Dopamine and 24-Epibrassinolide Upregulate Root Resilience, Mitigating Lead Stress on Leaf Tissue and Stomatal Performance in Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Growth Conditions
2.2. Plants, Containers, and Acclimation
2.3. Experimental Design
2.4. Dopamine (DOP) and 24-Epibrassinolide (EBR) Preparations and Applications
2.5. Plant Nutrition and Pb Treatment
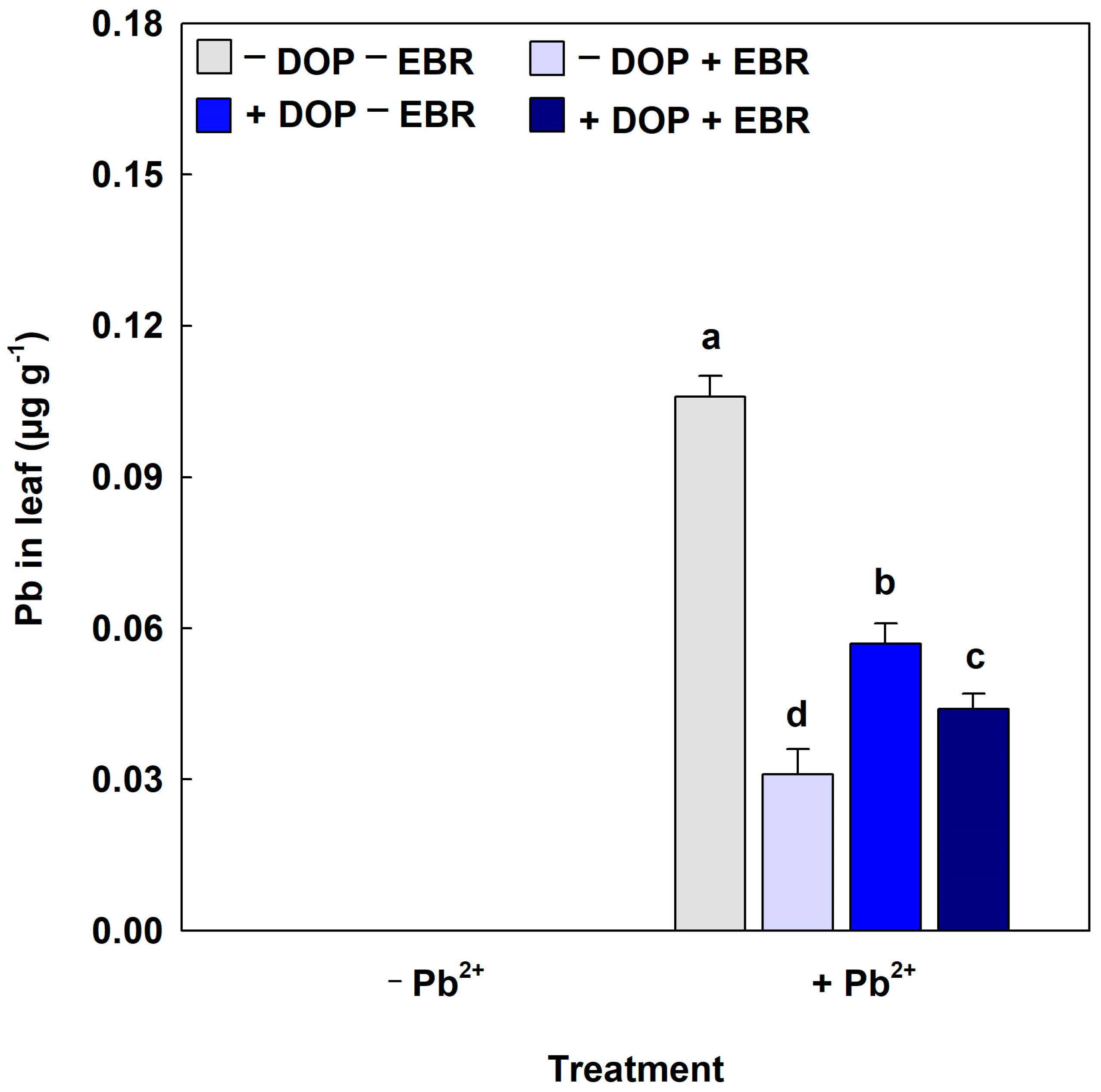
2.6. Pb Determination
2.7. Measurements of Anatomical Parameters
2.8. Data Analysis
3. Results
3.1. Dopamine and 24-Epibrassinolide Protect Root Against Lead Excess
3.2. Growth Regulators Minimized the Harmful Effects Linked to Lead on Leaf Tissue
3.3. Dopamine and 24-Epibrassinolide Positively Regulated Stomatal Characteristics in Lead-Stressed Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Yang, P.; Zhu, L. Preparation of modified atmosphere packaging based on the respiratory characteristics of cherry tomato and its freshness preservation application. Sci. Hortic. 2024, 333, 113286. [Google Scholar] [CrossRef]
- Meng, Z.; Du, X.; Xia, J.; Ma, Z.; Zhang, T. Real-time statistical algorithm for cherry tomatoes with different ripeness based on depth information mapping. Comput. Electron. Agric. 2024, 220, 108900. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Ling, J.; Xie, Z.; Song, L.; Wang, Y.; Zhao, L.; Zhao, T. Comparative analysis of physical traits, mineral compositions, antioxidant contents, and metabolite profiles in five cherry tomato cultivars. Food Res. Int. 2024, 194, 114897. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Hassan, S.A.; Javaid, H.; Zahid, M.; Naeem, M.; Bhat, Z.F.; Abdi, G.; Aadil, R.M. Factors responsible for spoilage, drawbacks of conventional packaging, and advanced packaging systems for tomatoes. J. Agric. Food Res. 2024, 15, 100962. [Google Scholar] [CrossRef]
- Li, J.; Sun, B.; Xu, Q.; Jiang, L.; Wang, N. Transcriptome-level analysis of gene expressions in different tissues of tomato and key gene identifications during seed germination. Sci. Hortic. 2024, 337, 113565. [Google Scholar] [CrossRef]
- Potestio, S.; Giannelli, G.; Degola, F.; Vamerali, T.; Fragni, R.; Cocconi, E.; Sandei, L.; Visioli, G. Salt stress mitigation and improvement in fruit nutritional characteristics of tomato plants: New opportunities from the exploitation of a Halotorelant agrobacterium strain. Plant Stress 2024, 13, 100558. [Google Scholar] [CrossRef]
- Rothan, C.; Diouf, I.; Causse, M. Trait discovery and editing in tomato. Plant J. 2019, 97, 73–90. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Qiu, Z.; Wang, C.; Zeng, Q. Ecotoxicological effects of polyethylene microplastics and lead (Pb) on the biomass, activity, and community diversity of soil microbes. Environ. Res. 2024, 252, 119012. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, Y.; Ren, Y.; Ma, L.; Tang, X.; Pan, S.; Duan, M.; Tian, H.; Mo, Z. Multi-walled carbon nanotubes affect yield, antioxidant response, and rhizosphere microbial community of scented rice under combined cadmium-lead (Cd–Pb) stress. Plant Physiol. Biochem. 2024, 213, 108826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Y.; Gu, H.; Lam, S.S.; Chen, X.; Sonne, C.; Peng, W. A Review of phytoremediation of environmental lead (Pb) contamination. Chemosphere 2024, 362, 142691. [Google Scholar] [CrossRef]
- Petit, J.C.J.; Mattielli, N.; De Jong, J.; Bouhoulle, E.; Debouge, W.; Maggi, P.; Hublet, G.; Fagel, N.; Pirard, C.; Charlier, C.; et al. High precision blood lead radiogenic isotope signatures in a community exposed to Pb contaminated soils and implications for the current Pb exposure of the European population. Sci. Total Environ. 2024, 950, 174763. [Google Scholar] [CrossRef] [PubMed]
- Vagnoni, G.; Bortolotti, E.; Checchi, S.; Saieva, C.; Berti, G.; Doccioli, C.; Caini, S. Lead (Pb) in biological samples in association with cancer risk and mortality: A systematic literature review. Cancer Epidemiol. 2024, 92, 102630. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Salmen, S.H.; Alharbi, S.A. Mitigating Pb-induced oxidative stress in rice plants by cerium oxide and iron oxide nanoparticles. S. Afr. J. Bot. 2024, 172, 544–555. [Google Scholar] [CrossRef]
- Hanif, S.; Farooq, S.; Kiani, M.Z.; Zia, M. Surface modified ZnO NPs by betaine and proline build up tomato plants against drought stress and increase fruit nutritional quality. Chemosphere 2024, 362, 142671. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Wu, Z.; Gu, S.; Gu, H.; Cheng, D.; Li, L.; Guo, X.; Wang, M.; He, S.; Li, M.; Chen, J. Physiological and transcriptomic analyses of brassinosteroid function in kiwifruit root. Environ. Exp. Bot. 2022, 194, 104685. [Google Scholar] [CrossRef]
- Zebosi, B.; Vollbrecht, E.; Best, N.B. Brassinosteroid biosynthesis and signaling: Conserved and diversified functions of core genes across multiple plant species. Plant Commun. 2024, 5, 100982. [Google Scholar] [CrossRef]
- Han, C.; Wang, L.; Lyu, J.; Shi, W.; Yao, L.; Fan, M.; Bai, M. Brassinosteroid signaling and molecular crosstalk with nutrients in plants. J. Genet. Genom. 2023, 50, 541–553. [Google Scholar] [CrossRef]
- Wen, Y.; Lei, A.-Q.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S.; Gao, X.-B. Foliar spraying of brassinolide affects leaf quality and secondary metabolite profiles of cold-stressed tea plants. Horticulturae 2024, 10, 639. [Google Scholar] [CrossRef]
- Gutiérrez-Villamil, D.A.; Magnitskiy, S.; Balaguera-López, H.E. Physiological and molecular functions of brassinosteroids during fruit development, ripening, and postharvest damage of horticultural products: A review. Postharvest Biol. Technol. 2024, 214, 112984. [Google Scholar] [CrossRef]
- Piacentini, D.; Bellini, C.; Peduzzi, A.; Casentini, B.; Tiraboschi, C.; Cacciotti, A. Plant stress arsenite and arsenate stress differently affect auxin distribution in rice roots and brassinosteroids restore it sustaining root system plasticity. Plant Stress 2024, 11, 100418. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, J.; Li, Q.; Quan, D.; Wang, Q.; Gao, Y. Auxin–Brassinosteroid crosstalk: Regulating rice plant architecture and grain shape. Crop J. 2024, 12, 953–963. [Google Scholar] [CrossRef]
- Gutkowska, M.; Buszewicz, D.; Zajbt-Łuczniewska, M.; Radkiewicz, M.; Nowakowska, J.; Swiezewska, E.; Surmacz, L. Medium-chain-length polyprenol (C45–C55) formation in chloroplasts of Arabidopsis is brassinosteroid-dependent. J. Plant Physiol. 2023, 291, 154126. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef]
- Kimura, M. Fluorescence histochemical study on serotonin and catecholamine in some plants. Jpn. J. Pharmacol. 1968, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X. Dopamine-induced abiotic stress tolerance in horticultural plants. Sci. Hortic. 2023, 307, 111506. [Google Scholar] [CrossRef]
- Akula, R.; Mukherjee, S. New insights on neurotransmitters signaling mechanisms in plants. Plant Signal. Behav. 2020, 15, 1737450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Ali, A.; Rizwan, M.; Ali, S. Dopamine alleviates hydrocarbon stress in Brassica oleracea through modulation of physio-biochemical attributes and antioxidant defense systems. Chemosphere 2021, 270, 128633. [Google Scholar] [CrossRef] [PubMed]
- Guedes, F.R.C.M.; Maia, C.F.; Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.S. Exogenous 24-epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine confers cadmium tolerance in apples by improving growth, reducing reactive oxygen species, and changing secondary metabolite levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Pontes, C.V.S.; Prestes, L.R.; Vieira Neto, A.F.; Lopes, L.K.C.; Barbosa, M.A.M.; Lobato, A.K.d.S. Single and combined applications of dopamine and 24-epibrassinolide in soybean seedlings under water deficiency: Tolerance mechanisms essential to inhibit deleterious effects. Plant Biosyst. 2024, 158, 1347–1357. [Google Scholar] [CrossRef]
- Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Bajguz, A.; Lobato, A.K.d.S. 24-Epibrassinolide simultaneously stimulates photosynthetic machinery and biomass accumulation in tomato plants under lead stress: Essential contributions connected to the antioxidant system and anatomical structures. Agronomy 2022, 12, 1985. [Google Scholar] [CrossRef]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective procedures for the determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and rare earth elements in plants and foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Book Co. Inc.: New York, NY, USA, 1940. [Google Scholar]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure: Principles and Selected Methods, 1st ed.; Termarcarphi Pty. Ltd.: Melbourne, Victoria, Australia, 1981. [Google Scholar]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.; Rampelotto, M.V.; Nicoloso, F.T. A technique for the anatomical study of potato leaf epidermis. Ciência Rural 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Tao, Q.; Liu, Y.; Li, M.; Li, J.; Luo, J.; Lux, A.; Kováč, J.; Yuan, S.; Li, B.; Li, Q.; et al. Cd-induced difference in root characteristics along root apex contributes to variation in cd uptake and accumulation between two contrasting ecotypes of Sedum Alfredii. Chemosphere 2020, 243, 125290. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Rafique, F.; Ahmed, K.; Khan, S.A.; Khan, N.; Akbar, M.; Zafar, M. Evaluation of heavy metals effects on morpho-anatomical alterations of wheat (Triticum aestivum L.) seedlings. Microsc. Res. Tech. 2021, 84, 2517–2529. [Google Scholar] [CrossRef]
- Lobato, S.M.S.; Santos, L.R.; Silva, B.R.S.; Paniz, F.P.; Batista, B.L.; Lobato, A.K.S. Root-differential modulation enhances nutritional status and leaf anatomy in pigeonpea plants under water deficit. Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 262, 151519. [Google Scholar] [CrossRef]
- Ribeiro, D.G.S.; Silva, B.R.S.; Lobato, A.K.S. Brassinosteroids induce tolerance to water deficit in soybean seedlings: Contributions linked to root anatomy and antioxidant enzymes. Acta Physiol. Plant. 2019, 41, 82. [Google Scholar] [CrossRef]
- Jung, J.K.H.; McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front. Plant Sci. 2013, 4, 186. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H.M. Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical changes, osmolytes accumulation and distribution in the native plants growing on Pb-contaminated sites. Environ. Geochem. Health 2020, 5, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Corrêa, F.F.; Pereira, M.P.; Kloss, R.B.; de Castro, E.M.; Pereira, F.J. Leaf ontogeny and meristem activity of Typha domingensis Pers. (Typhaceae) under different phosphate concentrations. Aquat. Bot. 2017, 136, 43–51. [Google Scholar] [CrossRef]
- Merced, A.; Renzaglia, K.S. Structure, function and evolution of stomata from a bryological perspective. Bryophyt. Divers. Evol. 2017, 39, 7–20. [Google Scholar] [CrossRef]
- Paulilo, M.T.S.; Viana, A.M.; Randi, Á.M. Fisiologia Vegetal; Viviane Mara Woehl, A.V., Nogueira, M.M., Eds.; Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2015; Volume 4, ISBN 07.007.007-7. [Google Scholar]
- Kulma, A.; Szopa, J. Catecholamines are active compounds in plants. Plant Sci. 2007, 172, 433–440. [Google Scholar] [CrossRef]
- Muñoz, A.W.; Hoyer, E.; Schumacher, K.; Grognot, M.; Taute, K.M.; Hacker, S.M.; Sieber, S.A.; Jung, K. Eukaryotic catecholamine hormones influence the chemotactic control of Vibrio campbellii by binding to the coupling protein CheW. Proc. Natl. Acad. Sci. USA 2022, 119, e2118227119. [Google Scholar] [CrossRef]
- Liang, B.; Gao, T.; Zhao, Q.; Ma, C.; Chen, Q.; Wei, Z.; Li, C.; Li, C.; Ma, F. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and water relations of Paulownia fortunei under photomixotrophic and photoautotrophic conditions. Biol. Plant. 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Sack, L.; Buckley, T.N. The developmental basis of stomatal density and flux. Plant Physiol. 2016, 171, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Segev, R.; Nannapaneni, R.; Sindurakar, P.; Kim, H.; Read, H.; Lijek, S. The effect of the stomatal index on the net rate of photosynthesis in the leaves of Spinacia oleracea, Vinca minor, Rhododendron spp., Epipremnum aureum, and Hedera spp. J. Emerg. Investig. 2015, 20, 2018. [Google Scholar] [CrossRef] [PubMed]
- Júnior, S.d.O.M.; de Andrade, J.R.; Nascimento, R.D.; de Lima, R.F.; de Castro Bezerra, C.V.; Ferreira, V.M. Brassinosteroid application increases tomato tolerance to salinity by changing the effects of stress on membrane integrity and gas exchange. Acta Sci.-Agron. 2022, 44, e55647. [Google Scholar] [CrossRef]


| Pb2+ | DOP | EBR | RET (µm) | RDT (µm) | RCT (µm) | VCD (µm) | RMD (µm) |
|---|---|---|---|---|---|---|---|
| − | − | − | 9.81 ± 0.48 e | 9.69 ± 0.12 c | 103.41 ± 5.14 e | 88.37 ± 2.71 d | 45.73 ± 2.03 d |
| − | − | + | 13.19 ± 0.64 a | 12.11 ± 0.54 a | 135.86 ± 5.62 a | 114.35 ± 3.98 a | 68.02 ± 2.57 a |
| − | + | − | 11.39 ± 0.23 c | 10.59 ± 0.29 b | 116.01 ± 2.74 c | 96.46 ± 1.83 c | 55.09 ± 2.58 c |
| − | + | + | 12.44 ± 0.31 b | 11.74 ± 0.28 a | 127.23 ± 1.08 b | 105.61 ± 2.99 b | 60.33 ± 3.78 b |
| + | − | − | 7.12 ± 0.43 g | 7.70 ± 0.37 e | 91.20 ± 5.51 f | 69.53 ± 1.57 f | 32.60 ± 1.65 g |
| + | − | + | 10.30 ± 0.12 d | 10.62 ± 0.37 b | 122.64 ± 6.85 c | 94.69 ± 3.79 c | 39.96 ± 1.51 e |
| + | + | − | 8.80 ± 0.31 f | 8.93 ± 0.32 d | 110.78 ± 5.54 d | 76.31 ± 3.91 e | 34.13 ± 1.15 g |
| + | + | + | 9.69 ± 0.42 e | 9.83 ± 0.48 c | 117.74 ± 6.75 c | 86.69 ± 1.63 d | 37.01 ± 1.10 f |
| Pb2+ | DOP | EBR | ETAd (µm) | ETAb (µm) | PPT (µm) | SPT (µm) | Ratio PPT/SPT |
|---|---|---|---|---|---|---|---|
| − | − | − | 5.06 ± 0.41 c | 4.71 ± 0.30 d | 23.71 ± 0.19 c | 29.39 ± 1.84 c | 0.80 ± 0.05 c |
| − | − | + | 6.40 ± 0.24 a | 6.64 ± 0.29 a | 25.79 ± 0.68 a | 35.97 ± 2.94 a | 0.72 ± 0.04 c |
| − | + | − | 5.81 ± 0.43 b | 5.41 ± 0.34 c | 24.40 ± 0.92 b | 31.23 ± 1.33 c | 0.78 ± 0.04 c |
| − | + | + | 5.97 ± 0.41 b | 5.81 ± 0.34 b | 24.66 ± 0.99 b | 32.94 ± 1.17 b | 0.75 ± 0.02 c |
| + | − | − | 3.48 ± 0.30 e | 3.55 ± 0.18 f | 19.12 ± 0.45 f | 20.79 ± 0.74 f | 0.92 ± 0.03 a |
| + | − | + | 5.06 ± 0.26 c | 5.01 ± 0.37 d | 23.19 ± 0.67 c | 29.48 ± 1.30 c | 0.78 ± 0.04 c |
| + | + | − | 4.04 ± 0.19 d | 4.31 ± 0.20 e | 20.95 ± 0.52 e | 23.30 ± 1.29 e | 0.90 ± 0.06 a |
| + | + | + | 4.27 ± 0.18 d | 4.79 ± 0.19 d | 22.02 ± 0.88 d | 26.03 ± 0.96 d | 0.84 ± 0.05 b |
| Pb2+ | DOP | EBR | SD (Stomata per mm2) | PDS (µm) | EDS (µm) | SF | SI (%) |
|---|---|---|---|---|---|---|---|
| Adaxial face | |||||||
| − | − | − | 530 ± 35 d | 13.62 ± 0.47 d | 10.11 ± 0.19 d | 1.35 ± 0.04 a | 8.17 ± 0.45 d |
| − | − | + | 630 ± 34 a | 11.78 ± 0.52 f | 8.57 ± 0.22 f | 1.38 ± 0.08 a | 11.36 ± 0.80 a |
| − | + | − | 570 ± 31 c | 12.66 ± 0.24 e | 9.31 ± 0.50 e | 1.36 ± 0.09 a | 9.62 ± 0.65 c |
| − | + | + | 603 ± 33 b | 11.90 ± 0.67 f | 8.73 ± 0.39 f | 1.37 ± 0.08 a | 10.30 ± 0.62 b |
| + | – | – | 272 ± 15 h | 17.82 ± 0.57 a | 14.58 ± 0.23 a | 1.22 ± 0.03 b | 5.69 ± 0.33 g |
| + | – | + | 457 ± 31 e | 13.59 ± 0.92 d | 10.28 ± 0.33 d | 1.32 ± 0.09 a | 7.95 ± 0.41 d |
| + | + | – | 357 ± 16 g | 15.79 ± 0.38 b | 12.49 ± 0.23 b | 1.26 ± 0.04 b | 6.60 ± 0.37 f |
| + | + | + | 384 ± 27 f | 14.94 ± 0.69 c | 11.39 ± 0.42 c | 1.31 ± 0.06 a | 7.31 ± 0.27 e |
| Abaxial face | |||||||
| – | − | − | 783 ± 36 c | 13.56 ± 0.41 e | 11.37 ± 0.37 d | 1.19 ± 0.04 a | 9.31 ± 0.24 d |
| – | − | + | 880 ± 29 a | 12.17 ± 0.69 f | 9.91 ± 0.65 f | 1.23 ± 0.10 a | 12.39 ± 0.45 a |
| – | + | − | 817 ± 24 b | 12.66 ± 0.24 f | 10.52 ± 0.30 e | 1.20 ± 0.03 a | 10.20 ± 0.49 c |
| – | + | + | 854 ± 39 a | 12.33 ± 0.54 f | 10.12 ± 0.63 f | 1.22 ± 0.08 a | 11.67 ± 0.48 b |
| + | – | – | 365 ± 9 g | 16.38 ± 0.45 a | 14.08 ± 0.56 a | 1.16 ± 0.03 a | 6.30 ± 0.11 f |
| + | – | + | 643 ± 36 d | 14.04 ± 0.39 d | 11.62 ± 0.49 d | 1.21 ± 0.06 a | 9.11 ± 0.50 d |
| + | + | – | 561 ± 32 f | 15.93 ± 0.62 b | 13.37 ± 0.32 b | 1.19 ± 0.02 a | 8.15 ± 0.53 e |
| + | + | + | 605 ± 27 e | 15.30 ± 0.18 c | 12.78 ± 0.44 c | 1.20 ± 0.03 a | 8.50 ± 0.24 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestes, L.R.; Silva, M.M.S.d.; Silva, S.G.A.d.; Gonçalves, M.A.F.; Batista, B.L.; Viana, I.B.; Lobato, A.K.d.S. Dopamine and 24-Epibrassinolide Upregulate Root Resilience, Mitigating Lead Stress on Leaf Tissue and Stomatal Performance in Tomato Plants. Agronomy 2025, 15, 239. https://doi.org/10.3390/agronomy15010239
Prestes LR, Silva MMSd, Silva SGAd, Gonçalves MAF, Batista BL, Viana IB, Lobato AKdS. Dopamine and 24-Epibrassinolide Upregulate Root Resilience, Mitigating Lead Stress on Leaf Tissue and Stomatal Performance in Tomato Plants. Agronomy. 2025; 15(1):239. https://doi.org/10.3390/agronomy15010239
Chicago/Turabian StylePrestes, Lohana Ribeiro, Madson Mateus Santos da Silva, Sharon Graziela Alves da Silva, Maria Andressa Fernandes Gonçalves, Bruno Lemos Batista, Ivan Becari Viana, and Allan Klynger da Silva Lobato. 2025. "Dopamine and 24-Epibrassinolide Upregulate Root Resilience, Mitigating Lead Stress on Leaf Tissue and Stomatal Performance in Tomato Plants" Agronomy 15, no. 1: 239. https://doi.org/10.3390/agronomy15010239
APA StylePrestes, L. R., Silva, M. M. S. d., Silva, S. G. A. d., Gonçalves, M. A. F., Batista, B. L., Viana, I. B., & Lobato, A. K. d. S. (2025). Dopamine and 24-Epibrassinolide Upregulate Root Resilience, Mitigating Lead Stress on Leaf Tissue and Stomatal Performance in Tomato Plants. Agronomy, 15(1), 239. https://doi.org/10.3390/agronomy15010239








