Abstract
CPPU, N-(2-Chloro-4-pyridyl)-N-phenylurea, is a synthetic cytokinin extensively used to enhance fruit size and overall quality in several crops, including kiwifruit. This study aimed to investigate the effects of three different CPPU application strategies (2.3, 3.0, and 4.6 ppm) and two crop load levels on key fruit quality parameters at harvest, as well as on post-harvest storage performance. Our results demonstrate that two applications of CPPU (4.6 ppm) significantly increased fruit weight, especially under standard crop-load conditions, likely due to more efficient resource allocation. Additionally, fruit firmness improved with two or three CPPU applications, probably because of enhanced cell wall development. Crop load consistently influenced fruit firmness, with lower loads resulting in softer fruits. The soluble solids content was not significantly affected by the two CPPU applications; however, it was notably influenced by crop load, with fruits from the standard crop load showing higher sugar accumulation. A similar trend was observed in fruit dry weight, where CPPU had a greater impact under standard crop loads. Regarding post-harvest performance, CPPU applications showed a limited effect on maintaining fruit firmness during the first five months of storage. Overall, CPPU can be a potential strategy to enhance fruit quality, but its effectiveness depends heavily on field management practices. Therefore, controlling field variables is essential to fully realize the benefits of CPPU and to avoid interference with the plant’s physiological responses.
1. Introduction
In the current scenario of global population growth along with farming surfaces shrinking and soil fertility depletion, overall production itself is not the major challenge to food security anymore, while the quest for sustainable and efficient farming practices has never been more imperative. In fact, a broad shift from labor-intensive cultural methods toward highly productive and renewable practices is instead demanded by many food production players (e.g., policymakers, farmers, and companies). Among the multiple solutions for crop management optimization, plant bioregulators, also known as plant growth regulators (PGRs), have emerged as a valuable innovation toward more effective, resilient, and environmentally conscious food production [1,2]. In this sense, plant growth regulators (PGRs) are natural or artificial compounds that mimic plant hormones or influence their biosynthesis, affecting metabolic pathways [3]. Additionally, PGRs are widely used in perennial fruit cultivation and can promote bud break, flower development, fruit set [4], disease resistance [5], yield, and fruit quality [6]. They are also used during post-harvest to enhance fruit storability [7]. At any rate, the effects of PGRs on plant hormonal balance are not fully understood, and factors such as application timing, rate, hormonal homeostasis, and environmental conditions can influence their performance.
Forchlorfenuron (1-(2-chloropyridin-4-yl)-3-phenylurea), also known as CPPU, is a synthetic cytokinin-based PGR that has been widely used to increase fruit size and quality traits in apple [8,9] cherry [10], and grape [11]. CPPU can be applied at bud break and enhances cell division and fruit setting, setting the foundation for improved fruit size. In detail, the pre-anthesis application is often used to promote uniform flowering and early fruit development, while post-bloom applications can directly affect fruit growth and quality, such as increasing fruit firmness and size. Generally, it is largely used during preharvest treatments in kiwifruit [12,13]. The CPPU action mechanism is complex and not yet fully elucidated [14]. Indeed, in kiwifruit, CPPU application resulted in a boost of gibberellin and cytokinin biosynthesis and signaling and in the repression of auxins and the ABA (abscisic acid) pathway; thus, CPPU application enhanced cell expansion of epidermal and parenchyma cells, promoting cell division of subepidermal cells [15]. Besides the multiple changes in fruit morphogenesis, carbohydrate metabolism, and fruit ripening [16], several fruit parameters with a crucial impact on overall fruit quality are likely to be affected by CPPU. However, since CPPU mechanisms on fruit development primarily affect its hormonal balance and signaling, the results are often contradictory. For example, some studies showed that CPPU application after full bloom increased fruit size and increased sugar accumulation, reduced acidity, and lowered firmness [17], whereas others showed that CPPU application in kiwifruit significantly reduced soluble solid content (SSC) and titratable acids [18].
Furthermore, the variability of CPPU effects in kiwifruit can be explained by the influence of several factors, such as the magnitude of pollination [19], pruning [20], or blooming stage when the compound is applied [21]. Moreover, the effects of Sitofex®, especially at high doses as indicated in the manufacturer’s labels (i.e., 800–1000 ppm containing CPPU 7.36–9.2 ppm), can also negatively affect fruit traits, histology, and metabolite partitioning, thus increasing softening and the incidence of post-harvest disorders and reducing storability. Finally, CPPU application to increase kiwifruit size may lead to residue accumulation, making fruit unmarketable [22]. In fact, although CPPU toxicity in mammals is clinically proven to be low, this PGR is degraded in kiwifruit to 3-hydroxyphenyl forchlorfenuron (3-OH-CPPU) and 4-hydroxyphenyl forchlorfenuron (4-OH-CPPU), which have been shown to be cytotoxic [23].
Considering the above-mentioned details, this study aimed to provide a comprehensive evaluation of the potential benefits and trade-offs associated with three distinct CPPU application strategies in commercial kiwifruit orchards. Differently from previous studies, we applied CPPU at dosages up to 4 times lower than the manufacturer label starting from the breaking/leaf emergence phenological stage (BBCH 12) instead of fruit development (BBCH 71) to minimize the potential side effects of CPPU on fruit development and residues risks. The specific objectives aimed at investigating how these strategies influence key fruit quality parameters (fresh weight, flesh firmness, soluble solid content, and dry matter) directly affecting market value. Additionally, this study aimed to determine the effects of CPPU application on fruit storability, with a focus on changes in firmness during storage. Field trials were conducted over three consecutive years in two commercial orchards, which were both managed under two different crop-load conditions (standard and high). By examining these variables, the research addressed the core question of how CPPU can be optimally applied to enhance fruit quality with considerations of marketability and storage potential. Furthermore, this study evaluated the hypothesis that early and low-dosage CPPU applications can mitigate some of the negative impacts associated with its use, such as fruit softening and reduced storability, while maximizing the benefits for growers and consumers alike.
2. Materials and Methods
2.1. Experimental Design
The experiment was carried out for three consecutive years from 2020 to 2022 (from this point onward, referred to as 2020, 2021, and 2022) in Latina province (Italy). Climate data are presented in Figure S1 and Table S1, described as Csa area by Köppen climate system. Two kiwifruit orchards with uniform growth and vigor were identified across the whole study area based on 10-year data provided by ZESPRI™ (Bologna, Italy)—the company leads this area, and orchards are cultivated following general management practices of pollination, soil fertilization, pruning, and irrigation. Kiwifruit plants belonged to the variety ‘Zespri® SunGold’ (Actinidia chinensis var. chinensis), were grafted onto ‘Hayward’, and were trained on either the ‘pergola’ or ‘tendone’ system; trees were spaced 5 m between the rows and 2.5 m or 3.5 m on the rows. Only uniform vines, with no evident signs of diseases or stress, were chosen. Vines that, during the time frame of the experiments, showed Psa or KVDS symptoms were replaced with new vines.
The experiment was laid out in a randomized complete block design of 12 replicates (4 trees × 3 blocks, plus control), and CPPU (Sitofex® a.i0.92%; AlzChem, Trostberg, Germany) was sprayed by foliar fertilization. Three different CPPU application strategies and two crop loads (CL) have been tested along the seasons. The first application strategy (A1) includes a single spray of 500 L/Hectare at 250 ppm of Sitofex®, corresponding to 2.3 ppm of CPPU when orchard reaches the bud-breaking/leaf emergence phenological stage (BBCH 12). The second strategy (A2) combines A1 with a second spray of 500 L/Hectare at 330 ppm of Sitofex®, corresponding to 3.0 ppm of CPPU performed 7–10 days before flowering and thinning of lateral branches was completed (BBCH 53). Finally, the third application strategy (A3) adds a 500 L/Hectare spray at 500 ppm of Sitofex®, corresponding to 4.6 ppm of CPPU when fruits reach nut size (approximately 35 days after full bloom) (BBCH 71). This strategy was applied only in one study year. The first crop load (CL), namely high CL (HCL), imposed a yield of around 45 Mg/ha with 45 fruits per cane, while the second crop load, described as standard CL (SCL), imposed a yield of around 60 Mg/ha with 60 fruits per cane.
Prior to the experiment, CPPU was never applied in those orchards since this compound was not yet included in allowed compounds in the Italian production guidelines for ‘Zespri® SunGold’.
2.2. Fruit Analysis and Storage Conditions
Harvest was carried out during the main harvest period, which was determined for each field using a combination of fruit ripening parameters and growth index. In every commercial orchard, a total of 25 kiwifruit samples without visible defects or deformations were randomly picked from different positions on the vines for each treatment and crop load. The kiwifruits were stored in international trays, wrapped in high-density polyethylene (HDPE) sheets, at 1 °C and 90% relative humidity in a normal atmosphere. The HDPE sheets had standard parameters, including a thickness of 20–30 microns, a size of 60 cm × 40 cm to fit standard trays, and a gas permeability rate of approximately 2000–3000 cm3/m2/day to ensure optimal gas exchange and moisture retention. Additionally, 50 fruits per time point were collected for the storability assessment.
The samples were processed within 24 h. The biometric parameters under investigation were fruit weight (FW), dry matter (DM), soluble solid content (SSC), and firmness (FF), which are known to be some of the most relevant parameters in fruit market pricing and orchard productivity.
2.3. Fruit Weight Analysis
Fruit fresh weight was measured by using a digital scale (Max = 1200 g; d = 0.01 g. Kern, Balingen, Germany). Dry matter was measured by comparing fresh weight and dry weight. A 2–3 mm equatorial slice was cut for each fruit. Fruit slices were singularly placed to dry in an oven at a constant temperature of 60–64 °C for 24 h.
2.4. Sugar Content Analysis and Firmness
Soluble solids content (°Brix) was measured by equatorially cutting the fruit, and 1–3 juice drops were squeezed on the prism surface of a digital refractometer (Atago, PAL-1, Sinergica soluzioni, Milano, Italy). Fruit firmness was measured using a Fruit Texture Analyser (FTA Guss, Ravenna, Italy) fitted with a 7.9 mm penetrometer probe after removal of skin and flesh to a depth of approximately 1 mm. The probe was driven into the flesh at 5 mm/s to a depth of 7.9 mm, and the maximum force was recorded as the firmness value. Firmness was measured twice at the equator of each fruit, with the two measurements taken at 90° to each other and averaged. Firmness was measured as kgf. Long storage assessment of fruit storability was carried out by monitoring the fruit firmness of 25 fruits per treatment every 40 days from the harvest time to 180 days after, following the same methodology of fruit quality analysis at harvest.
2.5. Statistical Analysis
Averages and standard deviations were calculated using the data from all repetition samples. Normality was carried out by applying the Shapiro–Wilk normality test. Barplots were created using R software, v. 2024, library ggplot2. At harvest, data were compared using an independent two-sample t-test. The analysis of post-harvest data differences was carried out via one-way Analysis of Variance (ANOVA) followed by Tukey HSD test with p < 0.05. All the statistical analyses were conducted on RStudio: Integrated Development for R.
3. Results
3.1. Application Strategy A1
Strategy A1 involved a single application of Sitofex® (250 ppm) at leaf emergence, resulting in a CPPU concentration of 2.3 ppm. In 2020, the highest fruit weight was recorded in Field 2 for both crop loads, with no significant impact from CPPU (Figure 1). Similarly, in 2021, field and crop load had a greater influence on fruit weight than CPPU treatment.
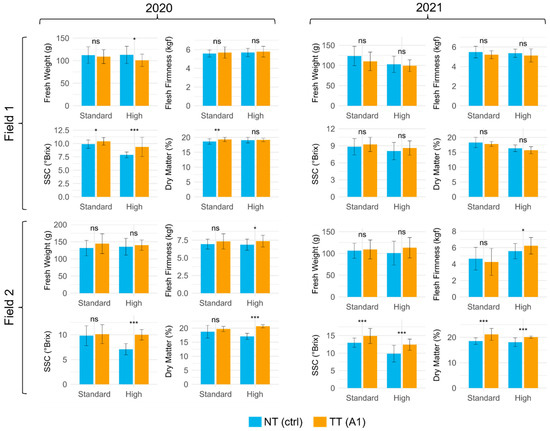
Figure 1.
Mean and SD of fresh fruit weight (g), flesh firmness (kgf), SSC (°Brix), and dry weight (%) of fruits from treated (TT) (A1) and non-treated (NT) plants of Field 1 and Field 2. Crop load: standard and high; years: 2020 and 2021. Statistical significance between TT and NT was determined using an independent two-sample t-test. Significant differences are indicated by the following codes: *** p < 0.001, ** p < 0.01, * p < 0.05, and ns p > 0.05.
Statistical analysis indicated significant interactions between field × crop load, field × treatment, and year × crop load × treatment (Figure 1, Table 1). While CPPU did not significantly affect fruit weight, it did influence other quality parameters, such as soluble solid content (SSC) and dry matter, depending on field conditions and crop management.

Table 1.
Multifactorial Analysis of Variance for CPPU 1 application (A1).
Concerning fruit quality characteristics, in both study years, the firmness showed the highest value in Field 2, regardless of CPPU treatment, in the high crop load. Year, field, and crop load statistically influenced this parameter (the treatment did not). Yet, a statistical interaction was found between year × field, year × crop load, year × treatment, field × treatment, and year × field × crop load. The highest values were found in 2020 (Figure 1 and Table 1). Regarding soluble solid content, in 2020, the highest level was found in the TT, only for the standard crop load, in both study fields. Regarding 2021, the highest values were recorded in Field 2 for the TT under the two crop loads. Again, year, field, and crop load statistically influenced this parameter together with the treatment. As for interactions, year × treatment, field × crop load, crop load × treatment, year × field × crop load, year × field × treatment, and year crop load per treatment influenced the SSC level (Figure 1 and Table 1). Lastly, concerning the fruit dry weight, in the 2020 study year, the highest value was found for TT in both testing, whilst in 2021, the highest recordings of °Brix was in TT in field crop loads. Here, every factor and linked interaction influenced this value, apart from year × treatment, crop load × treatment, and year × field × treatment (Figure 2 and Table 1).
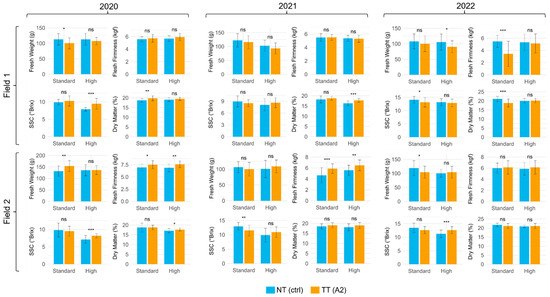
Figure 2.
Mean and SD of fresh fruit weight (g), flesh firmness (kgf), SSC (°Brix), and dry weight (%) of fruits from treated (TT) (A2) and non-treated (NT) plants of Field 1 and Field 2. Crop load: standard and high; years: 2020, 2021, and 2022. Statistical significance between TT and NT was determined using an independent two-sample t-test. Significant differences are indicated by the following codes: *** p < 0.001, ** p < 0.01, * p < 0.05, and ns p > 0.05.
3.2. Application Strategy A2
The second strategy, A2, consisted of two applications of CPPU. The first (CPPU2.3 ppm) performed at bud break was equal to A1. The following application (CPPU 3.0 ppm) was performed 7–10 days before flowering.
Considering this strategy and first investigating the fruit weight, in 2020, the TT of the standard crop load showed the highest value. Overall, field, crop load, and treatment had a statistical effect on this parameter, as well as the interaction year × field, field × treatment, and year × field × treatment (Figure 2 and Table 2).

Table 2.
Multifactorial Analysis of Variance for CPPU 2 applications (A2).
Regarding the fruit firmness, year, field, crop load, and CPPU application (treatment) showed statistical significance in influencing the considered parameter. Overall, the values of TT tended toward being higher compared to NT in both study years and fields. Moreover, the interactions field × treatment and year × treatment were statistically significant in determining firmness maintenance, and the same occurred for the year × field (Figure 2 and Table 2). Indeed, considering the fruit SSC, the highest values were found for the standard crop load in each study year without considering the treatment factor (as it was not significant), especially in Field 2. Thus, the year, field, and crop load showed a statistical effect on this parameter. Likewise, year × crop load, field × crop load, year × treatment, and crop load × treatment statistically influenced the sugar level (Figure 2 and Table 2). Considering the fruit dry weight, year, field, and crop load, once again, affected this value together with the following interactions: year × field, year × crop load, year × treatment, and crop load × treatment. Finally, since also, in this case, there was no effect made by the application of CPPU, the highest values were observed for the standard crop load in the 2022 study year in Field 2 (Figure 2 and Table 2).
3.3. Application Strategy A3
As the last strategy, three-time CPPU (A3) applications have been evaluated in one study year (4.6 ppm).
Concerning fruit weight, the overall highest value was found in Field 2 under the high crop load. At any rate, each variable did not show a statistical impact on the weight, whereas only the interactions crop load × field and field × crop load × treatment had a statistical impact on this parameter (Figure 3 and Table 3).
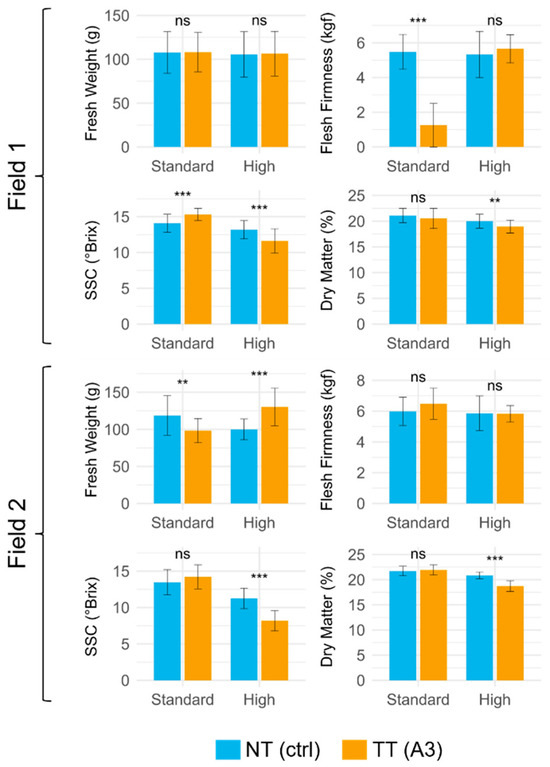
Figure 3.
Mean and SD of fresh fruit weight (g), flesh firmness (kgf), SSC (°Brix), and dry weight (%) of fruits from treated (TT) (A3) and non-treated (NT) plants of Field 1 and Field 2. Crop load: standard and high; year: 2022. Statistical significance between TT and NT was determined using an independent two-sample t-test. Significant differences are indicated by the following codes: *** p < 0.001, ** p < 0.01, and ns p > 0.05.

Table 3.
Multifactorial Analysis of Variance for CPPU 3 applications (A3).
Considering the firmness, the highest values were recorded for the standard crop load, and the CPPU-treated plants showed lower values compared to the non-treated under both crop loads in Field 2. Furthermore, each factor and interaction showed a statistical significance on this parameter (Figure 3 and Table 3). Regarding SSC overall, it was found to have the highest value in the standard crop load of both fields when CPPU is applied (TT). Yet, almost every factor and interaction had a statistical significance in this parameter, apart from field × crop load × treatment (Figure 3 and Table 3). Lastly, evaluating the fruit dry weight, also in this case, almost every factor and variable had a statistical influence on the measured parameter, with the sole exception of field × treatment. Specifically, the values were always higher in TT compared to NT. Values were generally higher in Field 2 (Figure 3 and Table 3).
3.4. Post-Harvest Performances
Quality parameters were analyzed along with the storage of kiwifruit at 45-day intervals up to 180 days (T: time; 1 to 4: time points), labeled as T1, T2, T3, and T4. Among the different quality parameters analyzed (FW, FF, SSC, DM), flesh firmness was chosen as the main evaluation parameter. Indeed, FF is the most important determinant of storage length since fruit reaching FF lower than 0.7 kgf is considered unmarketable. Flesh firmness was influenced by the year, crop yield, and field. Despite being at harvest time, the crop load had no influence on FF; during storage, fruit from HCL tended to have a faster softening. However, in all cases, FF values generally dropped in the first 90 days of storage (Table 4). In 2020, in Field 1, independently from crop load, CPPU application resulted in slight differences when considering the time point at which various treatments achieved the firmness marketability threshold. Overall, T2 (90 days after harvest) is the time point at which most of the stored fruits reach values of firmness around the limit, and differences at this point indicate the most relevant effect of forchlorfenuron.

Table 4.
Flesh firmness (kgf) of fruits from control or CPPU-treated vines in the three years of the experiment, field, and crop load. ctrl: non-treated (NT); SCL: standard crop load; HCL: high crop load. The heatmap displays the mean value expressed per field and year. The colour shows differences and similarities between values, ranging from red (lowest value) to green (highest value). Statistical significance of differences among treatments of the same crop load was assessed via one-way Analysis of Variance (ANOVA) followed by the Tukey HSD test with p < 0.05. Uppercase letters describe the difference between treatments in high crop load, whereas lowercase letters between standard crop loads.
CPPU treatments exhibit a slower decline over time, indicating a potential positive effect of the CPPU treatments in maintaining the fruit quality. Among the CPPU treatments, CPPU A2 in Field 1 of 2020 shows a more gradual decline, particularly for the high crop load (HCL), despite there being no statistical significance. In 2021, CPPU A2 consistently demonstrates favorable outcomes across both fields, again particularly under HCL conditions. This treatment shows higher measurements than control and A1 across multiple time points (T1–T4) of storage, although some values were not significant.
Finally, in 2022, kiwifruit storage performances were rather poor, and most of the fruit scored an FF of around 0.7 kgf starting from 45 days of storage. In Field 1, fruits from the vines that have received the double application of CPPU tended to have the highest FF values independently from crop load. In Field 2, both application strategies resulted in a slightly higher value of FF along storage, and the effect of CPPU was visible in comparison with the control on both fields; however, high significance values were not highlighted. Overall, the major boost of firmness derived from the A2 strategy rather than A3 is possible evidence of CPPU saturation of plant physiology pathways.
4. Discussion
The effect of CPPU on increasing fruit size and overall fruit features was investigated in this research, as previously carried out in different crops [24,25,26,27,28]. CPPU application can efficiently maintain fruit quality and delayed ripening [29,30]. In kiwifruit, the use of CPPU has been investigated primarily on A. chinensis var. deliciosa [17]. Thus, we aimed to optimize the use of CPPU in A. chinensis var. chinensis, taking into consideration its effects both at harvest and during post-harvest storage. In agricultural research, using multiple experimental fields and multiple years of study is crucial to account for the natural variability in environmental conditions, such as soil quality, climate, and pest pressures. This approach ensures that research findings are robust, reliable, and broadly applicable, making them useful across diverse agricultural settings. Observing year and field effects is normal and expected, reflecting real-world conditions and enhancing the overall resilience and adaptability of agricultural recommendations.
4.1. CPPU Effect on Fruit Fresh Weigh
In our study, the effect of CPPU on fruit weight was erratic and highly dependent both on the year and orchard (Table 1). This result highlights the principle that seasonal changes in climatic conditions and shifts in the time of agronomic practices can significantly affect plant physiology. In fact, variations in temperature, light, and humidity within the orchard directly influence the synthesis and degradation of plant hormones, disrupting their balance and thereby modifying the effectiveness of external inputs such as bioregulators [31,32]. Our experiments span over 3 consecutive years where the climatic conditions, and especially the rainfall distribution, were different (Table S1, Figure S1). For example, in the year 2020, rainfall was more frequent and intense from bud break until fruit development than in the year 2022, which was substantially drier (Figure S1). Moreover, biotic stresses, such as diseases and pests, can be indirect consequences of suboptimal growing conditions, further interfering with normal hormone function. In Italian conditions, most commercial orchards are prone to be damaged by Pseudomonas syringae pv. actinidiae (Psa) [33] and kiwifruit vine decline syndrome (KVDS) [34,35], whereas the pest Halyomorpha halys (BMSB) primarily affects fruit [36]. Damages from KVDS may remain unnoticed for a long time, and BMSB lesions on fruit are visible only after harvest; thus, their effects on plant and fruit physiology are difficult to take into account. Furthermore, despite the vine being infected, the symptoms of Psa cease to develop from July to November [37]. Therefore, the presence of biotic stressors on the tested vines cannot be excluded. These biotic stressors are well known to alter the vine hormonal balance by inducing stress-related signaling molecules such as ethylene [36], jasmonic acid, and VOCs [38]. Interestingly, several studies highlighted crosstalk between cytokinin and ethylene, which is dependent on the plant species, the organ, and the phenological stage [39]. For example, in Arabidopsis, the effects of cytokinin treatment were reduced by the application of inhibitors of ethylene signaling or biosynthesis [40]. Furthermore, in different plants, exogenous treatments with cytokinin on the ethylene-resistant mutants ein1 and ein2 showed that these mutants are also resistant to cytokinin-induced effects, such as the reduction of root and hypocotyl growth [41]. In addition to stress, the exogenous application of hormones to plants can also significantly disrupt the balance of their endogenous hormone homeostasis. Plants naturally regulate their growth and development through a complex network of hormone signaling pathways, maintaining homeostasis for their adaptative response to environmental stimuli [42]. However, when external hormones are introduced, this balance is often perturbed, leading to physiological changes that may not align with the plant’s developmental needs [4,32,43]. For instance, excess or deficient levels of a particular hormone can interfere with the plant’s ability to regulate processes such as cell division, elongation, or stress responses. In particular, in A. chinensis var. chinensis, the application of CPPU has been shown to increase gibberellin and cytokinin biosynthetic pathway and signaling and, at the same time, repress auxin and ABA biosynthetic pathways [44]. As a result, vine reproductive development can be altered. In our experiments, CPPU was applied at pre-anthesis applications, namely bud break (A1), flower buds growing (A2), and fruit cell multiplication (A3). Pre-anthesis applications were previously demonstrated to increase fruit size in ‘Hayward’, minimizing the risk of altered fruit shape or CPPU residues at harvest [45]. In our research, pre-anthesis applications of CPPU positively influenced the fruit weight only with a double application (A2) and primarily in standard crop load, suggesting that application at bud break (A1) either is too early or requires higher dosages to be effective. In high crop loads, the triple (A3) application of CPPU tended to increase fruit size in comparison to control. The lower effect of the double application of CPPU in high crop loads could have been related to an excessive number of fruits competing for limited resources [33,34]. In this condition, only a third application (A3) may hold positive results. This result suggests that the effect of biostimulants may also help counteract the negative effects of high crop loads if the application strategy is tailored to the fruit number per vine and the orchard management. In fact, a high crop load may reduce fruit quality, alter flower differentiation, and increase the susceptibility to post-harvest disorders [46]. On the other hand, a low crop load requires lower irrigation rates than a high crop load [47], yet fruit production depends directly on the vegetative growth of the preceding season because fruits develop from buds located in shoots of the previous year [48]. The carbon demand from fruits in a year of high crop load limits vegetative growth, thus restricting the potential yield of the following year [49]. Hence, it is the competition between vegetative and reproductive growth that is at the root of the alternate bearing behavior [50].
In a standard crop load, the maximum effect on fruit weight was observed in the double (A2) application and not in the triple (A3), suggesting a cumulative yet saturating effect reached with just two applications. Since CPPU is a synthetic cytokinin that promotes cell division and fruit growth, it acts primarily on meristematic cells, which are responsible for cell division and are most effective during the early stages of fruit development, particularly right after fruit set. During this phase, cell division is highly active, allowing CPPU to enhance fruit size by increasing the number of cells [51]. The observation that the maximum effect on fruit weight was seen with two applications (A2) rather than three (A3) suggests a cumulative yet saturating response. After two applications, the hormonal response could have reached a physiological limit where additional applications no longer increase fruit size. This is likely due to receptor saturation or the natural limit of cell division capacity. Therefore, beyond two applications, further CPPU treatments do not significantly enhance growth.
4.2. CPPU Effect on Fruit Firmness
Considering the fruit firmness, this parameter was positively and statistically influenced only by the two- and three-time CPPU applications. Previous studies performed on A. chinensis var. deliciosa ‘Hayward’ reported that CPPU applications after blooming reduced flesh firmness at harvest and, at the same time, increased sugar content and levering acidity, thus suggesting that the CPPU effect was mainly due to promotion of ripening However, pre-anthesis applications did not influence firmness [37]. The differences between our results and previous studies can be related not only to the fact that A. chinensis var. chinensis var is used but also to the different combinations of dosages and the time of application. The increase in fruit firmness may be related to differences in fruit histology and anatomy. In fact, in ‘Harward’, different studies reported that CPPU increased the volume and number of small isodiametric parenchyma cells in the outer pericarp, thus enlarging its depth [52,53]. In grapes, CPPU modulates hemicellulose and pectin cell wall composition, increasing calcium content in the cell wall and, thus, resulting in higher berry firmness [54]. Similar effects were also observed in apples, where CPPU influenced hemicellulose in the cell wall [55]. In our case, CPPU did not anticipate ripening, and we can hypothesize that the increase in firmness can be due to changes in the cell wall structure and the depth and structure of the outer pericarp. However, further studies aiming to investigate the cytological and histological changes induced by CPPU on fruit are needed to confirm this hypothesis. In our study, crop load was the factor primarily influencing berry firmness. This result is in accordance with previous studies that showed that crop load and canopy size may influence firmness and have an effect on the endogenous concentration of calcium inside fruit [56]. Similarly to other fruits, such as apples, endogenous calcium concentration, per se, does not directly correlate to firmness increase; however, calcium partitioning in the different tissues and cell compartments and exogenous CaCl2 or CaO application has a positive effect on firmness [56,57,58]. Furthermore, in high crop-load conditions, a possible resource competition could result in softer fruits due to the limited availability of other nutrients, in addition to calcium, for individual fruits [59,60].
4.3. CPPU Effect on Fruit Sugar Content
Taking into account fruit sugar content, the single and double applications of CPPU showed the trend of increasing SSC primarily in standard crop load, although the results were not always statistically significant. Also, in this case, crop load was the main driver of sugar content in the standard crop load; also, fruits had higher SSC values than the ones in the high crop load. In the end, for application 1, SSC was increased in both high and standard crop loads, whereas application strategy 2 showed only a trend toward an increment of the SSC due to application, and for application 3, no differences arose. This outcome could be valuable for orchard management practices, indicating that CPPU application may be particularly beneficial to enhance fruit, regardless of the crop load. However, again, there is an effect on the environment (field) that farmers must consider.
4.4. CPPU Effect on Fruit Dry Weight
Dry weight at harvest is a key parameter directly correlating with storability and consumer acceptance since it affects fruit sweetness and flavor at consumption [61]. The single application of CPPU showed the trend to increase dry weight at least in one of the two orchards (Field 1) included in this study; specifically, the dry weight tended toward showing higher parameters in both crop loads under the TT treatment. As for the previous analysis, CPPU application had varying effects on fruit dry weight depending on application frequency. This variability might stem from the differential regulation of fruit growth and development processes in response to both CPPU and environmental factors (i.e., study fields).
4.5. CPPU Effect on Storability
The use of CPPU has yielded promising results, especially with regard to the Brix and dry weight, across the years and with different applications. The efficacy of CPPU lies in its ability to serve as a simple and cost-effective strategy for bolstering overall fruit quality and yield. By stimulating the development and maturation processes, CPPU leads to fruits that boast enhanced size, sugar content, and structural integrity. The application of CPPU can significantly enhance the overall storability of kiwifruit, particularly at 45 and 90 days post-harvest. To optimize production and storage, a recommended program would include two applications of CPPU under standard crop-load conditions to improve fruit size, firmness, and sugar content. During storage, monitoring fruits treated with CPPU can ensure extended shelf-life without negatively impacting quality. Additionally, integrating CPPU treatments into routine crop management practices can maximize fruit quality and storability, supporting consistent market supply.
However, understanding the physiological mechanisms is crucial for optimizing CPPU application. In fact, it may also regulate enzymatic activity related to cell wall modification, enhancing firmness during fruit development and post-harvest storage. Furthermore, CPPU could impact sugar metabolism and translocation, potentially altering the expression of genes involved in carbohydrate transport and accumulation.
A mild red coloration in some plant shoots and leaves was observed during orchard sampling as a possible side-effect of CPPU treatments. This color shift is likely to be the result of physiological reactions leading to anthocyanin accumulation or other pigment alteration. Future research should aim to elucidate the potential effects on these pathways to provide a more comprehensive understanding of how CPPU affects plant and fruit quality at molecular and physiological levels.
5. Conclusions and Future Perspectives
The study indicates that the effect of CPPU is most pronounced under standard crop-load conditions, highlighting the need for a tailored application strategy based on orchard cultivation practices. This suggests that careful management of CPPU applications can maximize its benefits while avoiding overuse or potential saturation. The findings on maintaining fruit firmness during extended storage also support the use of CPPU as a tool to extend the marketability period of kiwifruit. For future research, studies could focus on optimizing CPPU application rates and timing across various crop loads and environmental conditions to establish best practices for different orchard systems. Additionally, investigating the long-term effects of CPPU on nutritional quality and consumer health, as well as exploring its interaction with other cultivation inputs, could provide a more comprehensive understanding of its role in kiwifruit production. Future directions should also include increasing the doses for A1 and studying the molecular mechanisms in both A1 and A2. Lastly, the extended use of CPPU may influence plant health by potentially altering hormonal balances critical to physiological processes such as flowering, fruit set, and senescence. However, there are no reports of this fact up till now. Additionally, while CPPU can enhance post-harvest storage quality by reducing weight loss and maintaining firmness, residual presence in fruit could pose concerns for consumer health if not properly regulated. Therefore, further studies are also essential to evaluate CPPU cumulative effects on soil and plant systems, as well as any implications for food safety, ensuring sustainable practices and consumer trust.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15010215/s1, Figure S1: Precipitation trends in the two field sides of the experiments between 2020 and 2022; Table S1: Climatic data (averages) in the two field sides of the experiments between 2020 and 2022.
Author Contributions
Conceptualization, F.S. and I.D.; methodology, F.S., A.C. and I.D.; formal analysis, I.D., A.C., M.C., T.M. and G.M.; investigation, I.D.; resources, F.S.; data curation, A.C.; writing—original draft preparation, G.M.; writing—review and editing, all authors; visualization, M.C. and G.M.; supervision, F.S.; project administration, F.S. and I.D.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zespri Group Limited on project GI23208: Biostimulants use on G3 & R19.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (the data are not publicly available due to privacy restrictions).
Acknowledgments
We are grateful to Niccolò Raule and Letizia Manzoni for their support in managing the field trials and in data collection and elaboration.
Conflicts of Interest
Authors Marco Mastroleo and Irene Donati were employed by the company Zespri Fresh Produce. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bons, H.K.; Kaur, M. Role of Plant Growth Regulators in Improving Fruit Set, Quality and Yield of Fruit Crops: A Review. J. Hortic. Sci. Biotechnol. 2020, 95, 137–146. [Google Scholar] [CrossRef]
- Kumar, A.; Rajan, R.; Pandey, K.; Ramprasad, R.R.; Kaur, G.; Vamshi, T.; Singh, T. Impact of New Generation Plant Growth Regulators on Fruit Crops—A Review. Hortic. Sci. 2024, 51, 1–22. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Costa, G.; Spinelli, F.; Soto, A.; Nardozza, S.; Asteggiano, L.; Vittone, G. Use of Plant Bioregulators in Kiwifruit Production. Acta Hortic. 2011, 913, 337–344. [Google Scholar] [CrossRef]
- Fischer, T.C.; Halbwirth, H.; Roemmelt, S.; Sabatini, E.; Schlangen, K.; Andreotti, C.; Spinelli, F.; Costa, G.; Forkmann, G.; Treutter, D.; et al. Induction of Polyphenol Gene Expression in Apple (Malus × Domestica) after the Application of a Dioxygenase Inhibitor. Physiol. Plant. 2006, 128, 604–617. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2020, 309, 111621. [Google Scholar] [CrossRef]
- Costa, G.; Rocchi, L.; Farneti, B.; Busatto, N.; Spinelli, F.; Vidoni, S. Use of Nondestructive Devices to Support Pre- and Postharvest Fruit Management. Horticulturae 2016, 3, 12. [Google Scholar] [CrossRef]
- Greene, D.W. CPPU Influences Fruit Quality and Fruit Abscission of ‘McIntosh’ apples. HortScience 2001, 36, 1292–1295. [Google Scholar] [CrossRef]
- Tartarini, S.; Sansavini, S.; Ventura, M. CPPU Control of Fruit Morphogenesis in Apple. Sci. Hortic. 1993, 53, 273–279. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M.D. Improving ‘Bing’ Sweet Cherry Fruit Quality with Plant Growth Regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- Azuara, M.; González, M.-R.; Mangas, R.; Martín, P. Effects of the Application of Forchlorfenuron (CPPU) on the Composition of Verdejo Grapes. BIO Web Conf. 2023, 56, 01022. [Google Scholar] [CrossRef]
- Costa, G.; Biasi, R.; Brigati, S.; Morigi, M.; Antognozzi, E. Effect of a Cytokinin-like Compound (CPPU) on Kiwifruit (Actinidia deliciosa) Ripening and Storage Life. Acta Hortic. 1995, 379, 421–428. [Google Scholar] [CrossRef]
- Antognozzi, E.; Famiani, F.; Palliotti, A.; Tombesi, A. Effects of CPPU (Cytokinin) on Kiwifruit Productivity. In Proceedings of the VII International Symposium on Plant Growth Regulators in Fruit Production 329, Jerusalem, Israel, 14–19 June 1992; pp. 150–152. [Google Scholar] [CrossRef]
- Xie, X.; Rahman, M.M.; Qiao, C.; Guo, L.; Xie, H.; Pang, R.; Tian, F. Effect of forchlorfenuron (CPPU) on fruits quality and residue analysis in kiwifruits. J. Food Compos. Anal. 2024, 133, 106383. [Google Scholar] [CrossRef]
- Luo, J.; Guo, L.; Huang, Y.; Wang, C.; Qiao, C.; Pang, R.; Li, J.; Pang, T.; Wang, R.; Xie, H.; et al. Transcriptome Analysis Reveals the Effect of Pre-Harvest CPPU Treatment on the Volatile Compounds Emitted by Kiwifruit Stored at Room Temperature. Food Res. Int. 2017, 102, 666–673. [Google Scholar] [CrossRef]
- Rana, V.S.; Bhardwaj, V.; Rana, N. Influence of CPPU Alone and in Combination with Other Plant Bio-Regulators on Fruit Yield, Quality, and Harvest Maturity of ‘Allison’ Kiwifruit. Acta Hortic. 2011, 913, 401–410. [Google Scholar] [CrossRef]
- Famiani, F.; Antognozzi, E.; Tombesi, A.; Moscatello, S.; Battistelli, A. CPPU Induced Alterations in Source-Sink Relationships in Actinidia deliciosa. In Proceedings of the VIII International Symposium on Plant Bioregulation in Fruit Production 463, Valencia, Spain, 1–4 April 1997; pp. 306–310. [Google Scholar]
- Kim, J.G.; Takami, Y.; Mizugami, T.; Beppu, K.; Fukuda, T.; Kataoka, I. CPPU application on size and quality of hardy kiwifruit. Sci. Hortic. 2006, 110, 219–222. [Google Scholar] [CrossRef]
- Ainalidou, A.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Diamantidis, G.; Matsi, T. CPPU Treatment and Pollination: Their Combined Effect on Kiwifruit Growth and Quality. Sci. Hortic. 2015, 193, 147–154. [Google Scholar] [CrossRef]
- Pramanick, K.K.; Kashyap, P.; Kishore, D.K.; Sharma, Y.P. Effect of Summer Pruning and CPPU on Yield and Quality of Kiwi Fruit (Actinidia deliciosa). J. Environ. Biol. 2015, 36, 351–356. [Google Scholar] [PubMed]
- Cruz-Castillo, J.G.; Woolley, D.J.; Famiani, F. Effects of Defoliation on Fruit Growth, Carbohydrate Reserves and Subsequent Flowering of ‘Hayward’ Kiwifruit Vines. Sci. Hortic. 2010, 125, 579–583. [Google Scholar] [CrossRef]
- Woodcock, S. A Review of Research and Development Undertaken on Psa. Kiwi Vine Health 2016, 5, 871–874. [Google Scholar]
- Zhang, Z.; Guo, K.; Bai, Y.; Dong, J.; Gao, Z.; Yuan, Y.; Wang, Y.; Liu, L.; Yue, T. Identification, Synthesis, and Safety Assessment of Forchlorfenuron (1-(2-Chloro-4-pyridyl)-3-phenylurea) and Its Metabolites in Kiwifruits. J. Agric. Food Chem. 2015, 63, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Du, C.L.; Cai, C.L.; Lu, Y.; Li, Y.M.; Xie, Z.S. Identification and expression analysis of invertase family genes during grape (Vitis vinifera L.) berry development under CPPU and GA treatment. Mol. Genet. Genom. 2023, 298, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.K.; Zheng, C.; Halaly, T.; Giacomelli, L.; Takebayashi, Y.; Jikumaru, Y.; Or, E. Abnormal endogenous repression of GA signaling in a seedless table grape cultivar with high berry growth response to GA application. Front. Plant Sci. 2017, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.C.; Greene, D.W. CPPU influences fruit quality, fruit set, return bloom, and preharvest drop of apples. HortScience 1993, 28, 115–119. [Google Scholar] [CrossRef]
- Wang, W.; Khalil-Ur-Rehman, M.; Feng, J.; Tao, J. RNA-Seq Based Transcriptomic Analysis of CPPU Treated Grape Berries and Emission of Volatile Compounds. J. Plant Physiol. 2017, 218, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y. Effect of Plant Growth Regulators on Banana Fruit and Broccoli During Storage. Sci. Hortic. 2012, 145, 62–67. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.; Feng, J.; Khalil-Ur-Rehman, M.; Tao, J. Transcriptome Sequencing Analyses Reveals Mechanisms of Eliminated Russet by Applying GA3 and CPPU on ‘Shine Muscat’ Grape. Sci. Hortic. 2019, 250, 94–103. [Google Scholar] [CrossRef]
- Huang, H.; Jing, G.; Wang, H.; Duan, X.; Qu, H.; Jiang, Y. The Combined Effects of Phenylurea and Gibberellins on Quality Maintenance and Shelf-Life Extension of Banana Fruit during Storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Dussi, M.C. Sustainable use of plant bioregulators in pear production. Acta Hortic. 2011, 909, 353–367. [Google Scholar] [CrossRef]
- Costa, G.; Botton, A. Plant bioregulators: Do we still need them? Acta Hortic. 2022, 1344, 193–202. [Google Scholar] [CrossRef]
- Michelotti, V.; Lamontanara, A.; Buriani, G.; Orrù, L.; Cellini, A.; Donati, I.; Vanneste, J.L.; Cattivelli, L.; Tacconi, G.; Spinelli, F. Comparative Transcriptome Analysis of the Interaction between Actinidia chinensis var. chinensis and Pseudomonas syringae pv. actinidiae in Absence and Presence of Acibenzolar-S-Methyl. BMC Genom. 2018, 19, 585. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Caldera, E.; Sorrenti, G.; Spinelli, F. Pathogens Associated to Kiwifruit Vine Decline in Italy. Agriculture 2020, 10, 119. [Google Scholar] [CrossRef]
- Mian, G.; Zuiderduin, K.; Barnes, L.S.; Loketsatian, S.; Bell, L.; Ermacora, P.; Cipriani, G. In Vitro Application of Eruca vesicaria subsp. sativa Leaf Extracts and Associated Metabolites Reduces the Growth of Oomycota Species Involved in Kiwifruit Vine Decline Syndrome. Front. Plant Sci. 2023, 14, 1292290. [Google Scholar] [CrossRef]
- Tamburini, G.; Laterza, I.; Nardi, D.; Mele, A.; Mori, N.; Pasini, M.; Scaccini, D.; Pozzebion, A.; Marini, L. Effect of landscape composition on the invasive pest Halyomorpha halys in fruit orchards. Agric. Ecosyst. Environ. 2023, 353, 108530. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, Infection Dynamics and Disease Epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef]
- Cellini, A.; Fiorentini, L.; Buriani, G.; Yu, J.; Donati, I.; Cornish, D.A.; Novak, B.; Costa, G.; Vanneste, J.L.; Spinelli, F. Elicitors of the Salicylic Acid Pathway Reduce Incidence of Bacterial Canker of Kiwifruit Caused by Pseudomonas syringae pv. actinidae. Ann. Appl. Biol. 2014, 165, 441–453. [Google Scholar] [CrossRef]
- El-Showk, S.; Ruonala, R.; Helariutta, Y. Crossing Paths: Cytokinin Signalling and Crosstalk. Dev. Camb. Engl. 2013, 140, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Cary, A.J.; Liu, W.; Howell, S.H. Cytokinin Action Is Coupled to Ethylene in Its Effects on the Inhibition of Root and Hypocotyl Elongation in Arabidopsis thaliana Seedlings. Plant Physiol. 1995, 107, 1075–1082. [Google Scholar] [CrossRef]
- Varma Penmetsa, R.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.C.; Nam, Y.W.; Cook, D.R. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef]
- Artner, C.; Benkova, E. Ethylene and Cytokinin: Partners in Root Growth Regulation. Mol. Plant 2019, 12, 1312–1314. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lan, J.; Xiang, X.; Xiang, H.; Jin, Z.; Khan, S.; Liu, Y. Transcriptome Sequencing and Endogenous Phytohormone Analysis Reveal New Insights in CPPU Controlling Fruit Development in Kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Castillo, J.G.; Baldicchi, A.; Frioni, T.; Marocchi, F.; Moscatello, S.; Proietti, S.; Battistelli, A.; Famiani, F. Pre-Anthesis CPPU Low Dosage Application Increases “Hayward” Kiwifruit Weight Without Affecting the Other Qualitative and Nutritional Characteristics. Food Chem. 2014, 158, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Naor, A. Crop load and irrigation interactions-a new dimension of RDI. In Proceedings of the VII International Symposium on Irrigation of Horticultural Crops 1038, Geisenheim, Germany, 16–20 June 2012; pp. 113–119. [Google Scholar] [CrossRef]
- Snowball, A.M. The Seasonal Cycle of Leaf, Shoot and Bud Development in Kiwifruit. J. Hortic. Sci. 1995, 70, 787–797. [Google Scholar] [CrossRef]
- Martín-Vertedor, A.I.; Rodríguez JM, P.; Losada, H.P.; Castiel, E.F. Interactive responses to water deficits and crop load in olive (Olea europaea L., cv. Morisca) I.—Growth and water relations. Agric. Water Manag. 2011, 98, 941–949. [Google Scholar] [CrossRef][Green Version]
- Burge, G.K.; Spence, C.B.; Marshall, R.R. Kiwifruit: Effects of Thinning on Fruit Size, Vegetative Growth, and Return Bloom. N. Z. J. Exp. Agric. 1987, 15, 317–324. [Google Scholar] [CrossRef]
- Ainalidou, A.; Tanou, G.; Belghazi, M.; Samiotaki, M.; Diamantidis, G.; Molassiotis, A.; Karamanoli, K. Integrated Analysis of Metabolites and Proteins Reveal Aspects of the Tissue-Specific Function of Synthetic Cytokinin in Kiwifruit Development and Ripening. J. Proteom. 2016, 143, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Castillo, J.G.; Woolley, D.J.; Lawes, G.S. Effects of CPPU and Other Plant Growth Regulators on Fruit Development in Kiwifruit. Acta Hortic. 1999, 498, 173–178. [Google Scholar] [CrossRef]
- Patterson, K.J.; Mason, K.A.; Gould, K.S. Effects of CPPU (N-(2-Chloro-4-pyridyl)-N′-phenylurea) on Fruit Growth, Maturity, and Storage Quality of Kiwifruit. N. Z. J. Crop Hortic. Sci. 1993, 21, 253–261. [Google Scholar] [CrossRef]
- Rojas, B.; Suárez-Vega, F.; Saez-Aguayo, S.; Olmedo, P.; Zepeda, B.; Delgado-Rioseco, J.; Defilippi, B.G.; Pedreschi, R.; Meneses, C.; Pérez-Donoso, A.G.; et al. Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides. Plants 2021, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.E.; Melton, L.D.; Jameson, P.E. Expansion during Early Apple Fruit Development Induced by Auxin and N-(2-Chloro-4-pyridyl)-N′-phenylurea: Effect on Cell Wall Hemicellulose. Plant Growth Regul. 1998, 26, 1–6. [Google Scholar] [CrossRef]
- Hopkirk, G.; Harker, F.R.; Harman, J.E. Calcium and the Firmness of Kiwifruit. N. Z. J. Crop Hortic. Sci. 1990, 18, 215–219. [Google Scholar] [CrossRef][Green Version]
- Antunes, M.D.C.; Neves, N.; Curado, F.; Rodrigues, S.; Franco, J.; Panagopoulos, T. The Effect of Calcium Applications on Kiwifruit Quality Preservation During Storage. Acta Hortic. 2007, 753, 727–732. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Corelli-Grappadelli, L.; Lakso, A.N. Fruit development in deciduous tree crops as affected by physiological factors and environmental conditions (keynote). In Proceedings of the XXVI International Horticultural Congress: Key Processes in the Growth and Cropping of Deciduous Fruit and Nut Trees 636, Toronto, ON, Canada, 11–17 August 2002; pp. 425–441. [Google Scholar] [CrossRef]
- Prudent, M.; Dai, Z.W.; Génard, M.; Bertin, N.; Causse, M.; Vivin, P. Resource Competition Modulates the Seed Number–Fruit Size Relationship in a Genotype-Dependent Manner: A Modeling Approach in Grape and Tomato. Ecol. Model. 2014, 290, 54–64. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Farinelli, D.; Cruz-Castillo, J.G.; Marocchi, F.; Mastroleo, M.; Moscatello, S.; Proietti, S.; Battistelli, A. Yield Affects Qualitative Kiwifruit Characteristics and Dry Matter Content May Be an Indicator of Both Quality and Storability. Sci. Hortic. 2012, 146, 124–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).