Abstract
Low light is an abiotic stress that has a significant impact on crop growth. However, the impact of low light on rapeseed yield has not been well documented. Our study aims to examine the potential effects of low light on the photosynthesis antioxidant capacity and yield composition of leaves by simulating low light environments. According to the study results, low light reduces key photosynthetic enzymes and sucrose synthase activity in rapeseed leaves, leading to a decrease in leaf carbohydrate accumulation. The reduced number of pods per plant and the number of pods per grain are the direct factors leading to the decrease in rapeseed grain yield, while the increase in grain weight compensates for some of the yield loss. In addition, low light increased the content of malondialdehyde in leaves and altered the activities of catalase (CAT) and superoxide dismutase (SOD). Low light inhibits photosynthesis in leaves, reduces leaf productivity, and leads to a decrease in yield. High-yielding varieties have stronger antioxidant capacity and lower production of malondialdehyde. By revealing the effects of low light on the photosynthesis and antioxidant capacity of rapeseed leaves, this studyprovide new insights into the composition of low light affecting rapeseed grain yield and explain significant guidance for the planting and management of different rapeseed varieties in low light areas.
1. Introduction
Light is one of the main energy source that promote plant growth and development. The intensity of light plays a crucial role in various physiological responses such as crop morphogenesis, flowering, and fruiting [1,2]. The growth of crops in natural environments is often influenced by different levels of light intensity. Compared to high light intensity, plants are more susceptible to light suppression under low light intensity [3]. Plants can adapt to different low light stress by changing their own morphology or physiological characteristics [4]. For example, increasing plant height to capture more light and provide more energy for photosynthesis [5].
In addition to affecting the morphological development of plants, low light can affect various physiological metabolic processes in plants, including photosynthetic characteristics and antioxidant activity, as well as carbon and nitrogen fixation [6]. Chlorophyll is an important photosynthetic pigment that participates in the absorption, transmission, and conversion of solar energy into electrochemical energy. Chlorophyll content directly affects photosynthetic efficiency and is an important indicator of photosynthetic capacity [7]. The N transfer in plants usually occurs in the form of amino acids, and the amino acid content in leaves has a significant impact on photosynthesis [8]. Amino acids can improve the ability of photosynthesis by triggering chlorophyll synthesis and enhancing plants’ carbon dioxide absorption [9]. A larger amount of amino acids distributed in the leaves would positively affect photosynthesis and the transport of sucrose.
However, little is known about the amino acid levels of the leaves of the rapeseed varieties in response to low light. ATP (adenosine triphosphate) in leaves plays a crucial role in photosynthesis. Meanwhile, amino acids in leaves are the fundamental units for protein synthesis, which requires the consumption of ATP. ATP is a product of the light reaction stage of photosynthesis and also a key energy source for the dark reaction stage [10]. Photosynthesis is divided into two stages (the photoreaction and dark reaction stage). The photoreaction stage mainly occurs on the thylakoid membrane of chloroplasts, where ATP and NADPH are generated through the absorption and conversion of light energy. In the dark reaction stage, ATP and NADPH generated by light reactions are used to fix CO2 and convert it into organic matter [11]. Therefore, the levels of ATP and NADPH have a significant impact on the accumulation of photosynthetic products. Rubisco engages in catalyzing the reaction with carbon dioxide and ribulose 1,5-diphosphate (RuBP), delivering carbon into autotrophic metabolism, which is the largest ATP and NADPH demand pathway in illuminated leaves. This means that the activity of rubisco largely determines the total ATP and NADPH requirements of chloroplasts and even the entire cell [12]. For a long time, simulating low-light environmental conditions to study the impact of reduced solar radiation on crop production has been widely used [13,14]. Numerous studies have shown that low light directly hinders photosynthesis in leaves, reduces crop canopy photosynthetic capacity, and leads to a decrease in the accumulation of photosynthetic compounds [15,16]. The major enzymes controlling photosynthetic carbon metabolism and efficiency are fructose 1, 6-bisphosphatase (FBP), and ribulose 1, 5-bisphosphate carboxylase/oxygenase (rubisco) [17]. Low light has a negative impact on rubisco activity, which may impair the production of ATP, thereby affecting the accumulation of photosynthetic products [18].
Photosynthetic antioxidant metabolism can well protect plants from abiotic stresses (high temperature, drought, cold damage, flooding, etc.) [19]. Malondialdehyde (MDA) is an important metabolite of oxygen-free radicals (OFRs) in organisms, which can reflect the degree of tissue peroxidation well. Plants can be easily damaged in adverse conditions, leading to membrane lipid peroxidation. The process is accompanied by an enhanced level of MDA as the final decomposition product, and the level denotes the plant damage degree caused by adversity [20]. Studies on purple cabbage and wheat have shown that low light alters antioxidant enzyme activity and increases MDA content [21,22]. An analysis of membrane lipid peroxidation in peach fruit showed that low light reduced the activities of catalase (CAT) but increased MDA content [23]. Nevertheless, the fundamental physiological mechanisms by which low-light stress affects the antioxidant capacity of rapeseed leaves have not been comprehensively studied. At present, there have been many research reports on the effects of abiotic stress (such as drought, cold damage, and high temperature) on the yield and quality of rapeseed [24]. Therefore, we assume that low light will affect yield by impairing the photosynthetic and antioxidant capacity of rapeseed leaves, and a two-year field experiment was conducted for validation. Three objectives are proposed here: (1) evaluate the effect of low light on the photosynthetic capacity of rapeseed leaves, (2) determine the antioxidant activity of low light on leaves, and (3) determine the impact of low light on the composition factors of rapeseed yield.
2. Materials and Methods
2.1. Experimental Site
The 2021–2023 field experiment was carried out at the rapeseed production base in Qiquan Town, Chongzhou City, China. This region features a subtropical humid monsoon climate, with an annual average temperature of 16.2 °C, an average sunshine hour of 995.9 h, and an annual precipitation of 1011.6 mm. The basic soil nutrients in the cultivation layer (0–20 cm) of the experimental base were pH 7.8, 23.6 g/kg of organic matter, 1.32 g/kg of total nitrogen, 204.5 mg/kg of available potassium, and 11.9 mg/kg of available phosphorus. The meteorological data during the two-year field experiment are shown in Figure 1.
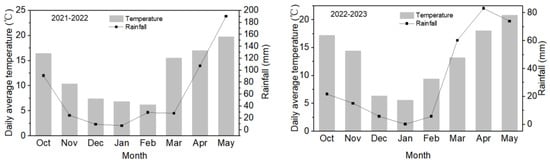
Figure 1.
Monthly temperature and rainfall during the growing season of rapeseed.
2.2. Experimental Design
Two cultivars (JYJS01 and CYZ1) were selected for this experiment. Each field experimental plot was 3 m × 6 m, including three replicates. The manual seed sowing method was employed, maintaining 20 cm plant–plant and 33 cm row–row distances. Two seedlings in each hole were maintained to meet the planting density of 300,000 plants ha−1. After sowing, 500 kg ha−1 of N, P, and K compound fertilizer (N: P2O5: K2O = 15%: 15%: 15%), 20 kgha−1 of borax as a basal fertilizer, and 120 kgha−1 of urea (urea, 46% N) at the five-leaf stage was applied. Nylon sunshade net (light reduction rate of 30%) was used for low-light treatment. The sunshade net was set 3 m above the ground in the plots and supported by steel pipes of uniform height around it. After sowing, herbicide was sprayed to control weeds. After maturity, crop was harvested manually to obtain the grain yield.
2.3. Sampling and Measurements
2.3.1. Chlorophyll Assessment
At the anthesis stage, leaves were collected to measure chlorophyll content. A mixture of anhydrous ethanol and acetone in a 1:1 ratio was used to extract for 24 h in the dark. A UV visible spectrophotometer (Mapada 722, Shanghai, China) was employed for measuring the absorbance at 645 nm and 663 nm, and the Lichtenthaler method was used for confirming the total chlorophyll content [25,26].
2.3.2. Activity of Key Photosynthetic and Antioxidant Enzymes
Ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco), fructose 1,6 bisphosphate aldolase (FBA), fructose-1, 6-bisphosphatase (FBP), sucrose synthase (SS), catalase (CAT), superoxide dismutase (SOD), and sucrose phosphate synthase (SPS) activity were measured using a test kit, as per the producer’s protocol (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China) [27].
2.3.3. Carbohydrate Content
The previously described methods were adopted for determining the sucrose, fructose, and glucose contents [28].
2.3.4. Amino Acid and Malondialdehyde Content
A test kit (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China) was used to determine the content of amino acids, proline, and malondialdehyde (MDA), following the procedure according to the manufacturer’s instructions [29].
2.3.5. ATP Content
High-performance liquid chromatography was used to determine the ATP content. We weighed 0.2 g of the sample, added 0.5 mL of 0.6 M perchloric acid solution, and grinded evenly. After that, extraction was performed with ultrasound for 1 h, as well as centrifugation at 8000× g for 10 min. Then, 0.5 mL of the supernatant was taken, and the pH was adjusted to neutral with NaOH solution, which was then filtered through a needle filter before testing [30].
2.4. Statistics Analysis
Data were collected and organized using Excel 2021 Analysis of variance (ANOVA) relied on the SPSS 21.0. The least significant difference (LSD) approach assisted in comparing the significance of distinctions between treatments, with a significance level of 0.05. Origin 2022 (Origin Lab Corp., Northampton, MA, USA) was used to draw the figures.
3. Results
3.1. Chlorophyll Content
Low light significantly increased the chlorophyll content in the leaves of JYJS01 and CYZ1, with the increase in JYJS01 being higher than in CYZ1 (Figure 2). Compared with CK, under low-light treatment, the contents of chlorophyll a, chlorophyll b, and the total chlorophyll of JYJS01 increased by 23.0%, 20.4%, and 21.9%, respectively, while CYZ1 increased by 24.5%, 25.2%, and 26.7%, respectively (based on two-year averages). Significant differences between varieties and treatment are show in Table 1.
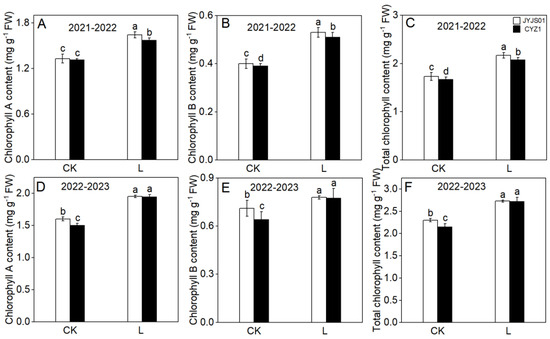
Figure 2.
The effect of low light on chlorophyll content. Chlorophyll A (A,D), chlorophyll B (B,E), total chlorophyll (C,F). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.

Table 1.
Variance table of leaf chlorophyll, LAI, and carbohydrate content.
3.2. Photosynthetic Enzymatic Activity
Low light significantly reduced the activity of rubisco, FBP, and FBA, in comparison of genotypes, JYJS01 showed higher content than CYZ1 (Figure 3). Compared with the CK, under low-light treatment, the rubisco, FBP, and FBA activities of JYJS01 decreased by 16.7, 68.2, and 13.1%, respectively, while those of CYZ1 decreased by 21.3, 69.2, and 19.8%, respectively. Significant differences between varieties and treatment are show in Table 2.
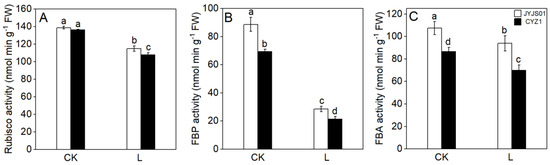
Figure 3.
The effect of low light on leaf photosynthetic enzymatic activity. Rubisco activity (A), FBP activity (B), FBA activity (C). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.

Table 2.
Variance table of rubisco, FBP, FBA, SS, and SPS activity.
3.3. Carbohydrate Content
Low-light treatment significantly reduced the leaf carbohydrate content of JYJS01 and CYZ1.JYJS01 genotype displayed higher content than CYZ1 (Figure 4). Compared with the CK, under low-light treatment, the glucose, fructose, and sucrose contents of JYJS01 decreased by 21.6, 27.1, and 20.0%, respectively, while CYZ1 decreased by 32.7, 28.4, and 41.4%, respectively (based on two-year averages). Significant differences between varieties and treatment are show in Table 1.
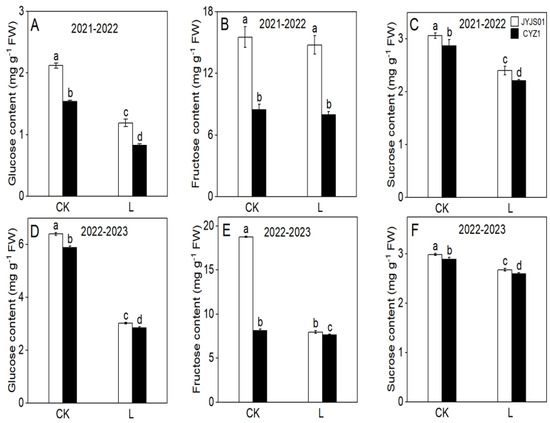
Figure 4.
The effect of low light on the carbohydrate content of leaves. Glucose content (A,D), fructose content (B,E), sucrose content (C,F). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.
3.4. Sucrose Synthase Activity
Low light significantly reduced the SS and SPS activities of JYJS01 and CYZ1 (Figure 5). Compared with the CK, JYJS01 presented decreases of 13.2 and 16.1% for SS and SPS activities, respectively, under low light, while the SS and SPS activities for CYZ1 decreased by 21.4 and 39.7%, respectively.
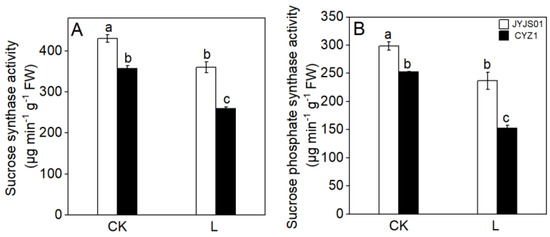
Figure 5.
The effect of low light on leaf sucrose synthase activity. Sucrose synthetase (A), sucrose phosphate synthase (B). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.
3.5. ATP, Amino Acid, and Proline Content
Low light has a significant impact on ATP, amino acid, and proline content, and there are significant differences between the two varieties (Figure 6). Compared with CK, under low-light treatment, the ATP, amino acid, and proline contents of JYJS01 decreased by 35.8, 12.5, and −37.8%, respectively, while CYZ1 decreased by 51.2, −12.3, and 13.7%, respectively. Under low-light conditions, JYJS01 presented higher ATP and proline contents, but lower amino acid content versus CYZ1. Significant differences between varieties and treatment are show in Table 3.
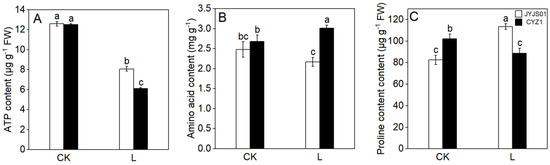
Figure 6.
The effect of low light on leaves in terms of ATP content (A), amino acid content (B), proline content (C). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.

Table 3.
Variance table of leaf ATP, amino acid, and proline content.
3.6. MDA Content CAT and SOD Activity
Although low light changed the MDA content of JYJS01 and CYZ1, there was no significant difference (Figure 7). Compared with the CK, the MDA content of JYJS01 decreased by 5.3% under low-light treatment, while that of CYZ1 increased by 13.3%. Low light altered the activity of CAT and SOD. Compared with the CK, the CAT and SOD activities of JYJS01 decreased by 4.5% and 45.5%, respectively, under low-light treatment, while the CAT activity of CYZ1 significantly decreased by 13.0%, and the SOD activity significantly increased by 33.6% under low-light treatment. Significant differences between varieties and treatment are show in Table 4.
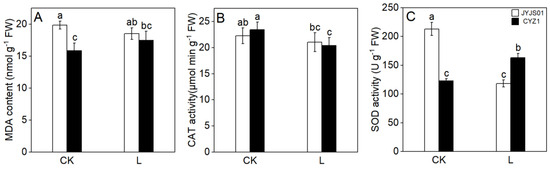
Figure 7.
The effect of low light on leaves in terms of MDA content (A), CAT activity (B), SOD activity (C). CK and L denote control and low light, respectively. Letters illustrate significant variations at the 0.05 level (LSD test) between treatments.

Table 4.
Variance table of leaf antioxidant indexes.
3.7. Yield Composition
Low light significantly reduced the number of pods per plant and seeds per pod for JYJS01 and CYZ1 (Table 5). Compared with the CK, JYJS01 showed a decrease of 26.7%, 23.2%, and 40.9% in the number of pods per plant, seed number, and yield, respectively, while CYZ1 showed a decrease of 42.0%, 32.0%, and 52.9%, respectively (based on the two-year average). JYJS01 showed a larger number of pods per plant and seed yield versus CYZ1. Low light significantly increased the thousand grain weight of JYJS01 and CYZ1. Compared with the CK, the thousand grain weight of JYJS01 and CYZ1 increased by 10.0% and 16.1%, respectively, under low-light treatment (Figure 8).

Table 5.
The effect of low light on the composition of rapeseed yield.
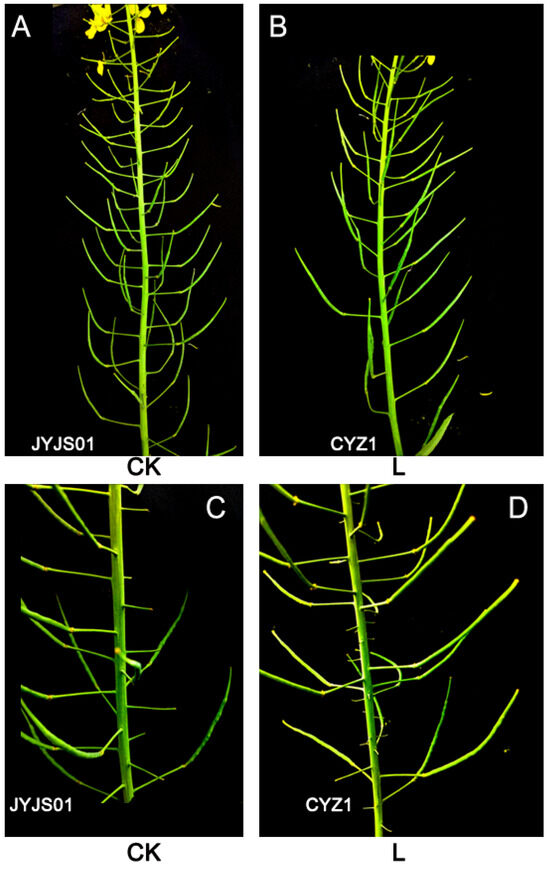
Figure 8.
The effect of low light on the development of rapeseed pod. JYJS01 (A,C), CYZ1 (B,D). CK and L denote control and low light, respectively.
3.8. Correlation Analysis
The correlation analysis showed that yield was significantly positively correlated with RuBP, FBP, FBA, SUC, SS, SPS, and ATP, while significantly negatively correlated with AA (Figure 9). In addition, CAT and SOD are significantly positively correlated with yield.
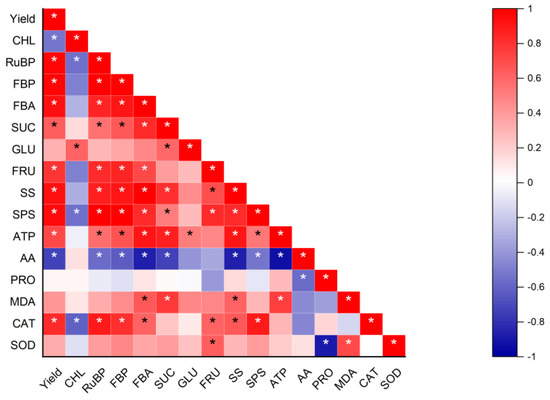
Figure 9.
Correlation analysis. Red and blue denote positive and negative correlations, respectively. CHL, chlorophyll; RuBP, ribulose-1,5-bisphosphate carboxylase; FBP, fructose 1,6-bisphosphatase; FBA, fructose 1,6 bisphosphate aldolase; SUC, sucrose; GLU, glucose; FRU, fructose; SS, sucrose synthase; SPS, sucrose phosphate synthase; ATP, adenosine triphosphate; AA, amino acid; PRO, proline; MDA, malondialdehyde; CAT, catalase; SOD, superoxide dismutase. * represents the significance level at p < 0.05.
4. Discussion
Chlorophyll plays a crucial role in the light reaction stage, and its content has a direct impact on the absorption and conversion efficiency of light energy, thereby affecting the entire process of photosynthesis [31]. In the current study, rapeseed leaves adapted to low-light conditions by increasing chlorophyll content. This reaction may be aimed at capturing more light energy, but this light energy is mainly converted to thermal energy consumption, leading to less light energy being available for photochemical reactions in the leaves, ultimately reducing the photosynthetic rate [32]. This response may also be from changes in chloroplasts, with anoectochilus roxburghii leaves grown in light shade containing more thylakoid. The chloroplast, grana, and stroma lamella of leaves are well developed, so they have higher chlorophyll content [33]. The loss of rubisco and FBP activities is thought to be a rapid crop response to shade stress and is directly related to the net photosynthetic rate under changing conditions [34]. The decrease in FBA activity will lead to a reduction in RuBP content, thereby inhibiting the photosynthetic rate [35]. Under low light, the photochemical efficiency of chloroplasts can be enhanced by biochemical regulation, but the light dependent supply of RuBP is inadequate for saturating the rubisco catalytic site, thereby reducing rubisco activity by decarboxylation and/or phosphate sugar inhibition [36,37]. Reduced rubisco activity can affect carbon assimilation and RuBP regeneration in the Calvin cycle [38]. Shade also reduces the ribulose-1,5-bisphosphate carboxylase/oxygenase activase (RAC) and down-regulates the transcript levels of most genes involved in the Calvin cycle (fructose-1,6-bisphosphatase, transketolase, and phosphoglycerate kinase), which in turn leads to a reduction in the CO2 assimilation rate and photosynthetic capacity of plants under low light [39,40]. It was observed in this study that low light altered the amino acid and proline content in the leaves of two varieties. However, JYJS01 and CYZ1 exhibited different response patterns, which may be related to their different low-light tolerance abilities. Previous research has reported that light intensity can affect amino acid content, such as in tea and ferns, where low light levels have been found to increase the amino acid content in their leaves [41,42]. A possible reason is that the photosynthetic capacity of leaves decreases under low light, leading to a decrease in the carbohydrate synthesis rate and a relative increase in amino acid content [43]. Amino acids are the main components of proteins and important components of living organisms. Usually, in situations where the carbohydrate supply is insufficient, plant cells can utilize the products of protein degradation, free amino acids, to further break down metabolism and produce energy (ATP) [44,45]. Previous studies have also shown that abiotic stress increases the transcription level of plant amino acid-degrading genes [46]. In addition, plants have also exhibited an increase in amino acid content under salt, drought, and high-temperature stress [47]. In the study of Azolla species, it was found that proline content significantly increased under salt stress [48]. The results of this study indicate that under low-light conditions, rapeseed may enhance the energy supply by increasing the accumulation of amino acids to support its growth and development. Therefore, amino acids can serve as important indicators for rapeseed to respond to low light stress.
A direct indicator of the efficiency of photosynthesis is the carbohydrate concentration. In the Calvin cycle, leaves utilize ATP and NADPH produced during photoreaction to fix carbon dioxide and generate carbohydrates. ATP can also be used to synthesize other organic compounds such as fats and proteins [49]. ATP plays a crucial role in photosynthesis. It not only forms during the light reaction stage but also provides the required energy and reducing force for the dark reaction stage [50]. Low light reduces ATP biosynthesis in leaves, thereby weakening their ability to fix carbon dioxide. This indicates that low light reduces the photosynthetic rate during the light response stage, leading to a decrease in ATP levels, which in turn cannot provide sufficient energy for plants to convert carbon dioxide into organic matter during the dark response stage. Additionally, studies have reported that low light levels lead to a decrease in ATP levels by reducing ATP synthase [51]. Previous studies have reported reduced sucrose content in leaves under low-light conditions in cloudy weather [52]. In the current study, low light also diminished the carbohydrate content of rapeseed leaves. The SS and SPS activities are crucial for regulating the synthesis of sucrose. Under low-light conditions, the SS and SPS activities were significantly reduced, resulting in reduced leaf sucrose accumulation. This indicates that low light makes photosynthetic products less accumulated in rapeseed leaves by decreasing sucrose synthase activity.
The increase in MDA content indicates that plant cells are subjected to excessive oxidative stress. This oxidative pressure can damage the membrane structure of plant cells and affect the energy conversion efficiency in photosynthesis [53]. Previous studies have shown a negative correlation between MDA content and the photosynthetic rate, which can affect the fixation process of carbon dioxide by reducing the activity of phosphoenolpyruvate carboxylase (PEPC) [54]. In addition, malondialdehyde can accelerate the degradation of chlorophyll and reduce the absorption and utilization of light energy, thereby inhibiting photosynthesis [55]. The increase in the MDA content of CYZ1 under low light indicates greater exposure to oxidative stress. Therefore, the weakened photosynthetic capacity of rapeseed leaves under low-light conditions is related to increased MDA content. The activities of CAT and SOD are closely related to the content of MDA. In oxidative stress response, the activity of SOD directly affects the production of MDA [56]. Low light reduced the SOD activity of JYJS01 but increased the SOD activity of CYZ1. On the one hand, this might be attributed to the differences in the response patterns of various rapeseed varieties to low-light pressure. On the other hand, it might be because of the lower antioxidant capacity of CYZ1, which accumulates more MDA under low light. Therefore, increasing SOD activity can eliminate superoxide anion radicals to reduce the likelihood of lipid peroxidation [57].
Several previous field experiments have reached a consistent conclusion that low-light conditions can cause a loss rate of over 50% in rapeseed seed yield [58,59], with the key factor being the reduction in the number of pods. This conforms to the results of this study. But in a field experiment in Chile, it was found that low-light treatment (reducing light by 70%) throughout the flowering period had no substantial effect on rapeseed grain yield, as the grains would compensate for the yield loss resulting from the fewer pods by doubling their weight [60]. Similar results were observed in this study, where the thousand grain weight of both varieties was significantly higher than that of the control under low-light treatment. However, the increase in grain weight did not fully compensate for the decrease in yield, indicating that rapeseed seeds have high plasticity. The specific physiological mechanism by which low light reduces the number of pods is still unclear, and some studies suggest that this is due to a shortage of assimilates encountered during the early stages of flower development and opening. Due to an insufficient supply of assimilates, the ovary may have fewer fertilized ovules and fewer cells that can expand in the husk [61].
5. Conclusions
Low light has a negative impact on the photosynthesis of rapeseed leaves, as it reduces their photosynthetic capacity and leads to a decrease in photoassimilates accumulation. The decrease in the number of pods is the main reason in reduction of rapeseed yield under low-light conditions, while the increase in seed weight compensates for some of the yield loss. Low light stimulated the antioxidant system of rapeseed leaves, increased MDA content, and altered CAT and SOD enzyme activities. The high-yield variety JYJS01 showed a stronger antioxidant capacity under low light, which is one of the physiological basis for low-light stress tolerance. In future research, further investigation should be conducted on the molecular- physiological mechanisms underlying the reduction in the number of pods caused by low light. Our findings will offer a foundation for future research to understand the impact of low light on the formation of rapeseed yield from new perspectives.
Author Contributions
Conceptualization: Y.W. and Y.H.; methodology: Y.H., Y.W. and H.H.J.; formal analysis and investigation: Y.H., L.L., Y.L. (Yalong Liu), X.Y., F.X., Y.L. (Ying Liu), X.P. and H.H.J.; writing—original draft preparation: Y.H.; writing—review and editing: H.H.J.; supervision Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
Sichuan Rapeseed Innovation Team of National Modern Agricultural Industrial Technology System (SCCXTD2024-03).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Xiao, L.; Shibuya, T.; Kato, K.; Nishiyama, M.; Kanayama, Y. Effects of light quality on plant development and fruit metabolism and their regulation by plant growth regulators in tomato. Sci. Hortic. 2022, 300, 111076. [Google Scholar] [CrossRef]
- Wang, N.; Ji, T.; Liu, X.; Li, Q.; Sairebieli, K.; Wu, P.; Song, H.; Wang, H.; Du, N.; Zheng, P. Defoliation significantly suppressed plant growth under low light conditions in two leguminosae species. Front. Plant Sci. 2022, 12, 777328. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Zhang, S.; Sun, Y. Artificial photosynthesis systems for solar energy conversion and storage: Platforms and their realities. Chem. Soc. Rev. 2022, 51, 6704–6737. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Sharkey, T.D. The end game (s) of photosynthetic carbon metabolism. Plant Physiol. 2024, 195, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Han, Y. Promotion of carbon dioxide biofixation through metabolic and enzyme engineering. Catalysts 2022, 12, 399. [Google Scholar] [CrossRef]
- Gardeström, P.; Igamberdiev, A.U. The origin of cytosolic ATP in photosynthetic cells. Physiol. Plant. 2016, 157, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kotilainen, T.; Robson, T.M.; Hernandez, R. Light quality characterization under climate screens and shade nets for controlled-environment agriculture. PLoS ONE 2018, 13, e0199628. [Google Scholar] [CrossRef]
- Andrade, F.H.; Ferreiro, M.A. Reproductive growth of maize, sunflower and soybean at different source levels during grain filling. Field Crops Res. 1996, 48, 155–165. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Atmospheric drought and low light impede mycorrhizal effects on leaf photosynthesis—A glasshouse study on tomato under naturally fluctuating environmental conditions. Mycorrhiza 2019, 29, 13–28. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Xie, Y.; Wang, B.; Kuai, J.; Zhou, G. An improvement in oilseed rape (Brassica napus L.) productivity through optimization of rice-straw quantity and plant density. Field Crop. Res. 2021, 273, 108290. [Google Scholar] [CrossRef]
- An, J.; Wei, X.; Huo, H. Transcriptome analysis reveals the accelerated expression of genes related to photosynthesis and chlorophyll biosynthesis contribution to shade-tolerant in Phoebe bournei. BMC Plant Biol. 2022, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv. Space Res. 2014, 53, 1557–1566. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Javed, H.H.; Hu, Y.; Raza, A.; Alabdallah, N.M.; Asghar, M.A.; Khan, K.S.; Peng, X.; Ghafoor, A.Z.; Ullah, A.; Wu, Y.C. Low light at specific growth stage affects photoassimilates transportation, seed quality and yield in Brassica napus L. J. Agron. Crop Sci. 2024, 210, e12735. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, L.Y.; He, Y.Y.; Hu, J.P.; Hu, G.W.; Zhu, Y.; Khan, A.; Xiong, Y.C.; Zhang, J.L. Potential roles of iron nanomaterials in enhancing growth and nitrogen fixation and modulating rhizomicrobiome in alfalfa (Medicago sativa L.). Bioresour. Technol. 2024, 391, 129987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qu, W.J. The Experimental Guide for Plant Physiology; Higher Education Press: Beijing, China, 2003. [Google Scholar]
- Chen, H.; Jin, Z.; Huang, R.; He, L.; Tian, W.; Zhao, L.; Zhang, Z. Promotion of growth of alfalfa by Erwinia persicina Cp2 exopolysaccharides under NaCl stress. Agronomy 2023, 13, 2129. [Google Scholar] [CrossRef]
- Cheng, B.; Zhou, M.; Tang, T.; Hassan, M.J.; Zhou, J.; Tan, M.; Li, Z.; Peng, Y. A Trifolium repens flavodoxin-like quinone reductase 1 (TrFQR1) improves plant adaptability to high temperature associated with oxidative homeostasis and lipids remodeling. Plant J. 2023, 115, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Servaites, J.C.; Parry, M.A.; Gutteridge, S.; Keys, A.J. Species variation in the predawn inhibition of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986, 82, 1161–1163. [Google Scholar] [CrossRef]
- Hu, L.; Liao, W.; Dawuda, M.M.; Yu, J.; Lv, J. Appropriate NH4+: NO3− ratio improves low light tolerance of mini Chinese cabbage seedlings. BMC Plant Biol. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Seemann, J.R.; Kirschbaum, M.U.; Sharkey, T.D.; Pearcy, R.W. Regulation of ribulose-1, 5-bisphosphate carboxylase activity in Alocasia macrorrhiza in response to step changes in irradiance. Plant Physiol. 1988, 88, 148–152. [Google Scholar] [CrossRef]
- Ernstsen, J.; Woodrow, I.E.; Mott, K.A. Responses of Rubisco activation and deactivation rates to variations in growth-light conditions. Photosyn. Res. 1997, 52, 117–125. [Google Scholar] [CrossRef]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated Rubisco gene expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Tang, W.; Guo, H.; Baskin, C.C.; Xiong, W.; Yang, C.; Li, Z.; Song, H.; Wang, T.; Yin, J.; Wu, X. Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa) seedlings. Plants 2022, 11, 1688. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cun, Z.; Chen, J.W. Photosynthetic performance and photosynthesis-related gene expression coordinated in a shade-tolerant species Panax notoginseng under nitrogen regimes. BMC Plant Biol. 2020, 20, 273. [Google Scholar] [CrossRef]
- Lazár, D.; Stirbet, A.; Björn, L.O.; Govindjee, G. Light quality, oxygenic photosynthesis and more. Photosynthetica 2022, 60, 25. [Google Scholar] [CrossRef]
- Mu, H.R.; Jiang, D.; Dai, T.B.; Jing, Q.; Cao, W.X. Effect of shading on photosynthesis and chlorophyll fluorescence characters in wheat flag leaves. Sci. Agric. Sin. 2008, 41, 599–606. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, H.; Guo, H.; Zhou, A.; Huang, Y.; Sun, Y.; Li, M. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS ONE 2014, 9, e85996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, e8354. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Li, J.; Zhou, X.; Xiao, Y.; Liao, Y.; Tang, J.; Dong, F.; Zeng, L. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Yu, M.; Xiao, H.; Wang, H.; Xie, H.; Zeng, X. Influences of silicon on content of soluble sugars, amino acids in Turf-grasses under shading stress. J. Huazhong Agric. Univ. 2010, 29, 317–320. [Google Scholar] [CrossRef]
- Zhu, X.; Galili, G. Lysine metabolism is concurrently regulated by synthesis and catabolism in both reproductive and vegetative tissues. Plant Physiol. 2004, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 2014, 5, 447. [Google Scholar] [CrossRef]
- Less, H.; Galili, G. Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol. 2008, 147, 316–330. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- van Kempen, M.M.; Smolders, A.J.; Bögemann, G.M.; Lamers, L.L.; Visser, E.J.; Roelofs, J.G. Responses of the Azolla filiculoides Stras.–Anabaena azollae Lam. association to elevated sodium chloride concentrations: Amino acids as indicators for salt stress and tipping point. Aquat. Bot. 2013, 106, 20–28. [Google Scholar] [CrossRef]
- Yu, A.; Xie, Y.; Pan, X.; Zhang, H.; Cao, P.; Su, X.; Chang, W.; Li, M. Photosynthetic phosphoribulokinase structures: Enzymatic mechanisms and the redox regulation of the Calvin-Benson-Bassham cycle. Plant Cell 2020, 32, 1556–1573. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef] [PubMed]
- Rott, M.; Martins, N.F.; Thiele, W.; Lein, W.; Bock, R.; Kramer, D.M.; Schöttler, M.A. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 2011, 23, 304–321. [Google Scholar] [CrossRef]
- Franklin, K.A. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z. Primary, secondary metabolites, H2O2, malondialdehyde and photosynthetic responses of Orthosiphon stimaneus Benth. to different irradiance levels. Molecules 2012, 17, 1159–1176. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Xu, B.; Li, J.; Huang, B. Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 2016, 7, 1500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.; Nan, S.; Li, Y.; Hu, J.; Zhao, K.; Guo, J.; Wang, S. PagSOD2a improves poplar salt tolerance by elevating superoxide dismutase activity and decreasing malondialdehyde contents. Front. Plant Sci. 2024, 15, 1456249. [Google Scholar] [CrossRef]
- Jie, Z.; Liu, J.; Shu, M.; Ying, Y.; Yang, H. Detection strategies for superoxide anion: A review. Talanta 2022, 236, 122892. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The critical period for yield and quality determination in canola (Brassica napus L.). Field Crop. Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Rondanini, D.P.; del Pilar Vilariño, M.; Roberts, M.E.; Polosa, M.A.; Botto, J.F. Physiological responses of spring rapeseed (Brassica napus) to red/far-red ratios and irradiance during pre-and post-flowering stages. Physiol. Plant. 2014, 152, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Labra, M.H.; Struik, P.C.; Evers, J.B.; Calderini, D.F. Plasticity of seed weight compensates reductions in seed number of oilseed rape in response to shading at flowering. Eur. J. Agron. 2017, 84, 113–124. [Google Scholar] [CrossRef]
- Huang, Y.; Schnurbusch, T. The birth and death of floral organs in cereal crops. Annu. Rev. Plant Biol. 2024, 75, 427–458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).