Early Detection of Verticillium Wilt in Cotton by Using Hyperspectral Imaging Combined with Recurrence Plots
Abstract
1. Introduction
2. Materials and Methods
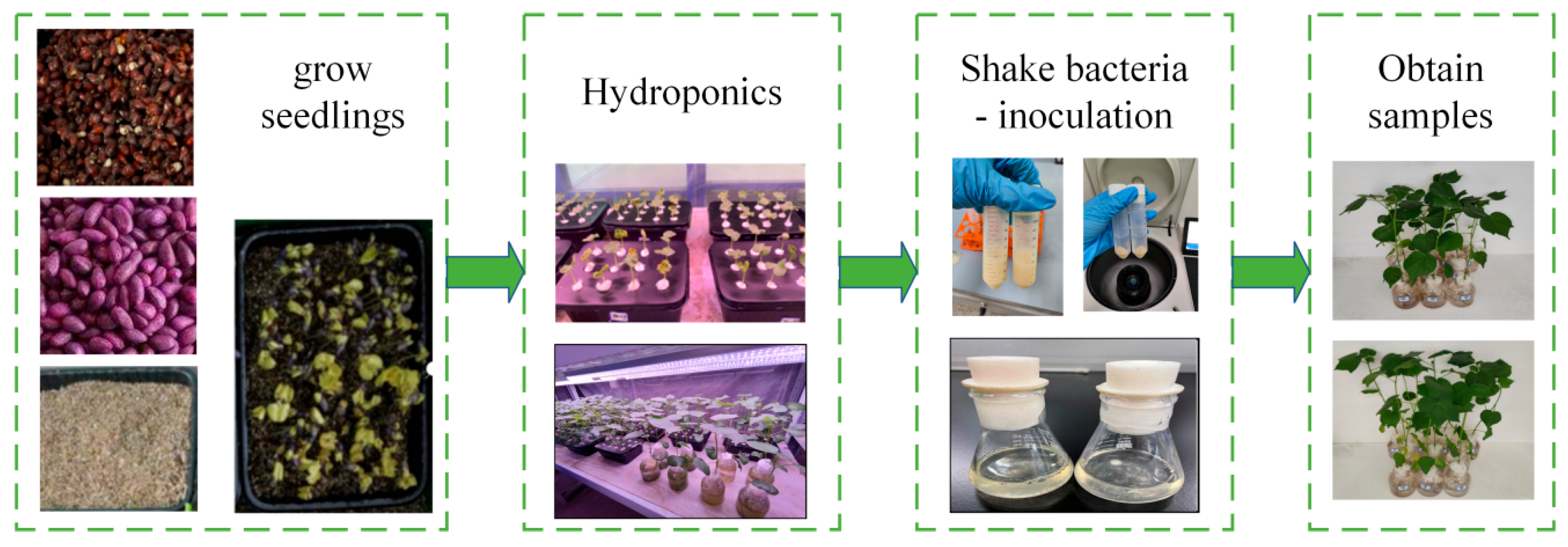
2.1. Experimental Sample Acquisition
2.2. Hyperspectral Image Acquisition and Preprocessing
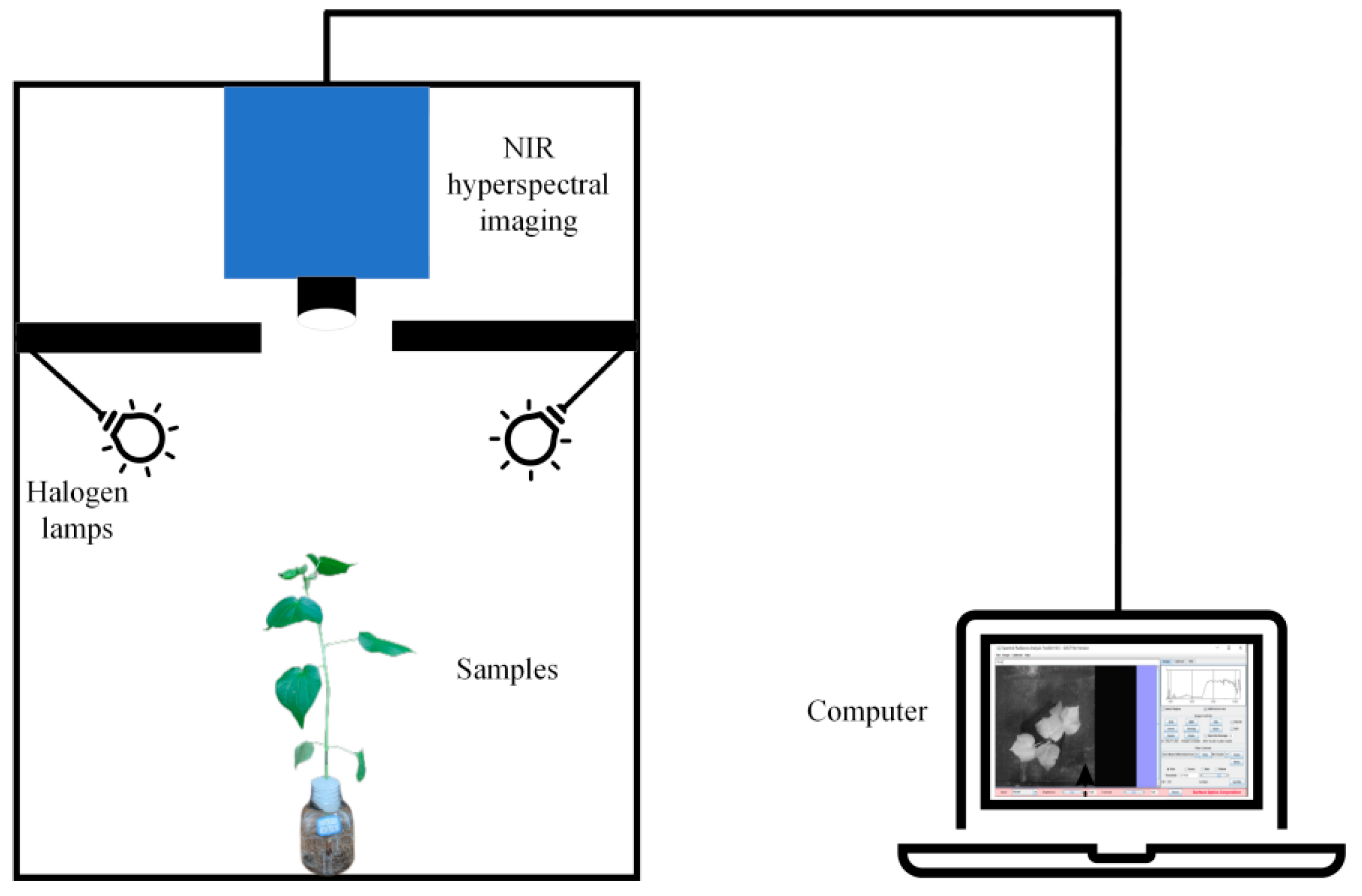
2.2.1. Hyperspectral Image Acquisition
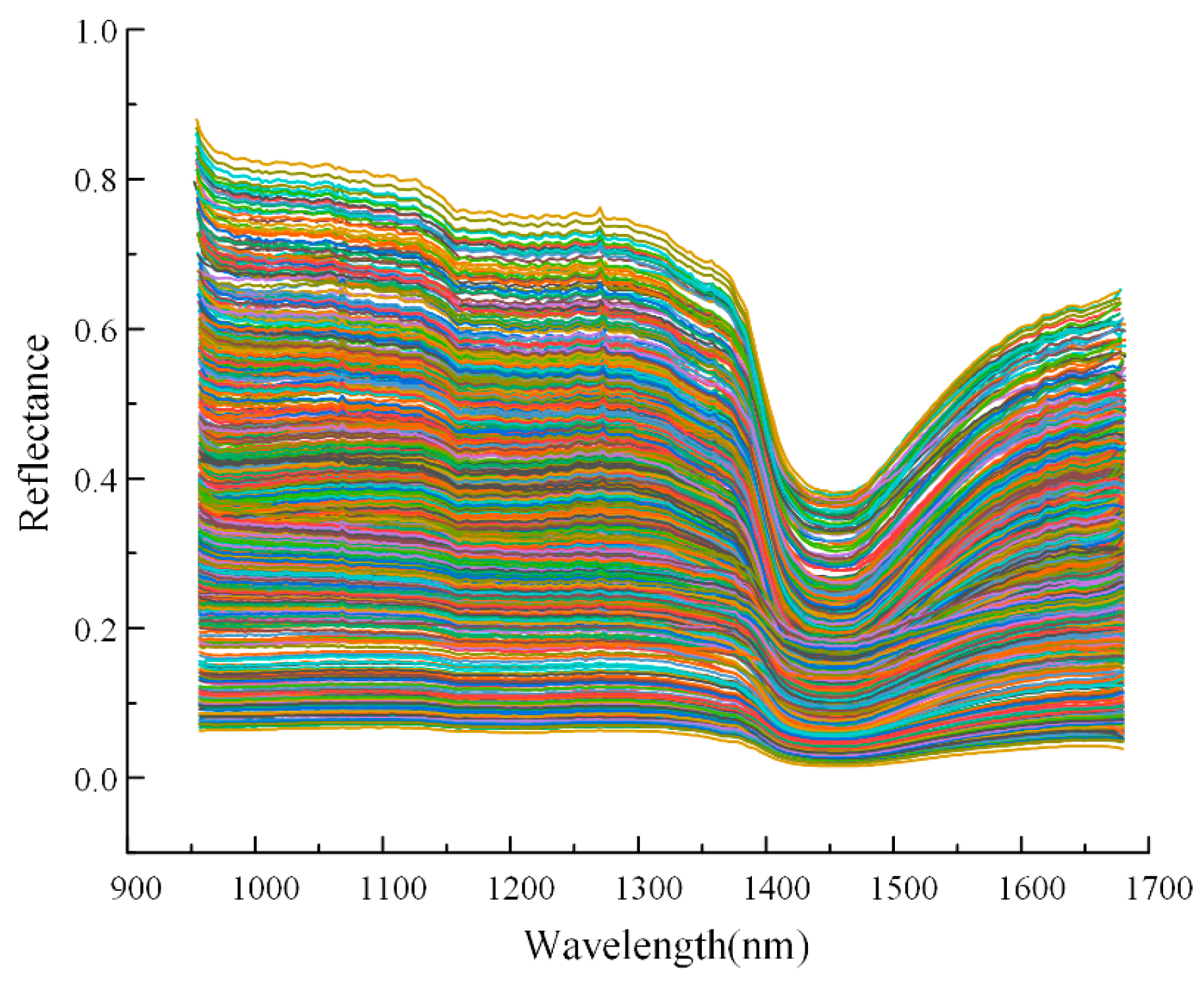
2.2.2. Hyperspectral Image Preprocessing
2.3. Recurrence Plots (RP)
2.4. Image Texture Feature
2.4.1. Image Texture Feature Extraction
2.4.2. Image Texture Feature Analysis
2.5. Model Building Method
2.5.1. K-Nearest Neighbors (KNNs)
2.5.2. Support Vector Machine (SVM)
2.5.3. Extreme Learning Machines (ELMs)
2.5.4. eXtreme Gradient Boosting (XGBoost)
2.6. Model Evaluation
3. Results and Discussion
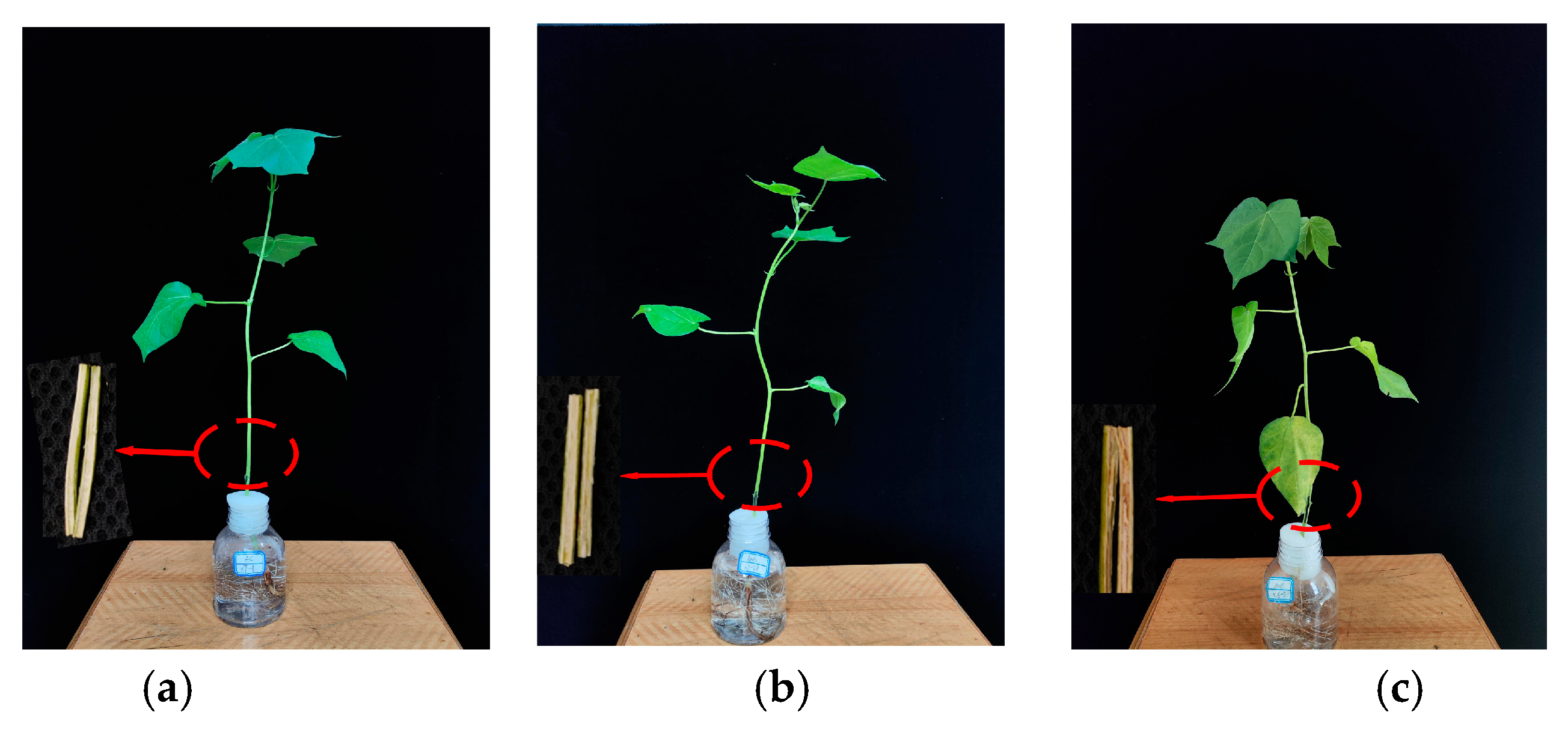
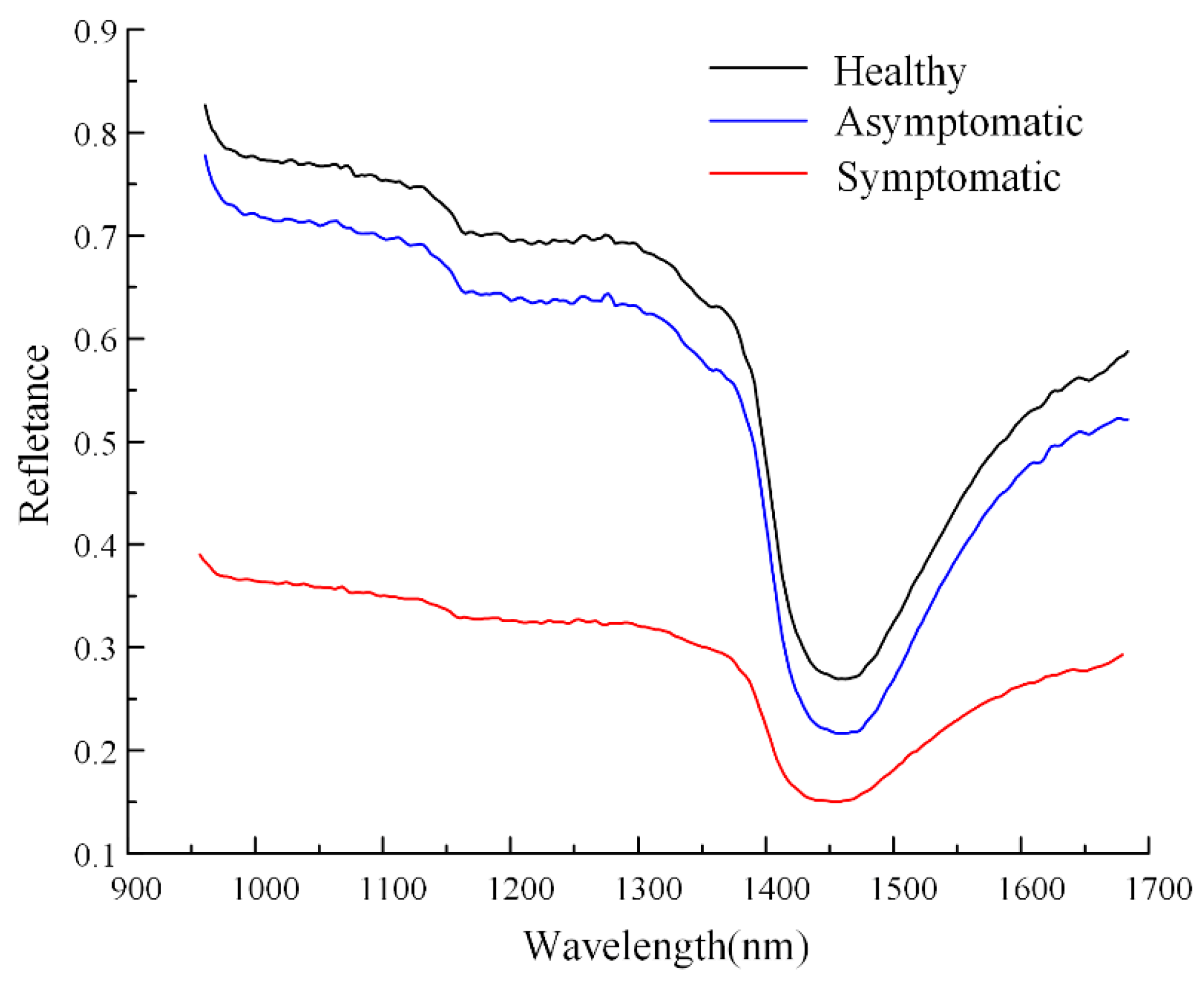
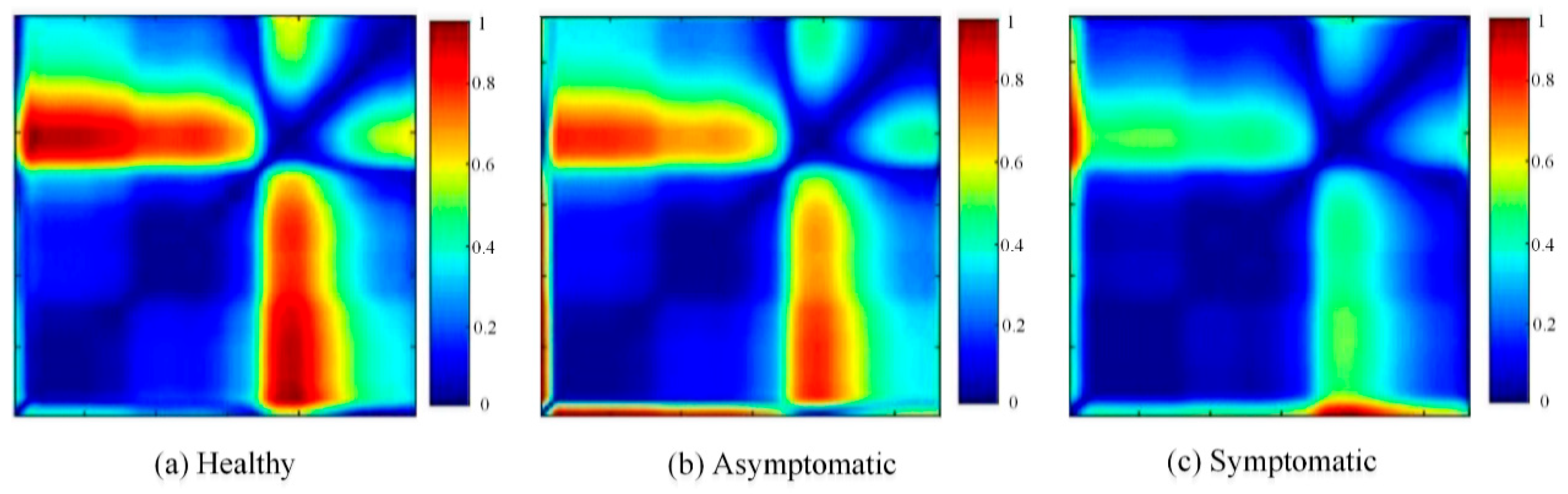
3.1. Different Infection States of Cotton Verticillium Wilt
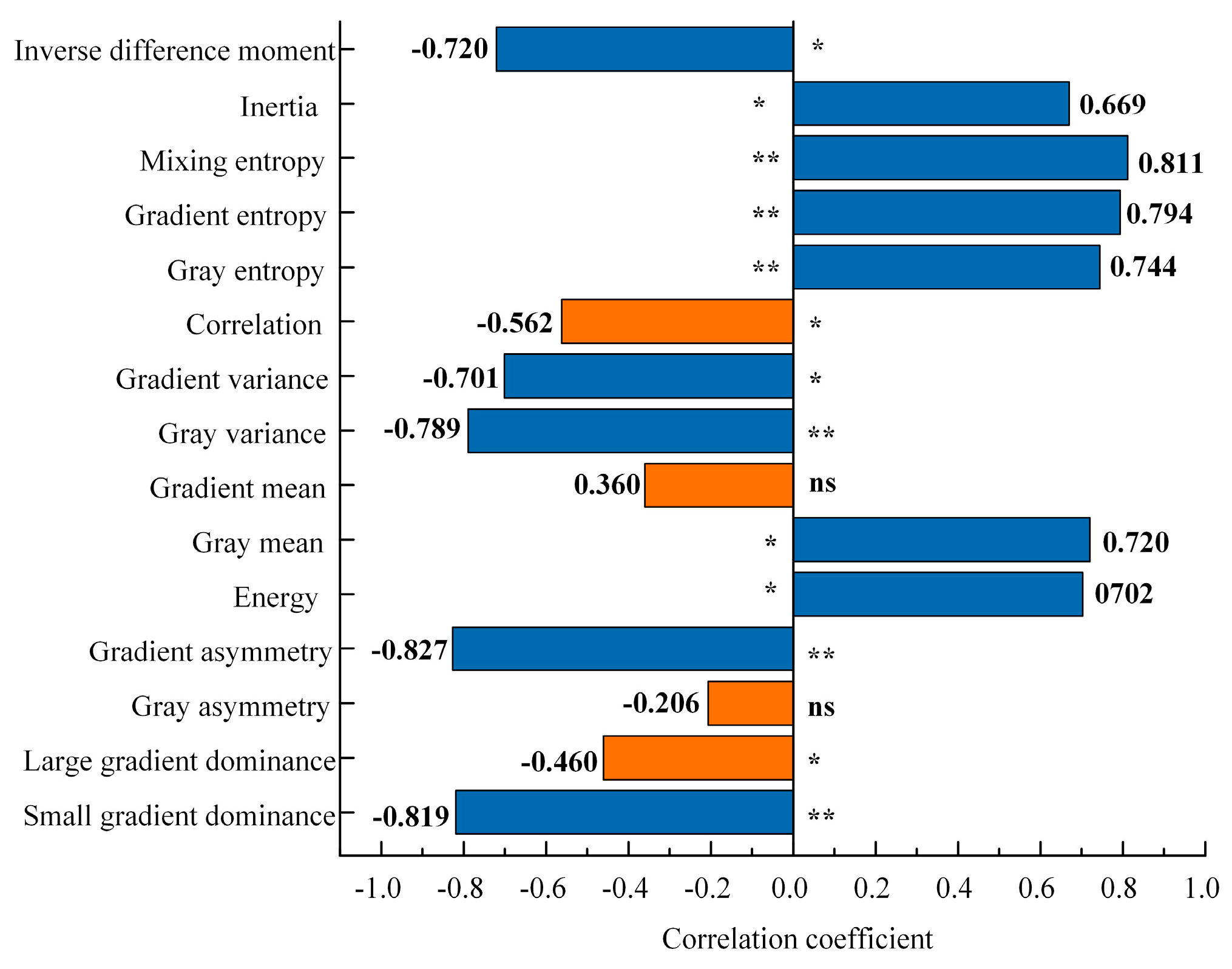
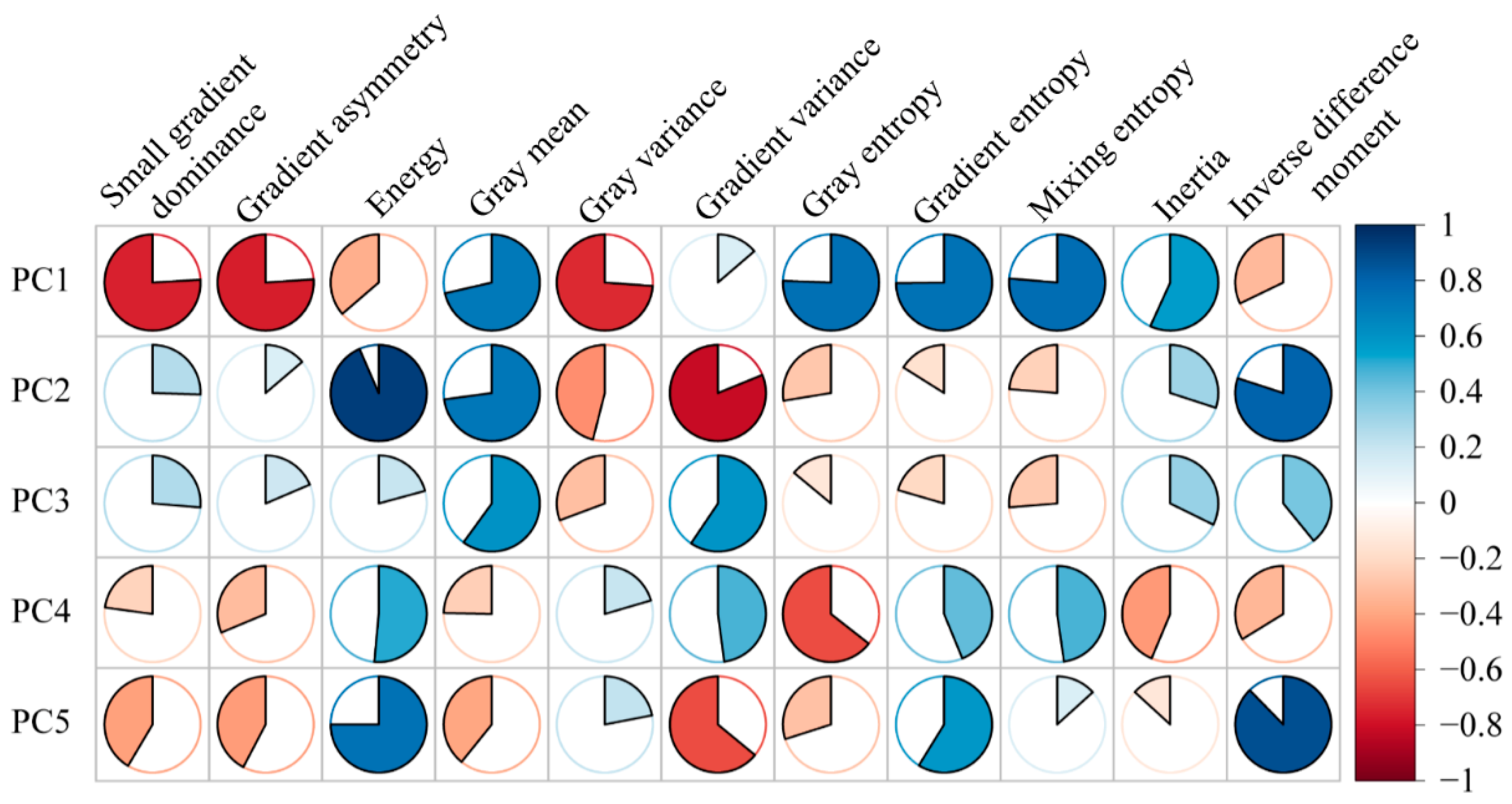
3.2. Correlation Between Texture Features
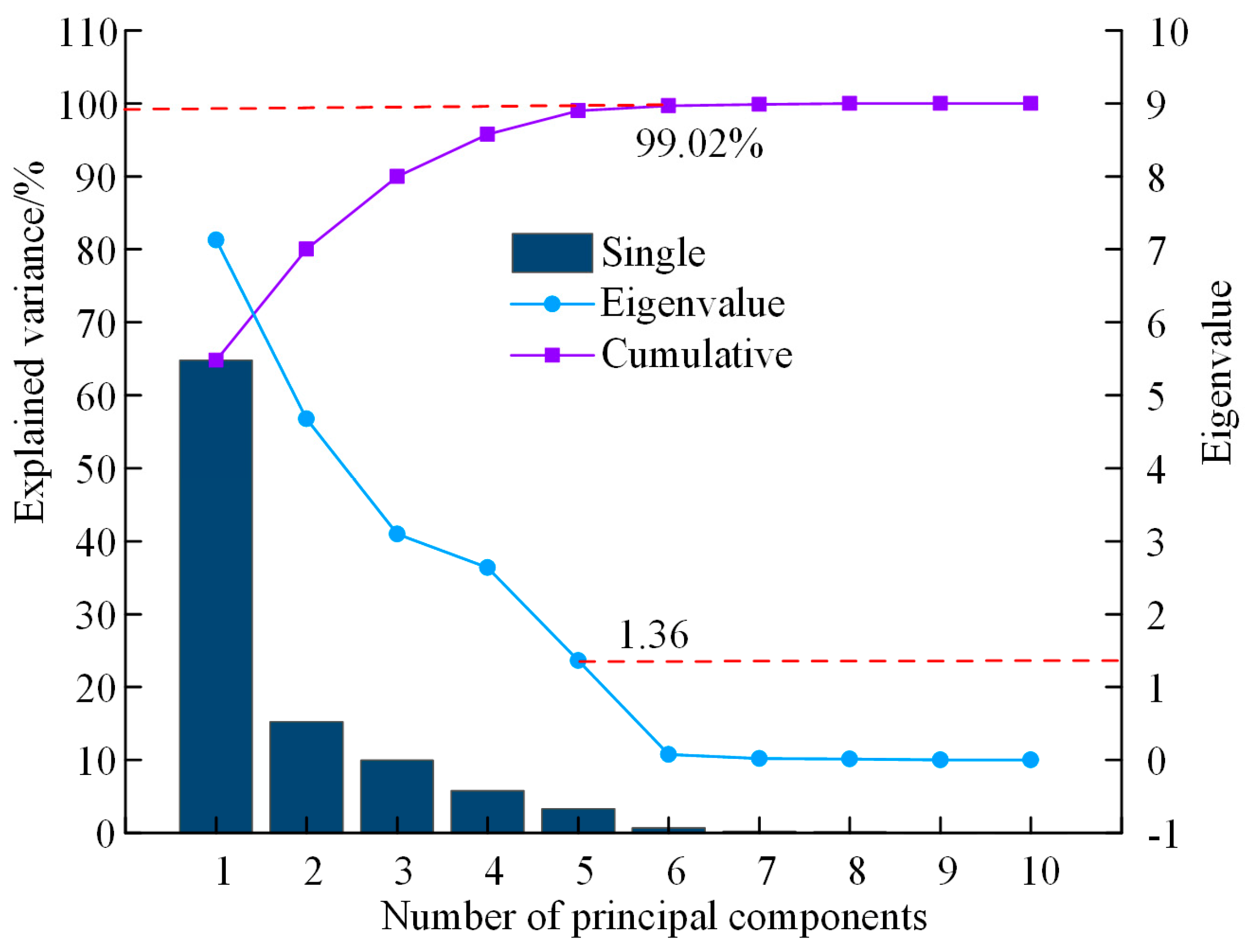
3.3. Determining the Principal Components That Describe the Texture Features
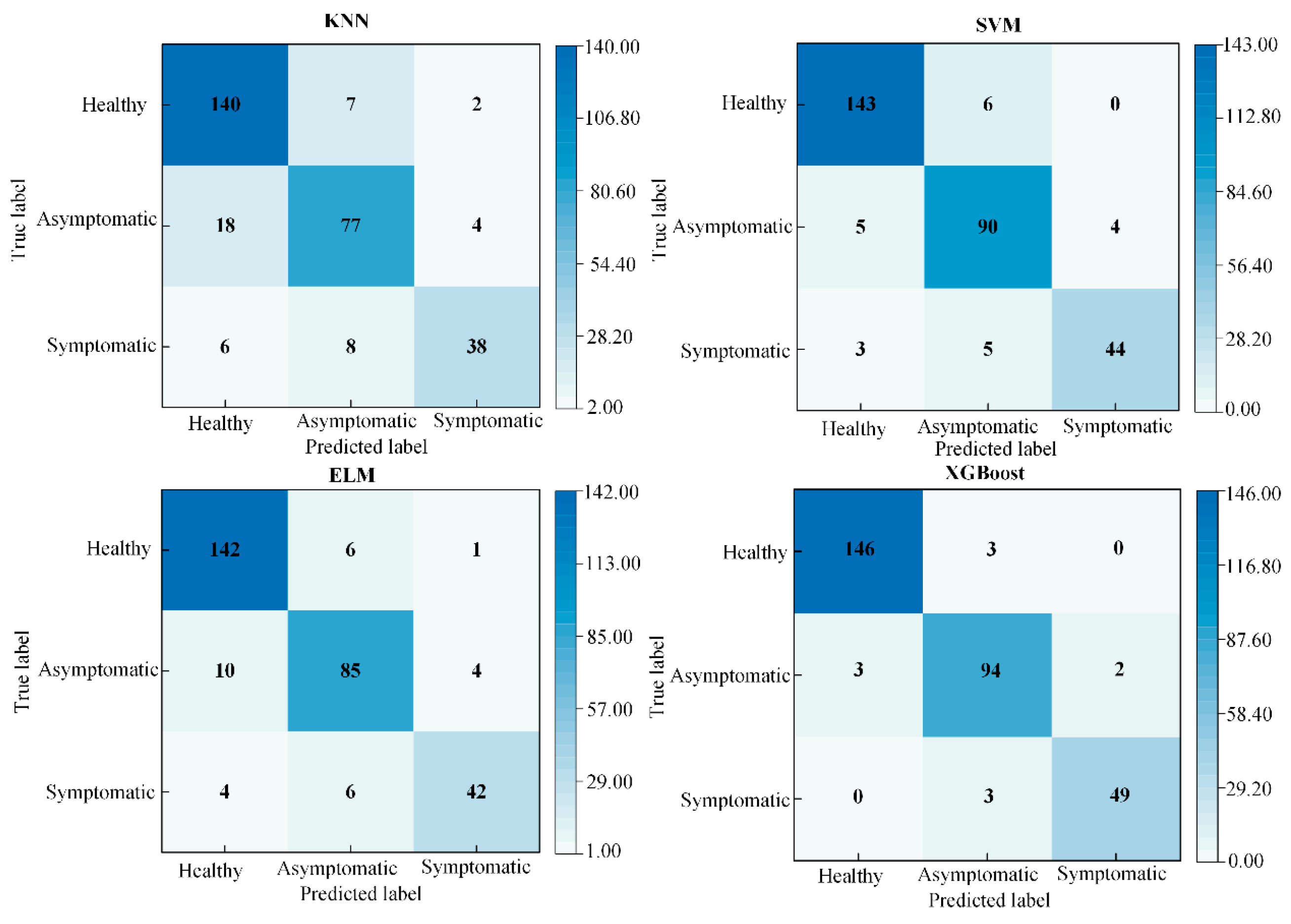
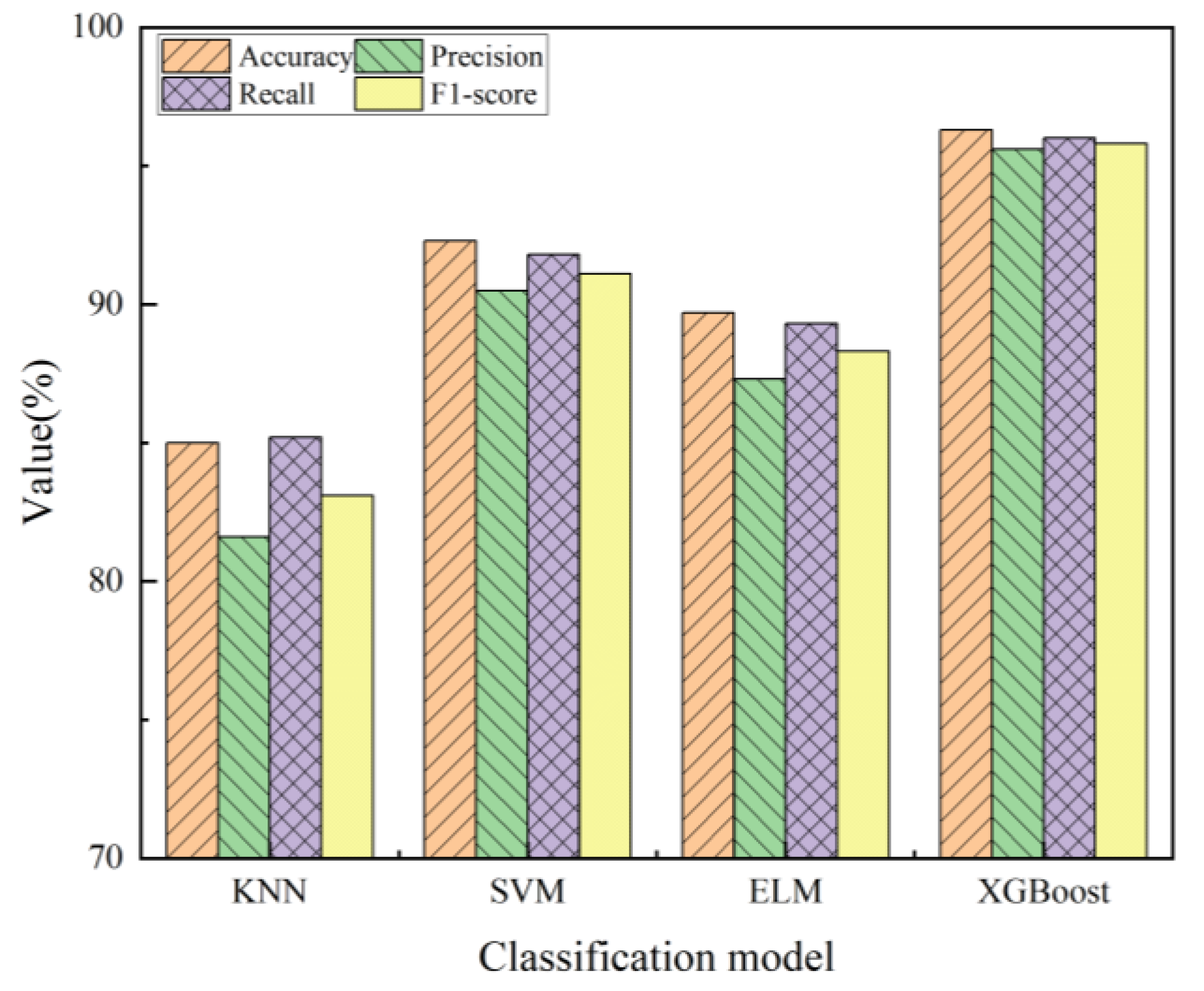
3.4. Comparison of Performance Between Different Classification Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardak, A.; Çelik, S.; Erdoğan, O.; Ekinci, R.; Dumlupinar, Z. Association Mapping of Verticillium wilt disease in a worldwide collection of cotton (Gossypium hirsutum L.). Plants 2021, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhang, H.; Gao, M.; Li, M.; Zhao, T.; Guan, X. Pseudomonas species isolated via high-throughput screening significantly protect cotton plants against verticillium wilt. AMB Express 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Xiao, H.L.; Gui, Y.J.; Zhang, D.D.; Li, L.; Bao, Y.M.; Dai, X.F. Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton-containing medium. Front. Microbiol. 2016, 7, 1709. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Early detection and quantification of Verticillium wilt in olive using hyperspectral and thermal imagery over large areas. Remote Sens. 2015, 7, 5584–5610. [Google Scholar] [CrossRef]
- Yuan, Y.; Feng, H.; Wang, L.; Li, Z.; Shi, Y.; Zhao, L.; Feng, Z.; Zhu, H. Potential of endophytic fungi isolated from cotton roots for biological control against verticillium wilt disease. PLoS ONE 2017, 12, e0170557. [Google Scholar] [CrossRef]
- Zahir, S.A.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Nguyen, C.; Sagan, V.; Maimaitiyiming, M.; Maimaitijiang, M.; Bhadra, S.; Kwasniewski, M.T. Early detection of plant viral disease using hyperspectral imaging and deep learning. Sensors 2021, 21, 742. [Google Scholar] [CrossRef]
- Shin, M.Y.; Viejo, C.G.; Tongson, E.; Wiechel, T.; Taylor, P.W.; Fuentes, S. Early detection of Verticillium wilt of potatoes using near-infrared spectroscopy and machine learning modeling. Comput. Electron. Agric. 2023, 204, 107567. [Google Scholar] [CrossRef]
- Jing, X.; Wang, J.; Song, X.; Xu, X.; Chen, B.; Huang, W. Continuous removal estimation method for the severity of cotton wilt disease. Trans. Chin. Soc. Agric. Eng. 2010, 26, 193–198. [Google Scholar] [CrossRef]
- Chen, B.; Wang, G.; Liu, J.; Ma, Z.; Wang, J.; Li, T. Extraction of Photosynthetic Parameters from Hyperspectral Diseases of Cotton Leaves. Spectrosc. Spectr. Anal. 2018, 38, 1834–1838. [Google Scholar]
- Zhang, N.; Zhang, X.; Shang, P.; Ma, R.; Yuan, X.; Li, L.; Bai, T. Detection of cotton verticillium wilt disease severity based on hyperspectrum and GWO-SVM. Remote Sens. 2023, 15, 3373. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, S.; Zhang, X.; Shi, Y.; Yang, W.; Zhu, L.; Huang, C. Intelligent identification on cotton verticillium wilt based on spectral and image feature fusion. Plant Methods 2023, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- Camino, C.; Calderón, R.; Parnell, S.; Dierkes, H.; Chemin, Y.; Román-Écija, M.; Montes-Borrego, M.; Landa, B.B.; Navas-Cortes, J.A.; Zarco-Tejada, P.J.; et al. Detection of Xylella fastidiosa in almond orchards by synergic use of an epidemic spread model and remotely sensed plant traits. Remote Sens. Environ. 2021, 260, 112420. [Google Scholar] [CrossRef]
- Dutta, A.; Tyagi, R.; Chattopadhyay, A.; Chatterjee, D.; Sarkar, A.; Lall, B.; Sharma, S. Early detection of wilt in Cajanus cajan using satellite hyperspectral images: Development and validation of disease-specific spectral index with integrated methodology. Comput. Electron. Agric. 2024, 219, 108784. [Google Scholar] [CrossRef]
- Riefolo, C.; Antelmi, I.; Castrignanò, A.; Ruggieri, S.; Galeone, C.; Belmonte, A.; Muolo, M.R.; Ranieri, N.A.; Labarile, R.; Gadaleta, G.; et al. Assessment of the hyperspectral data analysis as a tool to diagnose Xylella fastidiosa in the asymptomatic leaves of olive plants. Plants 2021, 10, 683. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, P.; Xu, H.; Martínez-Ortega, J.F.; Feng, J.; Zhai, Z. Detecting asymptomatic infections of rice bacterial leaf blight using hyperspectral imaging and 3-dimensional convolutional neural network with spectral dilated convolution. Front. Plant Sci. 2022, 13, 963170. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Yang, W.; Huang, L.; Zhang, J.; Peng, J.; Lan, Y. Severity Assessment of Cotton Canopy Verticillium Wilt by Machine Learning Based on Feature Selection and Optimization Algorithm Using UAV Hyperspectral Data. Remote Sens. 2024, 16, 4637. [Google Scholar] [CrossRef]
- He, Q.; McLellan, H.; Boevink, P.C.; Birch, P.R. All roads lead to susceptibility: The many modes of action of fungal and oomycete intracellular effectors. Plant Commun. 2020, 1, 12. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Trapero, C.; Alcántara, E.; Jiménez, J.; Amaro-Ventura, M.C.; Romero, J.; Koopmann, B.; Karlovsky, P.; Von Tiedemann, A.; Pérez-Rodríguez, M.; López-Escudero, F.J. Starch hydrolysis and vessel occlusion related to wilt symptoms in olive stems of susceptible cultivars infected by Verticillium dahliae. Front. Plant Sci. 2018, 9, 72. [Google Scholar] [CrossRef]
- Gao, Z.; Khot, L.R.; Naidu, R.A.; Zhang, Q. Early detection of grapevine leafroll disease in a red-berried wine grape cultivar using hyperspectral imaging. Comput. Electron. Agric. 2020, 179, 105807. [Google Scholar] [CrossRef]
- Yang, M.; Kang, X.; Qiu, X.; Ma, L.; Ren, H.; Huang, C.; Zhang, Z.; Lv, X. Method for early diagnosis of verticillium wilt in cotton based on chlorophyll fluorescence and hyperspectral technology. Comput. Electron. Agric. 2024, 216, 108497. [Google Scholar] [CrossRef]
- Pitsik, E.; Frolov, N.; Hauke Kraemer, K.; Grubov, V.; Maksimenko, V.; Kurths, J.; Hramov, A. Motor execution reduces EEG signals complexity: Recurrence quantification analysis study. Chaos Interdiscip. J. Nonlinear Sci. 2020, 30, 023111. [Google Scholar] [CrossRef]
- Seo, D.; Nam, H. Deep rp-cnn for burst signal detection in cognitive radios. IEEE Access 2020, 8, 167164–167171. [Google Scholar] [CrossRef]
- Mathunjwa, B.M.; Lin, Y.T.; Lin, C.H.; Abbod, M.F.; Shieh, J.S. ECG arrhythmia classification by using a recurrence plot and convolutional neural network. Biomed. Signal Process. Control 2021, 64, 102262. [Google Scholar] [CrossRef]
- Masalegoo, S.E.; Soleimani, A.; Saeedi Masine, H. Experimental fault detection of rotating machinery through chaos-based tools of recurrence plot and recurrence quantitative analysis. Arch. Appl. Mech. 2023, 93, 1259–1272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, M.; Zhu, R.; Ma, R. Adulteration discrimination and analysis of fresh and frozen-thawed minced adulterated mutton using hyperspectral images combined with recurrence plot and convolutional neural network. Meat Sci. 2022, 192, 108900. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, J.; Fan, P. A non-destructive dropped fruit impact signal imaging-based deep learning approach for smart sorting of kiwifruit. Comput. Electron. Agric. 2022, 202, 107380. [Google Scholar] [CrossRef]
- GB/T 22101.5-2009; Technical Specifications for Evaluation of Cotton Resistance to Diseases and Pests—Part 5: Verticillium Wilt. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2009.
- Eckmann, J.P.; Kamphorst, S.O.; Ruelle, D. Recurrence plots of dynamical systems. World Sci. Ser. Nonlinear Sci. Ser. A 1995, 16, 441–446. [Google Scholar] [CrossRef]
- Rogers, M.; Blanc-Talon, J.; Urschler, M.; Delmas, P. Wavelength and texture feature selection for hyperspectral imaging: A systematic literature review. J. Food Meas. Charact. 2023, 17, 6039–6064. [Google Scholar] [CrossRef]
- Guru, D.S.; Sharath, Y.H.; Manjunath, S. Texture features and KNN in classification of flower images. IJCA 2010, 37, 21–29. [Google Scholar] [CrossRef]
- Manavalan, R. Towards an intelligent approaches for cotton diseases detection: A review. Comput. Electron. Agric. 2022, 200, 107255. [Google Scholar] [CrossRef]
- Chorowski, J.; Wang, J.; Zurada, J.M. Review and performance comparison of SVM-and ELM-based classifiers. Neurocomputing 2014, 128, 507–516. [Google Scholar] [CrossRef]
- Munera, S.; Gómez-Sanchís, J.; Aleixos, N.; Vila-Francés, J.; Colelli, G.; Cubero, S.; Soler, E.; Blasco, J. Discrimination of common defects in loquat fruit cv. ‘Algerie’ using hyperspectral imaging and machine learning techniques. Postharvest Biol. Technol. 2021, 171, 111356. [Google Scholar] [CrossRef]
- Tian, J.; Kong, Z. Live-cell imaging elaborating epidermal invasion and vascular proliferation/colonization strategy of Verticillium dahliae in host plants. Mol. Plant Pathol. 2022, 23, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, N.; Xiao, Q.; Chen, S.; Gao, P.; He, Y.; Feng, L. Early detection of cotton verticillium wilt based on root magnetic resonance images. Front. Plant Sci. 2023, 14, 1135718. [Google Scholar] [CrossRef]


| Texture Features | Calculating Formula | Texture Features | Calculating Formula |
|---|---|---|---|
| Small gradient dominance | Gradient variance | ||
| Large gradient dominance | Correlation | ||
| Gray asymmetry | Gray entropy | ||
| Gradient asymmetry | Gradient entropy | ||
| Energy | Mixing entropy | ||
| Gray mean | Inertia | ||
| Gradient mean | Inverse difference moment | ||
| Gray variance |
| Model | Training Set | Test Set | ||||||
|---|---|---|---|---|---|---|---|---|
| Accuracy | Precision | Recall | F1-Score | Accuracy | Precision | Recall | F1-Score | |
| KNN | 86.8% | 83.5% | 85.9% | 84.7% | 85.0% | 81.6% | 85.2% | 83.1% |
| SVM | 93.7% | 91.2% | 92.5% | 91.9% | 92.3% | 90.5% | 91.8% | 91.1% |
| ELM | 91.4% | 88.7% | 89.8% | 89.3% | 89.7% | 87.3% | 89.3% | 88.3% |
| XGBoost | 98.4% | 96.3% | 97.1% | 96.7% | 96.3% | 95.6% | 96.0% | 95.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Gao, X.; Cang, H.; Wu, N.; Di, R.; Yan, J.; Li, C.; Gao, P.; Lv, X. Early Detection of Verticillium Wilt in Cotton by Using Hyperspectral Imaging Combined with Recurrence Plots. Agronomy 2025, 15, 213. https://doi.org/10.3390/agronomy15010213
Tan F, Gao X, Cang H, Wu N, Di R, Yan J, Li C, Gao P, Lv X. Early Detection of Verticillium Wilt in Cotton by Using Hyperspectral Imaging Combined with Recurrence Plots. Agronomy. 2025; 15(1):213. https://doi.org/10.3390/agronomy15010213
Chicago/Turabian StyleTan, Fei, Xiuwen Gao, Hao Cang, Nianyi Wu, Ruoyu Di, Jingkun Yan, Chengkai Li, Pan Gao, and Xin Lv. 2025. "Early Detection of Verticillium Wilt in Cotton by Using Hyperspectral Imaging Combined with Recurrence Plots" Agronomy 15, no. 1: 213. https://doi.org/10.3390/agronomy15010213
APA StyleTan, F., Gao, X., Cang, H., Wu, N., Di, R., Yan, J., Li, C., Gao, P., & Lv, X. (2025). Early Detection of Verticillium Wilt in Cotton by Using Hyperspectral Imaging Combined with Recurrence Plots. Agronomy, 15(1), 213. https://doi.org/10.3390/agronomy15010213






