using response surface methodology. Microorganisms 2023, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Hol, W.; de Boer, W.; Termorshuizen, A.J.; Meyer, K.M.; Schneider, J.; van Dam, N.M.; van Veen, J.A.; van der Putten, W.H. Reduction of rare soil microbes modifies plant-herbivore interactions. Ecol. Lett. 2010, 13, 292–301. [Google Scholar] [CrossRef]
- Li, C.X.; Fu, X.P.; Zhou, X.G.; Liu, S.W.; Xia, Y.; Li, N.H.; Zhang, X.X.; Wu, F.Z. Treatment with wheat root exudates and soil microorganisms from wheat/watermelon companion cropping can induce watermelon disease resistance against Fusarium oxysporum f. sp. niveum. Plant Dis. 2019, 103, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Nakajima, M. Complex plant-soil interactions enhance plant species diversity by delaying community convergence. J. Ecol. 2013, 101, 316–324. [Google Scholar] [CrossRef]
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, J.; He, X.; Lin, Y.; Huang, Q.; Shen, Q. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Blackburn, D.; Bol, R.; Klumpp, E.; Missong, A.; Nischwitz, V.; Haygarth, P.M. Citric acid effect on the abundance, size and composition of water-dispersible soil colloids and its relationship to soil phosphorus desorption: A case study. J. Soil Sci. Plant Nutr. 2021, 21, 2436–2446. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Zhao, Y.; Sun, C.; Hu, D. Mechanisms and regulation of organic acid accumulation in plant vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
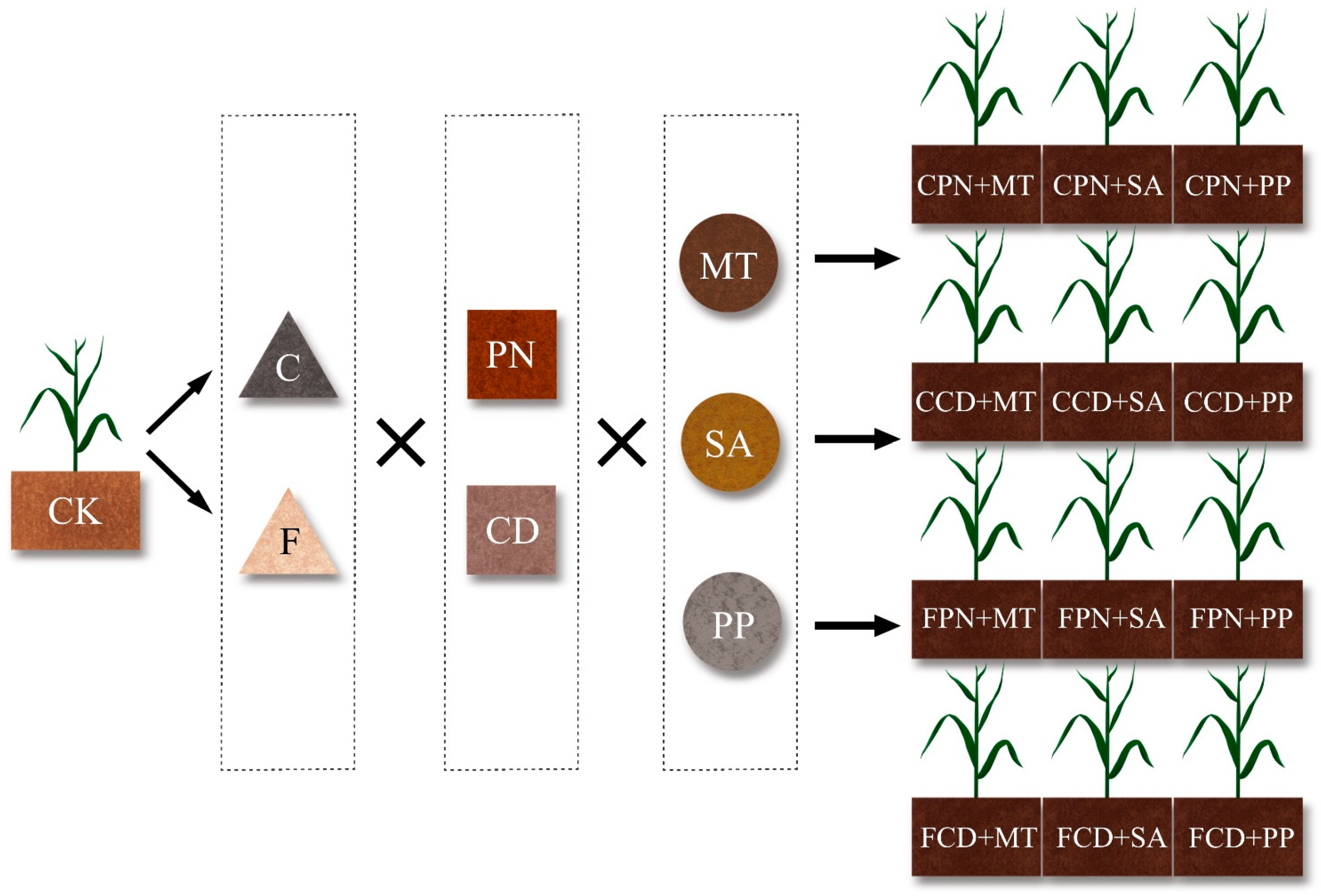


| Treatment | ||
|---|---|---|
| Citric acid (C) | Pine needle (PN) | Pr. megaterium 20065 + T. harzianum 13010 (MT) |
| B. subtilis 10089 + A. niger 2432 (SA) | ||
| B. pumilus 22096 + P. lilacinum 40210 (PP) | ||
| Cow dung (CD) | Pr. megaterium 20065 + T. harzianum 13010 (MT) | |
| B. subtilis 10089 + A. niger 2432 (SA) | ||
| B. pumilus 22096 + Pa. lilacinus 40210 (PP) | ||
| Fulvic acid (F) | Pine needle (PN) | Pr. megaterium 20065 + T. harzianum 13010 (MT) |
| B. subtilis 10089 + A. niger 2432 (SA) | ||
| B. pumilus 22096 + Pa. lilacinus 40210 (PP) | ||
| Cow dung (CD) | Pr. megaterium 20065 + T. harzianum 13010 (MT) | |
| B. subtilis 10089 + A. niger 2432 (SA) | ||
| B. pumilus 22096 + Pa. lilacinus 40210 (PP) | ||
| The Maximum Change Amplitude of Each Index Compared with CK (%) | CPN + MT | FPN + MT |
|---|---|---|
| SH | 213.22% | 48.28% |
| BP | 341.06% | 387.45% |
| RL | 424.18% | 204.35% |
| SD | 145.69% | 177.07% |
| FW | 341.06% | 386.27% |
| Chl | 160.01% | 208.94% |
| RA | 321.62% | 129.41% |
| SOD | 225.13% | 86.53% |
| POD | 110.89% | 116.69% |
| CAT | 90.86% | 115.23% |
| 82.43% | 89.23% | |
| MDA | 52.95% | 54.73% |
| PRO | 238.36% | 37.70% |
| pH | 10.31% | 12.68% |
| EC | 19.08% | 14.06% |
| SS | 19.08% | 14.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.; Yang, S.; Liu, X.; Qian, M.; Wang, H.; Yang, H.; Liu, X.; Shen, Y.; Li, J.; Sun, Z. Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy. Agronomy 2025, 15, 56. https://doi.org/10.3390/agronomy15010056
Xue W, Yang S, Liu X, Qian M, Wang H, Yang H, Liu X, Shen Y, Li J, Sun Z. Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy. Agronomy. 2025; 15(1):56. https://doi.org/10.3390/agronomy15010056
Chicago/Turabian StyleXue, Wei, Shengjie Yang, Xiaoyu Liu, Man Qian, Huiyan Wang, He Yang, Xinbao Liu, Yixin Shen, Jianlong Li, and Zhengguo Sun. 2025. "Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy" Agronomy 15, no. 1: 56. https://doi.org/10.3390/agronomy15010056
APA StyleXue, W., Yang, S., Liu, X., Qian, M., Wang, H., Yang, H., Liu, X., Shen, Y., Li, J., & Sun, Z. (2025). Enhanced Sweet Sorghum Growth and Soil Quality in Coastal Saline–Alkali Soils Through Organic Acid-Containing Bio-Based Materials and Microbial Synergy. Agronomy, 15(1), 56. https://doi.org/10.3390/agronomy15010056








