Effect of Experimental Warming on Forage Nutritive Value and Storage in Alpine Meadows at Three Different Altitudes of Nianqing Tanggula Mountain, Northern Tibet: A Long-Term Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experiment Design
2.2. Sampling and Analysis
2.3. Statistical Analyses
3. Results
3.1. Warming Effects on Soil Variables and AGB
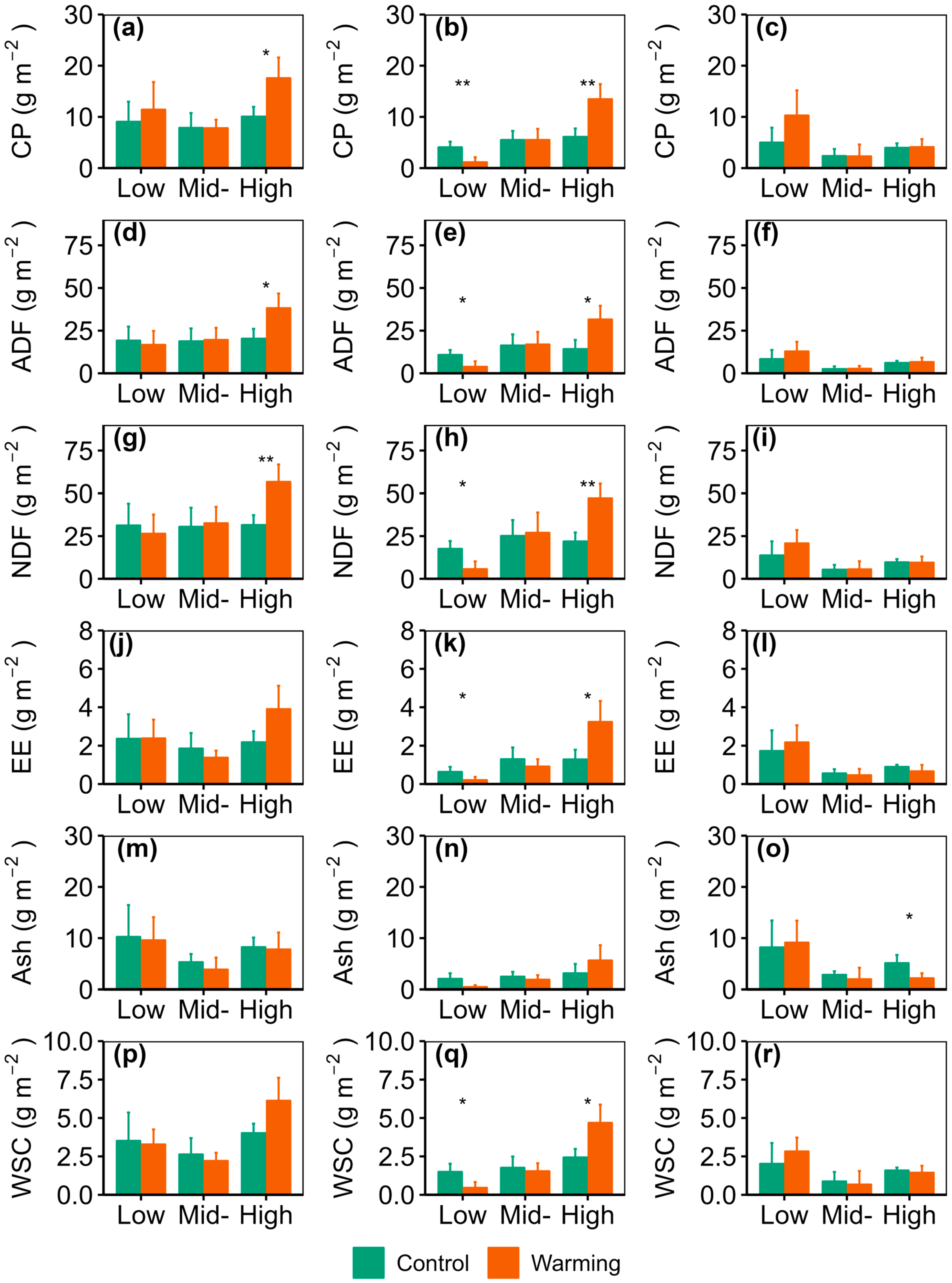
3.2. Warming Effects on Forage Nutrition Quality and Storage
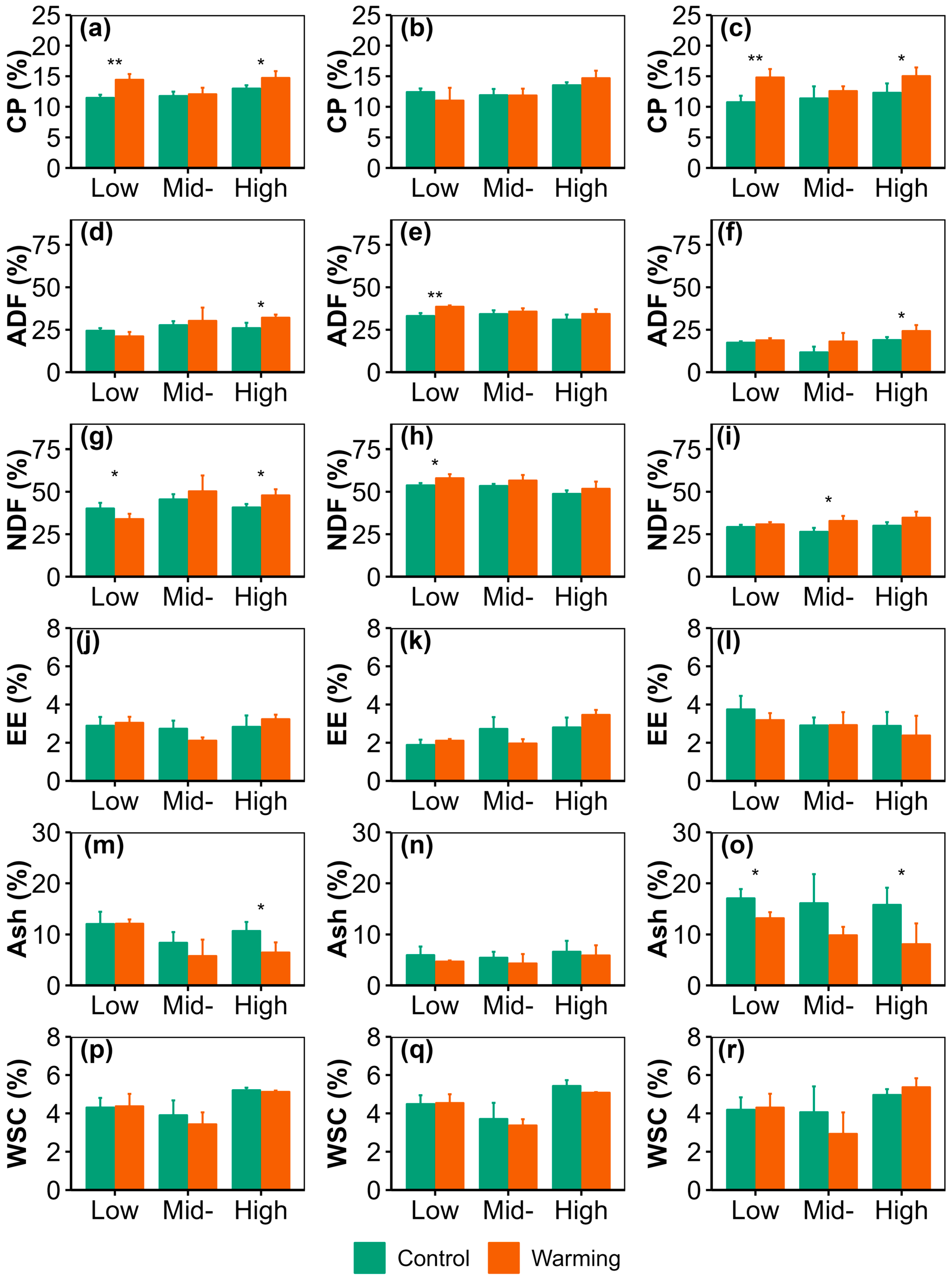
3.3. Comparison of Nutrition Quality Between Graminoid and Forb Forage

3.4. Relationships Between Nutrition Quality and Biotic (AGB and Diversity) and Abiotic (Microclimate and Soil Conditions) Variables
3.5. Relationships Between Nutrition Storage and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Zhu, M.Y.; Zhang, Z.H.; Ma, Z.Y.; Liu, H.Y.; Chen, L.T.; Cao, G.M.; Zhao, X.Q.; Schmid, B.; He, J.S. Experimentally simulating warmer and wetter climate additively improves rangeland quality on the Tibetan Plateau. J. Appl. Ecol. 2018, 55, 1486–1497. [Google Scholar] [CrossRef]
- White, S.R.; Cahill, J.F., Jr.; Bork, E.W. Implications of Precipitation, Warming, and Clipping for Grazing Resources in Canadian Prairies. Agron. J. 2014, 106, 33–42. [Google Scholar] [CrossRef]
- Arft, A.M.; Walker, M.D.; Gurevitch, J.; Alatalo, J.M.; Bret-Harte, M.S.; Dale, M.; Diemer, M.; Gugerli, F.; Henry, G.H.R.; Jones, M.H.; et al. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecol. Monogr. 1999, 69, 491–511. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; Gcte, N. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Xia, J.Y.; Wan, S.Q. Climate warming and biomass accumulation of terrestrial plants: A meta-analysis. New Phytol. 2010, 188, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E.; de Oliveira, E.A.D.; Contin, D.R.; Costa, J.V.C.P.; Costa, K.A.d.P.; Martinez, C.A. Warming offsets the benefits of elevated CO2 in water relations while amplifies elevated CO2-induced reduction in forage nutritional value in the C4 grass Megathyrsus maximus. Front. Plant Sci. 2022, 13, 1033953. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, Y.L.; Ma, W.H.; Liang, C.Z.; Zhao, X.Q.; Fang, J.Y.; He, J.S. Large scale patterns of forage yield and quality across Chinese grasslands. Chin. Sci. Bull. 2013, 58, 1187–1199. [Google Scholar] [CrossRef]
- Li, C.Y.; Peng, F.; Xue, X.; You, Q.G.; Lai, C.M.; Zhang, W.J.; Cheng, Y.X. Productivity and quality of alpine grassland vary with soil water availability under experimental warming. Front. Plant Sci. 2018, 9, 1790. [Google Scholar] [CrossRef]
- Leon-Sanchez, L.; Nicolas, E.; Prieto, I.; Nortes, P.; Maestre, F.T.; Ignacio Querejeta, J. Altered leaf elemental composition with climate change is linked to reductions in photosynthesis, growth and survival in a semi-arid shrubland. J. Ecol. 2020, 108, 47–60. [Google Scholar] [CrossRef]
- Yan, C.; Liu, Z.; Yuan, Z.; Shi, X.; Lock, T.R.; Kallenbach, R.L. Aridity modifies the responses of plant stoichiometry to global warming and nitrogen deposition in semi-arid steppes. Sci. Total Environ. 2022, 831, 154807. [Google Scholar] [CrossRef]
- Zha, X.J.; Tian, Y.; Ouzhu; Fu, G. Response of forage nutrient storages to grazing in alpine grasslands. Front. Plant Sci. 2022, 13, 991287. [Google Scholar] [CrossRef] [PubMed]
- Ni, J. Carbon storage in grasslands of China. J. Arid Environ. 2002, 50, 205–218. [Google Scholar] [CrossRef]
- Piao, S.; Tan, K.; Nan, H.; Ciais, P.; Fang, J.; Wang, T.; Vuichard, N.; Zhu, B. Impacts of climate and CO2 changes on the vegetation growth and carbon balance of Qinghai-Tibetan grasslands over the past five decades. Glob. Planet. Chang. 2012, 98-99, 73–80. [Google Scholar] [CrossRef]
- Piao, S.L.; Zhang, X.Z.; Wang, T.; Liang, E.Y.; Wang, S.P.; Zhu, J.T.; Niu, B. Responses and feedback of the Tibetan Plateau’s alpine ecosystem to climate change. Chin. Sci. Bull. 2019, 64, 2842–2855. (In Chinese) [Google Scholar] [CrossRef]
- Han, F.; Yu, C.; Fu, G. Temperature sensitivities of aboveground net primary production, species and phylogenetic diversity do not increase with increasing elevation in alpine grasslands. Glob. Ecol. Conserv. 2023, 43, e02464. [Google Scholar] [CrossRef]
- Yu, C.Q.; Han, F.S.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Moreno-Perez, A.J.; Pedroche, J.; Gallego-Tevar, B.; Cambrolle, J.; Matias, L.; Fernandez-Rebollo, P.; Martinez-Force, E.; Perez-Ramos, I.M. Climate change alters pasture productivity and quality: Impact on fatty acids and amino acids in Mediterranean silvopastoral ecosystems. Agric. Ecosyst. Environ. 2023, 358. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.Q. Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecol. Appl. 2007, 17, 541–557. [Google Scholar] [CrossRef]
- Wang, S.P.; Duan, J.C.; Xu, G.P.; Wang, Y.F.; Zhang, Z.H.; Rui, Y.C.; Luo, C.Y.; Xu, B.; Zhu, X.X.; Chang, X.F.; et al. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Dumont, B.; Andueza, D.; Niderkorn, V.; Lüscher, A.; Porqueddu, C.; Picon-Cochard, C. A meta-analysis of climate change effects on forage quality in grasslands: Specificities of mountain and Mediterranean areas. Grass Forage Sci. 2015, 70, 239–254. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Schoenbach, P.; Wan, H.; Gierus, M.; Loges, R.; Mueller, K.; Lin, L.; Susenbeth, A.; Taube, F. Effects of grazing and precipitation on herbage production, herbage nutritive value and performance of sheep in continental steppe. Grass Forage Sci. 2012, 67, 535–545. [Google Scholar] [CrossRef]
- Wangchuk, K.; Darabant, A.; Nirola, H.; Wangdi, J.; Gratzer, G. Climate Warming Decreases Plant Diversity but Increases Community Biomass in High-Altitude Grasslands. Rangel. Ecol. Manag. 2021, 75, 51–57. [Google Scholar] [CrossRef]
- An, Y.A.; Wan, S.Q.; Zhou, X.H.; Subedar, A.A.; Wallace, L.L.; Luo, Y.Q. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob. Chang. Biol. 2005, 11, 1733–1744. [Google Scholar] [CrossRef]
- Shi, L.N.; Lin, Z.R.; Wei, X.T.; Peng, C.J.; Yao, Z.Y.; Han, B.; Xiao, Q.; Zhou, H.K.; Deng, Y.F.; Liu, K.S.; et al. Precipitation increase counteracts warming effects on plant and soil C:N:P stoichiometry in an alpine meadow. Front. Plant Sci. 2022, 13, 1044173. [Google Scholar] [CrossRef]
- Shi, C.; Sun, G.; Zhang, H.; Xiao, B.; Ze, B.; Zhang, N.; Wu, N. Effects of warming on chlorophyll degradation and carbohydrate accumulation of alpine herbaceous species during plant senescence on the Tibetan Plateau. PLoS ONE 2014, 9, e107874. [Google Scholar] [CrossRef]
- Mei, L.; Yang, X.; Zhang, S.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alleviate phosphorus limitation by reducing plant N:P ratios under warming and nitrogen addition in a temperate meadow ecosystem. Sci. Total Environ. 2019, 686, 1129–1139. [Google Scholar] [CrossRef]
- Shi, G.X.; Yao, B.Q.; Liu, Y.J.; Jiang, S.J.; Wang, W.Y.; Pan, J.B.; Zhao, X.Q.; Feng, H.Y.; Zhou, H.K. The phylogenetic structure of AMF communities shifts in response to gradient warming with and without winter grazing on the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2017, 121, 31–40. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.L.; Chai, X. Effects of warming and nitrogen addition on nutrient resorption efficiency in an alpine meadow on the northern Tibetan Plateau. Soil Sci. Plant Nutr. 2018, 64, 482–490. [Google Scholar] [CrossRef]
- Ganjurjav, H.; Gao, Q.Z.; Gornish, E.S.; Schwartz, M.W.; Liang, Y.; Cao, X.J.; Zhang, W.N.; Zhang, Y.; Li, W.H.; Wan, Y.F.; et al. Differential response of alpine steppe and alpine meadow to climate warming in the central Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2016, 223, 233–240. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.Q. Decline in medicinal and forage species with warming is mediated by plant traits on the Tibetan Plateau. Ecosystems 2008, 11, 775–789. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.; Guo, R.; Guo, J. Warming and Nitrogen Addition Alter Photosynthetic Pigments, Sugars and Nutrients in a Temperate Meadow Ecosystem. PLoS ONE 2016, 11, e0155375. [Google Scholar] [CrossRef]
- Zhu, J.T.; Zhang, Y.J.; Jiang, L. Experimental warming drives a seasonal shift of ecosystem carbon exchange in Tibetan alpine meadow. Agric. For. Meteorol. 2017, 233, 242–249. [Google Scholar] [CrossRef]
- Shi, F.S.; Wu, Y.; Wu, N.; Luo, P. Different growth and physiological responses to experimental warming of two dominant plant species Elymus nutans and Potentilla anserina in an alpine meadow of the eastern Tibetan Plateau. Photosynthetica 2010, 48, 437–445. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.X.; Yang, L.D.; Guo, J.Y.; Li, N. Physiological responses of Kobresia pygmaea to warming in Qinghai-Tibetan Plateau permafrost region. Acta Oecol. 2012, 39, 109–116. [Google Scholar] [CrossRef]
- Augustine, D.J.; Blumenthal, D.M.; Springer, T.L.; LeCain, D.R.; Gunter, S.A.; Derner, J.D. Elevated CO2 induces substantial and persistent declines in forage quality irrespective of warming in mixedgrass prairie. Ecol. Appl. 2018, 28, 721–735. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.L.; Xu, W.H.; Li, W.; Dai, W.W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Aoki, R.; Kinugasa, T.; Sasaki, T. Predicted effects of simulated ambient warming and moisture on forage nutrient quality and community composition in Mongolian an arid grassland. Rangel. J. 2022, 44, 159–164. [Google Scholar] [CrossRef]
- Natali, S.M.; Schuur, E.A.G.; Rubin, R.L. Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. J. Ecol. 2012, 100, 488–498. [Google Scholar] [CrossRef]
- Jonsdottir, I.S.; Khitun, O.; Stenstrom, A. Biomass and nutrient responses of a clonal tundra sedge to climate warming. Can. J. Bot. Rev. Can. De Bot. 2005, 83, 1608–1621. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Blumenthal, D.; Morgan, J.A.; Pendall, E.; Carrillo, Y.; Follett, R.F. Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytol. 2010, 187, 426–437. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Zong, N.; Chai, X.; Shi, P.L.; Yang, X.C. Effects of warming and nitrogen addition on plant photosynthate partitioning in an alpine meadow on the Tibetan Plateau. J. Plant Growth Regul. 2018, 37, 803–812. [Google Scholar] [CrossRef]
- Gardarin, A.; Garnier, E.; Carrere, P.; Cruz, P.; Andueza, D.; Bonis, A.; Colace, M.-P.; Dumont, B.; Duru, M.; Farruggia, A.; et al. Plant trait-digestibility relationships across management and climate gradients in permanent grasslands. J. Appl. Ecol. 2014, 51, 1207–1217. [Google Scholar] [CrossRef]
- Shi, C.G.; Silva, L.C.R.; Zhang, H.X.; Zheng, Q.Y.; Xiao, B.X.; Wu, N.; Sun, G. Climate warming alters nitrogen dynamics and total non-structural carbohydrate accumulations of perennial herbs of distinctive functional groups during the plant senescence in autumn in an alpine meadow of the Tibetan Plateau, China. Agric. For. Meteorol. 2015, 200, 21–29. [Google Scholar] [CrossRef]
- Salazar-Tortosa, D.; Castro, J.; Villar-Salvador, P.; Vinegla, B.; Matias, L.; Michelsen, A.; Rubio de Casas, R.; Querejeta, J.I. The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob. Chang. Biol. 2018, 24, 4069–4083. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; LeCain, D.R.; Follett, R.F.; Williams, D.G. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef] [PubMed]

| Community | Graminoids | Forbs | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variable | CP | ADF | NDF | EE | Ash | WSC | βBrayN | CP | ADF | NDF | EE | Ash | WSC | βBrayN | CP | ADF | NDF | EE | Ash | WSC | βBrayN | |
| Nutrition content | AGB | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.2 | 0.2 | 0.8 | 0.0 |
| SR | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | |
| Shannon | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | |
| Simpson | 0.0 | 0.1 | 0.0 | 0.1 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.3 | 0.0 | |
| Pielou | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | |
| PD | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | |
| MNTD | 0.0 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.3 | 0.2 | 0.0 | |
| βBray | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.2 | 0.4 | 0.0 | |
| βMNTD | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| Ts | 0.0 | 0.1 | 0.1 | 0.1 | 0.4 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 | |
| SM | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.4 | 0.0 | 0.1 | 0.2 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 | |
| Ta | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.3 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | |
| VPD | 0.0 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.3 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 | |
| SOC | 0.0 | 0.0 | 0.0 | 0.1 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.6 | 0.1 | 0.0 | |
| TN | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | 0.5 | 0.1 | 0.1 | |
| TP | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | |
| NH4+-N | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.5 | 0.1 | 0.1 | |
| NO3−-N | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | |
| AP | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| C:N | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | |
| C:P | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | |
| N:P | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | |
| NH4+-N:NO3−-N | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| Available N:P | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| pH | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 | 0.2 | 0.2 | 0.0 | |
| Nutrition yield | AGB | 5.1 | 4.0 | 3.7 | 5.3 | 1.2 | 5.0 | 3.9 | 18.2 | 17.6 | 18.1 | 15.7 | 12.4 | 17.6 | 18.1 | 13.2 | 12.2 | 13.4 | 12.0 | 9.4 | 15.1 | 13.0 |
| SR | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | |
| Shannon | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | |
| Simpson | 0.0 | 0.2 | 0.1 | 0.1 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.2 | 0.0 | |
| Pielou | 0.1 | 0.2 | 0.1 | 0.2 | 0.4 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.3 | 0.3 | 0.0 | 0.1 | 0.2 | 0.1 | 0.3 | 0.3 | 0.2 | 0.0 | |
| PD | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| MNTD | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | 0.1 | 0.3 | 0.0 | 0.7 | 0.7 | 0.2 | 0.0 | |
| βBray | 0.0 | 0.1 | 0.1 | 0.1 | 0.5 | 0.1 | 0.1 | 0.4 | 0.2 | 0.1 | 0.3 | 0.6 | 0.1 | 0.1 | 0.3 | 0.4 | 0.2 | 0.7 | 0.3 | 0.6 | 0.1 | |
| βMNTD | 0.0 | 0.2 | 0.1 | 0.1 | 0.4 | 0.1 | 0.1 | 1.1 | 0.4 | 0.1 | 1.1 | 1.9 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.2 | 0.2 | 0.0 | |
| Ts | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.6 | 0.2 | 0.1 | |
| SM | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | 0.3 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.3 | 0.2 | 0.0 | 0.2 | 0.3 | 0.1 | 0.2 | 0.2 | 0.3 | 0.0 | |
| Ta | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.2 | 0.7 | 0.3 | 0.0 | |
| VPD | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.2 | 0.4 | 0.1 | 0.0 | |
| SOC | 0.1 | 0.1 | 0.1 | 0.1 | 0.7 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.9 | 0.2 | 0.0 | |
| TN | 0.1 | 0.1 | 0.1 | 0.1 | 0.8 | 0.2 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.2 | 0.8 | 0.2 | 0.0 | |
| TP | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| NH4+-N | 0.0 | 0.1 | 0.1 | 0.2 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.0 | |
| NO3−-N | 0.1 | 0.2 | 0.1 | 0.1 | 0.7 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.3 | 0.1 | |
| AP | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.3 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | |
| C:N | 0.0 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.5 | 0.4 | 0.0 | |
| C:P | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | |
| N:P | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.3 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.0 | |
| NH4+-N:NO3−-N | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.5 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | |
| Available N:P | 0.1 | 0.1 | 0.2 | 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.4 | 0.4 | 0.2 | 0.0 | |
| pH | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, F.; Sun, W.; Li, S.; Yu, C.; Xu, J.; Li, T.; Deng, Y.; Dorblha; Chen, C.; Dawaqiongda; et al. Effect of Experimental Warming on Forage Nutritive Value and Storage in Alpine Meadows at Three Different Altitudes of Nianqing Tanggula Mountain, Northern Tibet: A Long-Term Experience. Agronomy 2025, 15, 186. https://doi.org/10.3390/agronomy15010186
Han F, Sun W, Li S, Yu C, Xu J, Li T, Deng Y, Dorblha, Chen C, Dawaqiongda, et al. Effect of Experimental Warming on Forage Nutritive Value and Storage in Alpine Meadows at Three Different Altitudes of Nianqing Tanggula Mountain, Northern Tibet: A Long-Term Experience. Agronomy. 2025; 15(1):186. https://doi.org/10.3390/agronomy15010186
Chicago/Turabian StyleHan, Fusong, Wei Sun, Shaowei Li, Chengqun Yu, Jun Xu, Tianyu Li, Yujie Deng, Dorblha, Chuhong Chen, Dawaqiongda, and et al. 2025. "Effect of Experimental Warming on Forage Nutritive Value and Storage in Alpine Meadows at Three Different Altitudes of Nianqing Tanggula Mountain, Northern Tibet: A Long-Term Experience" Agronomy 15, no. 1: 186. https://doi.org/10.3390/agronomy15010186
APA StyleHan, F., Sun, W., Li, S., Yu, C., Xu, J., Li, T., Deng, Y., Dorblha, Chen, C., Dawaqiongda, Luobu, & Fu, G. (2025). Effect of Experimental Warming on Forage Nutritive Value and Storage in Alpine Meadows at Three Different Altitudes of Nianqing Tanggula Mountain, Northern Tibet: A Long-Term Experience. Agronomy, 15(1), 186. https://doi.org/10.3390/agronomy15010186






