Abstract
To comprehensively investigate the impacts of increased atmospheric CO2 concentrations on the growth, development and reproduction of the meadow moth Loxostege sticticalis when fed on pea plants (Pisum sativum), in this experiment, we simulated the two CO2 conditions: ambient CO2 (i.e., 400 μL/L designated as aCO2) and elevated CO2 (i.e., 800 μL/L designated as eCO2) by using light-CO2 climate chambers. Subsequently, the changes in several key nutrients and defensive compounds present in pea seedlings were assessed. Moreover, we assessed the growth, development, reproduction and changes in the nutritional components and enzyme activities of L. sticticalis as they fed on pea seedlings grown under aCO2 and eCO2. The results showed that the CO2 level significantly affected the measured indexes of pea seedlings and L. sticticalis. Host pea seedlings grown under eCO2 exhibited significant increases in soluble sugar (SS), soluble protein (SP) and total amino acid (TAA) contents by 42.52%, 77.06% and 62.50%, respectively, relative to those grown under aCO2. In addition, total phenol (TP), ethylene (ET) and jasmonic acid (JA) contents grown under eCO2 increased significantly by 20.60%, 71.72% and 36.22%, respectively, under eCO2 compared to aCO2. Furthermore, the duration of egg, larva and pupa of L. sticticalis was significantly shortened by 16.63%, 10.66% and 10.12%, respectively, while the adult longevity was significantly prolonged by 19.07% after feeding on pea seedlings grown under eCO2 in contrast to aCO2. Furthermore, for L. sticticalis, the content of SS, SP, TAA and free fatty acid was significantly increased, by 60.02%, 77.06%, 91.67% and 27.19%, respectively. Moreover, the enzyme activity of CAT, POD, CarE and GST was also enhanced by 56.70%, 63.89%, 128.08% and 93.45%, respectively, as they fed on pea seedlings grown under eCO2 in contrast to aCO2. The findings of our study revealed that eCO2 not only improved the nutritional quality but also altered the defensive compounds in the seedlings, which in turn affected the growth, development, reproduction and changes in the physiology of L. sticticalis.
1. Introduction
The current climate situation is severe, with climate change characterized by increasing atmospheric carbon dioxide (CO2) concentration that directly affects human living environments and socio-economic development (IPCC, 2023). According to the annual reports from the U.S. National Oceanic and Atmospheric Administration (NOAA), atmospheric CO2 concentration is rising annually, especially at an accelerating rate. As of 2023, the atmospheric carbon dioxide levels have been increasing by over 2 ppm for twelve consecutive years [1]. Results from multiple observation stations indicate that the average atmospheric CO2 concentration in April 2024 reached 426.57 ppm, which is 53% higher than the pre-industrial level (about 278 ppm in 1750), and it could potentially double by the end of the 21st century.
For plants, CO2 serves as a substrate for photosynthesis. The increase in atmospheric CO2 concentration can directly enhance the photosynthetic rate, carbon (C) assimilation rate, carbon-to-nitrogen (N) ratio (i.e., C/N ratio) and crop yield while reducing N-content in plant tissues, which in turn leads to the assimilation and redistribution of C- and N-sources within plant tissues, further influencing the production and allocation of primary and secondary metabolites, and ultimately altering the content and composition of plant nutrients [2,3,4,5]. Qian et al. [6] reported an increase in soluble sugar (SS), soluble protein (SP) and free fatty acid (FFA) in kidney bean (Phaseolus vulgaris) plants grown under elevated CO2 (eCO2) compared to ambient CO2 (aCO2), indicating a shift in the plant’s metabolic condition. Li et al. [7] further found that doubling the CO2 concentration led to a significant increase in SS and polyphenol, along with a significant decrease in FFA and caffeine in tea seedlings, demonstrating the diverse responses of plant metabolites to eCO2. Goufo et al. [8] reported that the rice (Oryza sativa) plants grown under eCO2 conditions exhibited increased contents of non-structural carbohydrates (including total phenols and flavonoids) throughout the growth period as compared to the rice plants grown under aCO2 conditions. The increased photosynthetic rate due to eCO2 can allocate excess C sources in plants and facilitate the synthesis of structural compounds (e.g., pectin and cellulose) and C-based secondary metabolites (e.g., phenols and terpenes), thus affecting plant metabolism [9]. Robinson et al. [10] found that eCO2 enhanced the content of total phenols, tannins and flavonoids in plant tissues by 19%, 22% and 27%, respectively, while reducing the content of terpenes by 13%, indicating significant implications for the plant defense mechanisms. Regarding the plant defense mechanisms, the induced defense is mainly regulated by three signaling pathways, i.e., jasmonic acid (JA), salicylic acid (SA) and ethylene (ET). These pathways are involved in regulating diverse defensive compounds. Total phenols encompass phenolic compounds that contribute to antioxidant properties and defensive functions [11]. Additionally, saponins categorized as secondary metabolites can act as a defensive shield against pests and pathogens [12]. Ethylene plays an instrumental role in implicating both defense mechanisms and developmental processes. Salicylic acid is crucial for plant defense against necrotrophic pathogens, while jasmonic acid is critical for plant defense against insect herbivores [13]. Elevated CO2 can affect these three types of plant hormone-dependent induced defense signaling pathways [14]. Sun et al. [15] found that eCO2 upregulated SA defense genes and downregulated JA and ET defense genes in Arabidopsis (Arabidopsis thaliana), showing a modulation of the plant’s defense response. Such alterations in plant chemical composition can have cascading effects on herbivorous insects, thereby influencing their growth, development, reproduction and feeding and oviposition behaviors [16,17].
Numerous studies have vividly illustrated that eCO2 adversely influenced the growth, development and reproduction of chewing insects, such as Lepidopterans. Xie et al. [18] found that eCO2 significantly delayed the growth and development of the Asian corn borer (Ostrinia furnacalis), accompanied by a reduction in body weight and an increase in mortality, suggesting a negative impact on its fitness. Li et al. [19] found that eCO2 reduced the body weight of the 5th instar larvae of rice leafroller (Cnaphalocrocis medinalis) by 16.1%, shortened the pupal duration by 28.7% and reduced the pupal weight by 9.9%, which further emphasized the developmental disruptions caused by the increased CO2 concentration. Zhang et al. [20] noted a prolonged development duration and increase in body weight of the Tobacco cutworm (Spodoptera litura) under eCO2, highlighting the complex and species-specific responses to eCO2. Satishchandra et al. [21] found that eCO2 delayed the larval development of American tomato moth (Tuta absoluta). The research also highlighted a significant increase in the larval mortality rate as well as fecundity of the American tomato moth, suggesting a significant shift in the pest’s life history strategy and population regulatory mechanisms under eCO2. These collective findings from previous research provide a comprehensive framework that justifies and shapes our current investigation into the interactions between plants and insects under the influence of eCO2.
The meadow moth (Loxostege sticticalis), a species belonging to the family Crambidae of the order Lepidoptera, is a major global agricultural pest, mainly distributed in the broad region between 36 °N and 55 °N [22]. In China, it primarily causes severe damage in the North, Northeast and Northwest regions, collectively referred to as the “Three North” regions of China. The polyphagous larvae of L. sticticalis consume a wide variety of host plants, specifically 259 species belonging to 48 distinct families [23]. In the latest edition of the “List of the First-Class Crop Pests” released by the State Ministry of Agriculture and Rural Affairs of China in 2023, L. sticticalis is ranked as the 3rd most significant pest. Currently, L. sticticalis has entered into a 4th outbreak cycle in China, which poses a severe threat to food security [24]. The majority of the studies emphasize the influence of environmental factors such as temperature [25] and humidity [22] and their impacts on the growth, development and reproduction of L. sticticalis. Despite the importance of pea plants as a vegetable crop, there are limited studies investigating the alterations in nutrient contents and defensive compounds in these plants when grown in eCO2 conditions. Additionally, the impacts of these changes in host pea plants on the growth, development and reproduction of L. sticticalis also remain scarcely explored.
In order to clarify the impacts of eCO2 on the growth, development and reproduction of L. sticticalis fed on pea plants under global climate change, the ambient and elevated CO2 were simulated by using light-CO2 culture chambers. The changes in nutrients and defensive compounds in pea seedlings, as well as the growth, development, reproduction and changes in the nutritional components and enzyme activities of L. sticticalis fed on pea seedlings grown under aCO2 and eCO2, were also measured. This study aims to clarify the outbreak patterns and population dynamics of L. sticticalis under future high CO2 climate conditions, providing scientific support and a practical basis for the long-term prediction and integrated management of L. sticticalis.
2. Materials and Methods
2.1. Pea Plants
The broad-leaf green pea (Pisum sativum) was used, and the pea plants were grown in double-layer hydroponic seedling trays (length:width:height = 32 cm:24 cm:3.5 cm) with nutrients solutions recommended for the hydroponic cultivation (components of hydroponic nutrient solution: 0.7 g/L of potassium nitrate, 0.0006 g/L of boric acid, 0.7 g/L of calcium nitrate, 0.0006 g/L of manganese sulfate, 0.8 g/L of superphosphate, 0.0006 g/L of zinc sulfate, 0.28 g/L of magnesium sulfate, 0.0006 g/L of copper sulfate, 0.12 g/L of iron sulfate and 0.0006 g/L of ammonium molybdate). Before germination, the pea seeds were covered with seed germination paper and sprayed with water daily to promote sprouting. After germination, the seed germination paper was removed, and the nutrient solution was replaced every 5 days. The pea plants were grown in light-CO2 climate chambers (GDN-400D-4-CO2, Ningbo Southeast Instrument, Ningbo, China) under controlled CO2 concentrations of 400 μL/L (i.e., the current atmospheric CO2 concentration as control of ambient CO2 (aCO2)) and 800 μL/L (i.e., the projected CO2 concentration by the end of the 21st century as elevated CO2 (eCO2)) [26], respectively. The required CO2 concentration in the light-CO2 climate chambers was controlled with CO2 gas supplied from a CO2 cylinder. The growth conditions in the climate chambers were set to a temperature of (22 ± 1) °C, relative humidity of (75 ± 5)% and photoperiod of 16 L:8 D, with a light intensity of 20,000 lx. After 20 days of seedling growth, the leaves and stems of pea plants were collected and stored in liquid nitrogen for the following testing.
2.2. Meadow Moths
The larvae of tested meadow moth L. sticticalis were collected from Kangbao County, Hebei Province of China (41.85° N, 114.26° E), in Sept 2023. A total of 100 larvae were initially used to initiate the lab colony development. The collected larvae were reared and bred in the laboratory, using broad-leaf green pea (P. sativum) as food, for three generations to ensure uniformity and eliminate the influence of genetic and environmental variability from the wild population. The test colony was maintained in light-CO2 climate chambers (GDN-400D-4-CO2, Ningbo Southeast Instrument, Ningbo, China) under the same conditions for the used pea plants, with a temperature of (22 ± 1) °C, relative humidity of (75% ± 5) % and a photoperiod of 16 L:8 D, with a light intensity of 20,000 lx.
2.3. Determination of Nutrients and Defensive Substances in Pea Seedlings Grown Under aCO2 and eCO2
To clarify the changes in the contents of nutritional components and defensive substances in pea seedlings grown under different CO2 concentrations, this experiment was set up with two CO2 concentrations, i.e., aCO2 and eCO2. The pea seedlings grown under aCO2 and eCO2 for 20 days were randomly collected and ground into powder, and 0.1 g plant tissues were weighed and ground into a 10% homogenate with ultrapure water. The nutritional components in pea seedlings were measured according to the instructions of the assay kits for soluble sugar (A145), soluble protein (A045-2), free fatty acid (A042-2-1) and total amino acid (A026-1-1). The defensive compounds present in pea seedlings were also measured according to the instructions of the assay kits for total phenol (A143-1-1) and saponin (BR500741). All the used assay kits except that for saponin were from the Nanjing Jiancheng Bioengineering Institute in the Jiangsu Province of China, and the assay kit for saponin was supplied from Shanghai Bioleaper Bio-Technology Co., Ltd., Shanghai, China.
The hormone levels in pea seedlings were determined using enzyme-linked immunosorbent assay (ELISA) according to the instructions of the assay kits for ethylene (M133388), salicylic acid (M133352) and jasmonic acid (M133322); all these ELISA kits were purchased from Beijing MREDA Technology Co., Ltd., of Beijing, China. The absorbance (optical density, OD) of the reaction products was, respectively, measured at wavelengths of 620 (SS), 595 (SP), 546 (FFA), 650 (AA), 760 (TP), 589 (SP) and 450 (ELISA for ET, SA and JA) nm using a microplate reader, and the corresponding concentration of these compounds was calculated. Each experimental treatment was repeated three times with each replicate using a distinct 0.1 g sample of pea seedlings.
2.4. Determination of Growth, Development and Reproduction of L. sticticalis Fed on Pea Seedlings Grown Under aCO2 and eCO2
To clarify the effects of eCO2 on the growth, development and reproduction of L. sticticalis, 20 female and male pairs of newly eclosed 1-day-old adults were randomly selected for each treatment of aCO2 and eCO2. The female and male pairs were, respectively, placed in plastic cups for mating, with cotton balls and oviposition egg strips placed in each cup. A solution with 10% glucose was added to the cotton balls at 18:00 daily to feed the moths. After egg-laying, the oviposition egg strips were removed and placed individually into glass jars (12.6 cm high, 8.3 cm in diameter), each containing 30 eggs. The jar was covered with gauze, and a moist towel was placed over it to maintain humidity. The towel was moistened daily, and the hatching status of the eggs was observed and recorded daily.
The egg duration was calculated based on the egg-laying date and the hatching date. Each glass jar was treated as a single replicate, with three replicates per treatment (N = 30). After hatching, fresh pea seedlings were provided daily at 18:00, and the developmental stages and other related characteristics of the larvae were observed and recorded daily. When the larvae stopped feeding and entered the pupal stage, they were removed from the glass jars and individually reared in test tubes. The dates of pupation and adult emergence were respectively recorded, and the developmental duration of larvae and pupae was also respectively calculated. Each test tube was considered as one single replicate, with 50 replicates per treatment. The pupae were weighed on the 3rd day after pupation using a Huachi electronic balance with a maximum capacity of 200 g, an accuracy of 0.001 g and a readability of 0.0001 g. A total of 50 pupae were weighed per treatment. After emergence, 20 pairs of newly emerged male and female moths were paired and placed into plastic cups for mating and egg-laying, and the dates of emergence and death of the moths were recorded to calculate the adult longevity. The status of female moths was observed daily, and the number of eggs laid per day was recorded until the female moths died, and the total egg count per female moth was calculated. The eggs laid on the same day were placed in glass jars to observe hatching. If no larvae were hatched for three consecutive days, it was assumed that all viable eggs had been hatched, and the number of unhatched eggs was also counted. The fecundity (number of eggs per female) and egg hatchability were calculated based on the number of larvae hatched and the number of unhatched eggs. The formulae used were as follows: number of eggs per female = number of hatched larvae + number of unhatched eggs; egg hatchability = (number of hatched larvae/number of eggs per female) × 100%.
2.5. Bioassay for Nutritional Components and Enzyme Activity in L. sticticalis Fed on Pea Seedlings Grown Under aCO2 and eCO2
To clarify the changes in the contents of nutritional components and enzyme activity in L. sticticalis larvae fed on pea seedlings grown under different CO2 concentrations, five 2nd-day 5th instar larvae were randomly selected for each treatment of aCO2 and eCO2. After freezing the larvae with liquid nitrogen, their weights were recorded. Then, ultrapure water was added, and the larvae were ground into a 10% homogenate. The content of soluble sugar (A145), soluble protein (A045-2), free fatty acid (A042-2-1) and total amino acid (A026-1-1) in L. sticticalis larvae was measured according to the insect test kit instructions. The enzyme activity of carboxylesterase (A133-1-1), glutathione S-transferase (BC0350), acetylcholinesterase (A024-1-1), superoxide dismutase (A001-3-1), catalase (A007-1-1) and peroxidase (A084-3-1) in L. sticticalis larvae was also measured, following the respective test kit instructions. The optical density (OD) value of the reactants was measured using a microplate reader at 620 (SS), 595 (SP), 546 (FFA), 650 (AA), 450 (CarE), 340 (GST), 412 (AChE), 450 (SOD), 405 (CAT) and 420 (POD) nm wavelengths, respectively, and the corresponding concentrations of various compounds were also calculated respectively. Each experimental treatment was repeated three times, with each replicate using a distinct 0.1 g sample of larvae. All of the assay kits employed in the study, except the glutathione S-transferase assay kit, were procured from the Nanjing Jiancheng Bioengineering Institute in the Jiangsu Province of China, and the glutathione S-transferase assay kit was procured from Beijing Solarbio Bio-Technology Co., Ltd., Shanghai, China.
2.6. Data Analysis
The experimental data were summarized and analyzed using Excel 2019 software. One-way analysis of variance (ANOVA) was performed on the experimental data to identify significant differences among treatments by using SPSS 23.0 (SPSS Inc., Chicago, IL, USA), and the independent samples t-test was used for determining the significance levels between treatments at a probability level of 0.05 (i.e., p < 0.05).
3. Results
3.1. Impact of eCO2 on the Nutritional Components and Defensive Substances of Pea Seedlings
One-way ANOVA showed that CO2 level significantly affected the contents of nutritional components in pea seedlings, including soluble sugar (F(1,8) = 3.958, p = 0.017), soluble protein (F(1,8) = 2.971, p = 0.041) and total amino acid (F(1,8) = 3.064, p = 0.038) in pea seedlings (Table 1), but did not significantly impact the free fatty acid content (F(1,8) = 0.541, p = 0.642; Table 1). Compared to aCO2, eCO2 significantly increased the contents of soluble sugar by 42.52%, soluble protein by 77.06% and total amino acid by 62.50% (p < 0.05), except that there was a slight decrease in free fatty acid content in pea seedlings grown under eCO2, in contrast to aCO2 (p > 0.05; Table 1). It also indicated that CO2 levels significantly affected the contents of total phenol (F(1,8) = 2.920, p = 0.027), ethylene content (F(1,8) = 2.688, p = 0.036) and jasmonic acid (F(1,8) = 2.551, p = 0.043) but had no significant impact on the contents of saponin (F(1,8) = 1.984, p = 0.095) and salicylic acid (F(1,8) = 0.632, p = 0.547) in pea seedlings (Table 1). Compared to aCO2, eCO2 significantly increased the contents of total phenol by 20.60%, ethylene by 71.72% and jasmonic acid by 36.22% (p < 0.05; Table 1). The contents of saponin and salicylic acid were also increased as they grew under eCO2, in contrast to aCO2, but the changes were not significant (p > 0.05; Table 1).

Table 1.
The contents of nutritional components and defensive substances in pea seedlings grown under ambient CO2 (aCO2) and elevated CO2 (eCO2).
3.2. Impact of eCO2 on the Growth, Development and Reproduction of L. Sticticalis
One-way ANOVA revealed that CO2 levels significantly affected the duration of egg (F(1,178) = 2.420, p = 0.020), larvae (F(1,98) = 6.782, p < 0.001), pupae (F(1,98) = 4.707, p < 0.001) and adult longevity (F(1,78) = 2.723, p = 0.008), but did not significantly affect the number of eggs laid per female (F(1,38) = 0.671, p = 0.512) or pupal weight (F(1,98) = 0.287, p = 0.755) of L. sticticalis fed on pea seedlings grown under aCO2 and eCO2 (Table 2). The duration of egg (aCO2: 4.63 d; eCO2: 3.86 d), larva (aCO2: 25.70 d; eCO2: 22.96 d) and pupae (aCO2: 17.68 d; eCO2: 15.89 d) were significantly shortened by 16.63% (p < 0.05), 10.66% (p < 0.001) and 10.12% (p < 0.001), respectively, as they fed on pea seedlings grown under eCO2 in contrast to aCO2 (Figure 1a–c). Moreover, the adult longevity under eCO2 (22.42 d) was significantly prolonged by 19.07% than under aCO2 (18.83 d; p < 0.01, Figure 1d). The number of eggs laid per female under eCO2 slightly increased than under aCO2, while the pupal weight slightly decreased under eCO2 than under aCO2 (p > 0.05; Figure 1e,f).

Table 2.
One-way ANOVAs for the effects of CO2 level (aCO2 vs. eCO2) on the growth, development and reproduction of L. sticticalis, and the contents of nutritional components and enzyme activity in L. sticticalis larvae fed on pea seedlings grown under aCO2 and eCO2.
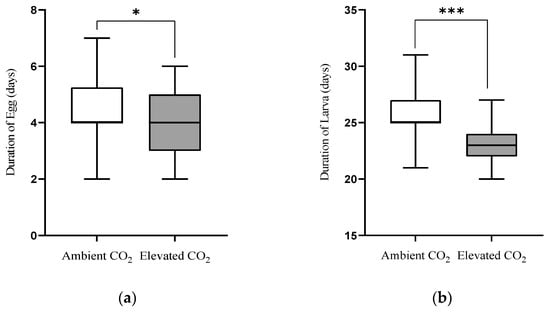
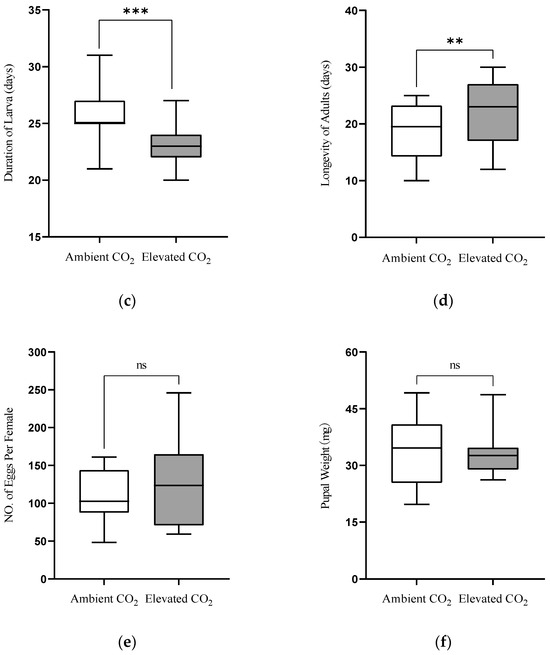
Figure 1.
Egg (a), larvae (b), pupae (c), adult longevity (d), number of eggs laid per female (e) and pupal weight (f) of Loxostege sticticalis fed on pea seedlings grown under ambient CO2 (aCO2) and elevated CO2 (eCO2) (Note: *, ** and *** indicate being significantly different between aCO2 and eCO2 by the independent samples t-test at p < 0.05, 0.01 and 0.001, respectively. While ns indicates being no significant difference.
3.3. Impact of eCO2 on the Nutritional Composition and Enzyme Activity in L. sticticalis Larvae
One-way ANOVA showed that the CO2 level significantly affected the contents of soluble sugar (F(1,8) = 7.441, p = 0.002), soluble protein (F(1,8) = 5.036, p = 0.007), total amino acid (F(1,8) = 11.007, p < 0.001) and free fatty acid (F(1,8) = 2.894, p = 0.046) in L. sticticalis larvae fed on pea seedlings grown under aCO2 and eCO2 (Table 3). Compared to aCO2, eCO2 significantly increased the contents of soluble sugar by 60.02%, soluble protein by 77.06% and total amino acid by 91.67%, and significantly decreased the free fatty acid content by 27.19% as L. sticticalis larvae fed on pea seedlings (p < 0.05; Table 3). It also indicated that the CO2 level significantly affected the activity of catalase (CAT) (F(1,8) = 3.461, p = 0.026), peroxidase (POD) (F(1,8) = 4.573, p = 0.010), carboxylesterase (CarE) (F(1,8) = 2.926, p = 0.043) and glutathione S-transferase (GST)(F(1,8) = 4.581, p = 0.037), but did not significantly influence the activity of superoxide dismutase (SOD) (F(1,8) = 1.657, p = 0.238) and acetylcholinesterase (AChE) (F(1,8) = 0.218, p = 0.838) in L. sticticalis larvae fed on pea seedlings grown under aCO2 and eCO2, respectively (Table 3). Compared to aCO2, eCO2 significantly increased the activity of CAT by 56.70%, POD by 63.89%, CarE by 128.08% and GST by 93.45%, respectively (p < 0.05; Table 3). Moreover, the activity of SOD and AChE also slightly increased as L. sticticalis larvae fed on pea seedlings grown under eCO2 in contrast to aCO2, respectively, but the changes were not significant (p > 0.05; Table 3).

Table 3.
The contents of nutritional components and enzyme activity in L. sticticalis fed on pea seedlings grown under aCO2 and eCO2.
4. Discussion and Conclusions
The impact of increased atmospheric CO2 concentration in the environment on plants primarily manifests as changes in their chemical composition [27]. Previous studies have consistently demonstrated that eCO2 leads to a higher C:N ratio in plants, which subsequently alters the nutrient and chemical composition within plant tissues [28,29]. In this study, eCO2 significantly augmented the contents of soluble sugar, soluble protein and total amino acid, while simultaneously leading to a slight reduction in the free fatty acid content in pea seedlings compared with aCO2. This can be attributed to the enhanced photosynthetic and nitrogen metabolism under eCO2. The increased carbon dioxide availability stimulates photosynthesis for greater carbohydrate accumulation and enables more efficient utilization and storage of nitrogenous compounds due to altered nitrogen metabolism. The slight decrease in free fatty acid content might be a consequence of the redirection of carbon skeletons towards the synthesis of sugars and proteins, which are energetically and functionally more crucial under the new metabolic regime. These findings are consistent with previous research findings, which demonstrated that eCO2 markedly increased the content of soluble sugars, soluble proteins and total amino acids in kidney bean (P. vulgaris) [6] while simultaneously decreasing the free fatty acid content in tea seedlings [7].
Secondary metabolites, such as phenols and saponins, form a complex defense system in plants to resist harmful organisms (e.g., insect pests) during evolution. The strong antioxidant capacity of total phenols can reduce oxidative stress induced by the damage of insect pests, thereby enhancing the pest resistance of plants [30]. In our study, eCO2 significantly elevated the total phenol content in pea seedlings, corroborating the conclusions of Ryan et al. [31]. In their comprehensive review of 343 studies conducted in 2010, it was indicated that over half of the studies reported an increase in total phenol content in response to eCO2. Saponins, a class of secondary metabolites widely found in legumes, were also found to increase in broad bean leaves under eCO2 in contrast to aCO2, as observed by Li [32]. Our study also found that eCO2 significantly increased the saponin content in pea seedlings compared with aCO2. Ethylene, salicylic acid (SA) and jasmonic acid (JA) are three key plant hormones involved in regulating plant defense mechanisms. Many documents have shown that eCO2 can influence the growth and development of herbivorous insects by altering the plant hormone-mediated induced pest-resistance response mechanisms [33]. For instance, Sun et al. [15] found that eCO2 enhanced the salicylic acid-induced defense pathway in Arabidopsis (A. thaliana) while reducing the JA-induced defense pathway, thereby affecting the expression of downstream resistance genes and promoting aphid population outbreaks. In this study, eCO2 significantly increased the contents of ethylene and JA in pea seedlings, with SA content also slightly increased compared with aCO2. The divergence from Sun et al. [15] might result from the metabolic pathway differences between legumes and cruciferous plants under eCO2. Guo et al. [34] found that in Medicago truncatula, the jasmonate pathway is involved in promoting nodule symbiosis while suppressing host plant defense, which highlights the specialized and unique nature of hormone regulation in legumes. Considering these differences in hormonal mechanisms compared to other plant families like cruciferous plants, it is likely that legumes have their own characteristic hormone synthesis and signaling regulatory mechanisms, which result in their disparate responses when exposed to elevated CO2 levels.
Regarding the role of these compounds, the increased soluble sugars, proteins and amino acids in pea seedlings grown under eCO2 offer more resources for L. sticticalis larvae. They fuel the energy-consuming processes of growth and metamorphosis, but a rushed development might cause some larvae to miss out on full utilization. For the defensive compounds, higher total phenols and saponins in pea seedlings grown under eCO2 force L. sticticalis larvae to allocate resources to counteract the stress and maintain gut integrity, which can disrupt normal development rhythms.
Changes in biochemical substances in plant tissues can affect the nutritional quality of food for herbivorous insects, which in turn influences the growth, development, reproduction, nutrition content and enzyme activity in the bodies of these insect pests [18,21,33,35,36]. Pang [37] found that prolonged exposure to high CO2 concentrations accelerated the population growth of cotton bollworms and significantly shortened the mean generation time. In this study, eCO2 significantly shortened the duration of eggs, larvae and pupae of L. sticticalis while slightly reducing the pupal weight. The reduction in pupal weight, consistent with Li et al. [19], though not statistically significant, may result from a trade-off between rapid development and resource allocation. Under eCO2, the larvae might prioritize energy expenditure toward accelerated growth and metamorphosis rather than towards building substantial pupal mass. This could be due to the fact that the enhanced nutrient availability in pea seedlings grown under eCO2 triggers a physiological response in L. sticticalis larvae to hasten their development, with the consequence that the resources allocated to pupal formation are relatively limited. The contents of soluble sugars, soluble proteins and total amino acids in L. sticticalis larvae were significantly increased as they fed on pea seedlings grown under eCO2 in contrast to aCO2. This indicates that eCO2 not only increased the nutrient content in pea seedlings but also enhanced the uptake of these essential nutrients by L. sticticalis larvae, thereby accelerating its generational turnover. However, it is speculated that the shortened developmental period may lead to reduced growth resources for some individuals during the larval stage, potentially affecting their development quality. In this study, eCO2 significantly prolonged adult longevity. This could be mainly due to the enhanced nutritional quality of pea seedlings grown under eCO2. The increased levels of soluble sugars, proteins and amino acids in the seedlings provided adults with a more stable and sufficient energy and nutrient supply, better maintaining their physiological functions and potentially enhancing stress resistance, thus contributing to the extended lifespan. While the observed trends in adult longevity might have certain implications, it is premature to conclude that eCO2 may positively influence the dispersal and reproduction of L. sticticalis and potentially exacerbate their threat to agricultural ecosystems, as other factors in the field environment, such as natural predators, parasites and abiotic stresses, which were not considered in this study, could have a significant impact on the population dynamics of L. sticticalis and might mitigate any potential increase in their threat. Although the increase in the number of eggs laid per female under eCO2 was not statistically significant, it could potentially be related to the improved nutritional quality of pea seedlings. The elevated levels of soluble sugars, proteins and amino acids in the seedlings might provide a more favorable nutritional environment for the female insects. This could enhance their physiological condition and reproductive capacity to some extent, leading to a marginal increase in egg production. However, the complex interplay of various factors in the ecosystem, such as the potential changes in plant defense mechanisms and the insects’ own physiological regulatory systems, may limit the magnitude of this effect and prevent it from reaching statistical significance.
Enzymes, such as CarE, GST and AchE, are important detoxification enzymes in insects, playing a crucial role in breaking down exogenous toxins and maintaining normal physiological metabolism [38]. Studies have demonstrated that CarE plays a critical role in the metabolism of plant secondary metabolites in various insects [39]. In this particular study, it could be pivotal in handling the altered chemical composition of pea seedlings under eCO2. For GST, its activity is often associated with the detoxification of xenobiotics and maintaining redox homeostasis [40]. The increased production of reactive oxygen species (ROS) in L. sticticalis might lead to a compensatory increase in GST activity to prevent oxidative damage under eCO2. SOD, CAT and POD are vital protective enzymes in insects. SOD promotes the generation of O2, removes excess superoxide anion radicals in the body and ultimately produces H2O2, which CAT and POD can decompose into H2O [41]. Therefore, detoxification and protective enzymes are of great significance to the growth and development of herbivorous insects. Particularly, SOD, as the foremost defense against oxidative stress, plays a crucial role in scavenging superoxide anions under eCO2. [42]. The upregulation of SOD activity under eCO2 could be a response to the perturbed intracellular environment. Subsequently, CAT and POD will work in coordination to further break down the H2O2 produced by SOD [43]. The coordinated action of these enzymes is essential for the proper development and survival of L. sticticalis larvae. Research shows that under high CO2 conditions, the activities of detoxification enzymes (GST, CarE) and protective enzymes (SOD, CAT, POD) in Frankliniella occidentalis and F. intonsa generally trend upwards [44]. In this study, the activities of protective enzymes (CAT, POD) and detoxification enzymes (CarE, GST) in L. sticticalis larvae significantly increased as they fed on pea seedlings grown under eCO2 in contrast to aCO2, and the activities of SOD and AchE in L. sticticalis larvae also showed a slight rise as they fed on pea seedlings grown under eCO2 in contrast to aCO2, which is consistent with previous research findings. Biologically, the enhanced enzyme activities in L. sticticalis larvae might improve their survival and growth by facilitating the synthesis of metabolic compounds in pea seedlings grown under eCO2. At the organism level, it could affect the larvae’s physiological state, development and potential reproductive capacity. In terms of population, the increased enzyme activities may reduce the effectiveness of traditional pest control and require new strategies to manage L. sticticalis, as its population growth might be enhanced under eCO2, posing a greater threat to agricultural ecosystems.
The results of this study indicate that eCO2 can improve the nutritional quality of pea seedlings, promoting the growth and development of L. sticticalis. However, in terms of reproductive performance, although there were some changes in the number of eggs laid per female and pupal weight under eCO2 compared to aCO2, these differences were not statistically significant, suggesting that the impact of eCO2 on the reproduction of L. sticticalis may be limited or requires further investigation. Moreover, the changes in the contents of defensive substances in pea seedlings further stimulated the changes in enzyme activity in L. sticticalis larvae, altering its environmental adaptability. Our study provides a comprehensive understanding of the impact of eCO2 on the interaction between pea plants and L. sticticalis, which adds to the growing body of research on the ecological consequences of climate change. Also, we combined the changes in the contents of nutrient components and defensive substances in pea seedlings with the changes in growth, development, and reproduction of L. sticticalis, as well as changes in the nutritional substances and enzyme activities in its body (seen in Figure 2), to predict that the changes in plant nutrients and defensive compounds will shorten the developmental period of L. sticticalis, increase its generational output and improve its adaptability to environmental changes, thereby increasing the risk of L. sticticalis outbreaks and their migration and dispersal under the continuous rise in atmospheric CO2 concentrations in the future. Nevertheless, it is essential to acknowledge the limitations of our current study. We have only considered the singular impact of eCO2 on pea seedlings and meadow moths, without accounting for the interactions with other environmental factors (e.g., temperature, humidity, light, etc.). These interactions could potentially modify or even reverse the observed effects, thereby restricting the generalizability of our findings. Future research should strive to incorporate a more comprehensive environmental context to better understand the complex ecological dynamics. Additionally, our study has not delved into the intricate molecular mechanisms underlying the changes in defensive substances in pea seedlings. Future investigations could leverage molecular biology techniques to dissect the effects of eCO2 on plant-defensive signaling pathways. This would not only deepen our understanding of the fundamental processes but also provide valuable insights for the development of more effective pest management strategies in the context of climate change. Future research should incorporate molecular biology approaches to explore the effects of eCO2 on various plant-defensive signaling pathways.
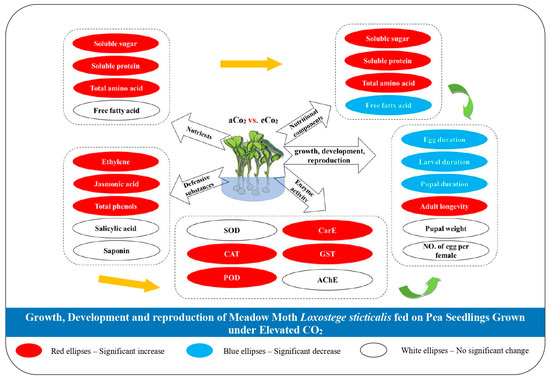
Figure 2.
Elevated CO2 alters the interaction between pea plants and L. sticticalis by impacting plant nutritional quality and promoting the growth, development and reproduction, thereby facilitating the population occurrence of L. sticticalis, simultaneously influencing plant defensive substances and further stimulating the changes in enzyme activity in larvae and altering the environmental adaptability of L. sticticalis.
Author Contributions
J.Z.: Writing—Original draft, Investigation and Formal analysis; Z.X., Z.Y., H.M. and J.H.: Investigation and Formal analysis; F.C. and X.C.: Project administration, Funding acquisition and Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFD1400600).
Data Availability Statement
The data obtained in this study are presented “as is” in at least one of the figures or tables embedded in the manuscript.
Acknowledgments
We would like to thank Sabin Saurav Pokharel (pokharelsabin93@gmail.com) in our group of the Insect-Information Ecology Lab, Department of Entomology, College of Plant Protection, Nanjing Agricultural University, for his help revising the revision of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Montzka, S. The NOAA Annual Greenhouse Gas Index (AGGI). NOAA Global Monitoring Laboratory Website. 2022. Available online: https://gml.noaa.gov/aggi/aggi.html (accessed on 30 April 2023).
- Dafellowese, S. Influence of elevated CO2 on interspecific interactions at higher trophic levels. Glob. Chang. Biol. 2002, 8, 668–678. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Niziolek, O.K.; Berenbaum, M.R.; Delucia, E.H. Impact of elevated CO2 and increased temperature on Japanese beetle herbivory. Insect Sci. 2013, 20, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; He, S.Q.; Liu, Y.J.; Ke, R.; Hao, R.Q.; Gui, F.R. Comparative development and reproduction of Frankliniella occidentalis and F. intonsa (Thysanoptera: Thripidae) under elevated CO2 concentration. J. Environ. Entomol. 2015, 37, 701–709. [Google Scholar]
- Qian, L.; Chen, F.J.; Liu, J.N.; He, S.Q.; Liu, J.Y.; Li, Z.Y.; Gui, F.R. Effects of elevated CO2 on life-history traits of three successive generations of Frankliniella occidentalis and F. intonsa on kidney bean, Phaseolus vulgaris. Entomol. Exp. Et Appl. 2017, 165, 50–61. [Google Scholar] [CrossRef]
- Li, L.K.; Wang, M.F.; Pokharel, S.S.; Li, C.X.; Parajulee, M.N.; Chen, F.J.; Fang, W.P. Effects of elevated CO2 on foliar soluble nutrients and functional componenfigts of tea, and population dynamics of tea aphid, Toxoptera aurantii. Plant Physiol. Biochem. 2019, 145, 84–94. [Google Scholar] [CrossRef]
- Goufo, P.; Pereira, J.; Moutinho-Pereira, J.; Correia, C.M.; Figueiredo, N.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ. Exp. Bot. 2014, 99, 28–37. [Google Scholar] [CrossRef]
- Sun, Y.C.; Cao, H.F.; Yin, J.; Kang, L.; Ge, F. Elevated CO2 changes the interactions between nematode and tomato genotypes differing in the JA pathway. Plant Cell Environ. 2010, 33, 729–739. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlight the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer Nature Singapore Pte., Ltd.: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.D.; Qiu, D.L. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.A.; Gog, L.; Giacometti, R. Anthropogenic increase in carbon dioxide modifies plant–insect interactions. Ann. Appl. Biol. 2017, 170, 68–77. [Google Scholar] [CrossRef]
- Sun, Y.C.; Guo, H.J.; Zhu-Salzman, K.; Ge, F. Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 2013, 210, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Shinya, T.; Hojo, Y.; Desaki, Y.; Christeller, J.T.; Okada, K.; Shibuya, N.; Galis, I. Modulation of plant defense responses to herbivores by simultaneous recognition of different herbivore-associated elicitors in rice. Sci. Rep. 2016, 6, 32537. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Effects of elevated CO2 on resistant and susceptible rice cultivar and its primary host, brown planthopper (BPH), Nilaparvata lugens (Stål). Sci. Rep. 2021, 11, 8905. [Google Scholar] [CrossRef]
- Xie, C.H.; Zhao, L.; Yang, Q.F.; Wang, Z.Y.; He, K.L. Direct Effects of Elevated CO2 Levels on the Fitness Performance of Asian Corn Borer (Lepidoptera:Crambidae) for Multigenerations. Environ. Entomol. 2015, 44, 1250–1257. [Google Scholar] [CrossRef]
- Li, B.P.; Guo, Q.; Meng, L. Effects of elevated CO2 concentration on development, reproduction, and food utilization of the Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). Sci. Agric. Sin. 2013, 46, 4464–4470. (In Chinese) [Google Scholar]
- Zhang, Y.F.; Dai, Y.; Wan, G.J.; Liu, B.; Xing, G.N.; Chen, F.J. Effects of Elevated CO2 on Plant Chemistry, Growth, Yield of Resistant Soybean, and Feeding of a Target Lepidoptera Pest, Spodoptera litura (Lepidoptera: Noctuidae). Environ. Entomol. 2018, 47, 848–856. [Google Scholar]
- Satishchandra, N.K.; Vaddi, S.; Naik, S.O.; Chakravarthy, A.K.; Atlihan, R. Effect of Temperature and CO2 on Population Growth of South American Tomato Moth, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on Tomato. J. Econ. Entomol. 2018, 111, 1614–1624. [Google Scholar] [CrossRef]
- Luo, L.Z.; Cheng, Y.X.; Tang, J.H.; Zhang, L.; Jiang, X.F. Temperature and humidity as key factors influencing the occurrence and damage of Mythimna separata. Plant Prot. 2016, 42, 1–8. (In Chinese) [Google Scholar]
- Chen, Z.Y.; Zhang, Z.; Zhang, Y.H. Research progress on the occurrence, damage, monitoring, and early warning technologies of the meadow moth. Chin. J. Appl. Entomol. 2021, 58, 552–564. (In Chinese) [Google Scholar]
- Zhang, L.; Jiang, X.F. Occurrence tendency and management strategies of the beet webworm, Loxostege sticticalis in China. Plant Prot. 2022, 48, 68–72. (In Chinese) [Google Scholar]
- Tang, J.H.; Luo, L.Z.; Jiang, J.X.; Cheng, Y.X.; Zhang, L. Effect of flight temperature on flight capacity, energy substances and fecundity of the beet webworm (Loxostege sticticalis L.). Chin. J. Appl. Entomol. 2023, 60, 1669–1678. (In Chinese) [Google Scholar]
- IPCC. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Gunderson, C.A.; Norby, R.J.; Wullschleger, S.D. Foliar gas exchange responses of two deciduous hardwoods during 3 years of growth in elevated CO2: No loss of photosynthetic enhancement. Plant Cell Environ. 2010, 16, 797–807. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Orla, D.; Mihai, A.; Zangerl, A.R.; Alistair, R.; Berenbaum, M.R.; Delucia, E.H. Anthropogenic changes in tropospheric composition increase susceptibility of soybean to insect herbivory. Environ. Entomol. 2005, 34, 479–485. [Google Scholar] [CrossRef]
- Mndela, M.; Tjelele, J.T.; Madakadze, I.C.; Mangwane, M.; Samuels, I.M.; Muller, F.; Pule, H.T. A global meta-analysis of woody plant responses to elevated CO2: Implications on biomass, growth, leaf N content, photosynthesis and water relations. Ecol. Process 2022, 11, 52. [Google Scholar] [CrossRef]
- He, P.; Hou, B.; Li, Y.; Xu, C.; Ma, P.; Lam, S.M.; Gil, V.; Yang, X.; Yang, X.; Zhang, L.; et al. Lipid Profiling Reveals Browning Heterogeneity of White Adipose Tissue by Β3-Adrenergic Stimulation. Biomolecules 2019, 9, 444. [Google Scholar] [CrossRef]
- Ryan, G.D.; Rasmussen, S.; Newman, J.A. Global atmospheric change and trophic interactions: Are there any general responses? In Plant Communication from an Ecological Perspective: Signaling and Communication in Plants; Baluška, F., Ninkovic, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 179–214. [Google Scholar] [CrossRef]
- Li, R.H. The Mechanism of Elevated CO2 Concentration on the Interaction of Two-Color Morphs of Pea Aphid and Vicia faba. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2017. (In Chinese). [Google Scholar]
- Moreno-Delafuente, A.; Fereres, A.; Viñuela, E.; Medina, P. Elevated carbon dioxide reduces Aphis gossypii intrinsic increase rates without affecting Aphidius colemani parasitism rate. Biol. Control 2021, 163, 104741. [Google Scholar] [CrossRef]
- Guo, D.; Li, J.R.; Wang, Y.Z.; Cao, N.; Fang, X.L.; Wang, T.; Dong, J.L. The jasmonate pathway promotes nodule symbiosis and suppresses host plant defense in Medicago truncatula. Mol. Plant 2024, 17, 1183–1203. [Google Scholar] [CrossRef]
- Pan, L.H.; Miao, H.Y.; Wang qm Walling, L.L.; Liu, S.S. Virus-induced phytohormone dynamics and their effects on plant–insect interactions. New Phytol. 2021, 230, 1305–1320. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Aguirre, N.M.; Helms, A.M. The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores. Plants Life Sci. 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Pang, P. Effects of Sustained High CO2 Concentration Stress on the Growth and Development of Helicoverpa armigera and Functional Role of Bacillus safensis AN1. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2019. (In Chinese). [Google Scholar]
- Richard, D. Human Glutathione Transferases Structure, Function, and Implications in Health and Disease. Pharm. Bioprocess. 2023, 11, 4. [Google Scholar]
- Zhang, Y.F.; Deng, F.; Fan, Y.L.; Zhao, Z.W. Effects of carboxylesterase gene silence on wheat aphid Sitobion avenae (Fabricius). J. Asia-Pac. Entomol. 2016, 19, 341–345. [Google Scholar] [CrossRef]
- Zhuge, X.L.; Xu, H.; Xiu, Z.J.; Yang, H.L. Biochemical Functions of Glutathione S-Transferase Family of Salix babylonica. Front. Plant Sci. 2020, 11, 364. [Google Scholar] [CrossRef]
- Gao, Y.P.; Luo, M.; Wang, X.Y.; He, X.Z.; Lu, W.; Zheng, X.L. Pathogenicity of Beauveria bassiana PfBb and Immune Responses of a Non-Target Host, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insect 2022, 13, 914. [Google Scholar] [CrossRef]
- Nahrstedt, A. The Significance of Secondary Metabolites for Interactions between Plants and Insects. Planta Med. 1989, 55, 333–338. [Google Scholar] [CrossRef]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef]
- Fan, Z.F.; Qian, L.; Chen, Y.P.; Fan, R.; He, S.Q.; Gao, Y.L.; Gui, F.R. Effects of elevated CO2 on activities of protective and detoxifying enzymes in Frankliniella occidentalis and F. intonsa under spinetoram stress. Pest Manag. Sci. 2022, 78, 274–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).