Optimum Nitrogen and Density Allocation for Trade−Off Between Yield and Lodging Resistance of Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
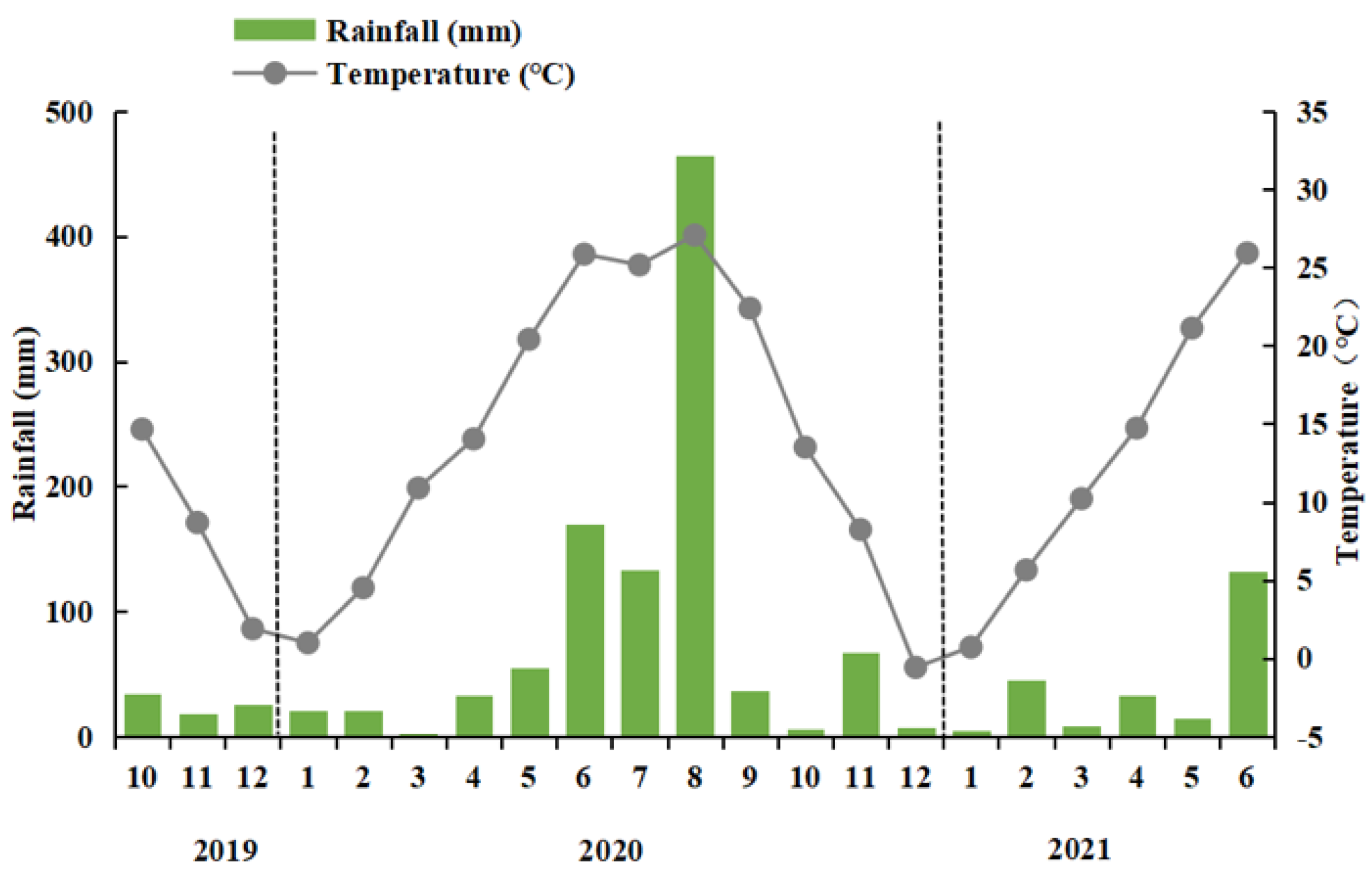
2.1. Site Description
2.2. Experimental Design
2.3. Sampling and Measurement Methods
2.4. Determination Parameters and Methods
2.4.1. Internode Length, Fresh Weight of Internode, and Stem Breaking Strength
2.4.2. Calculation of Mechanics Parameters
2.4.3. Cellulose Content
2.4.4. Hemicellulose Content
2.4.5. Lignin Content
2.4.6. Lignin Subunit Content
2.5. Determination of Yield and Its Components
2.6. Statistical Analysis
3. Results
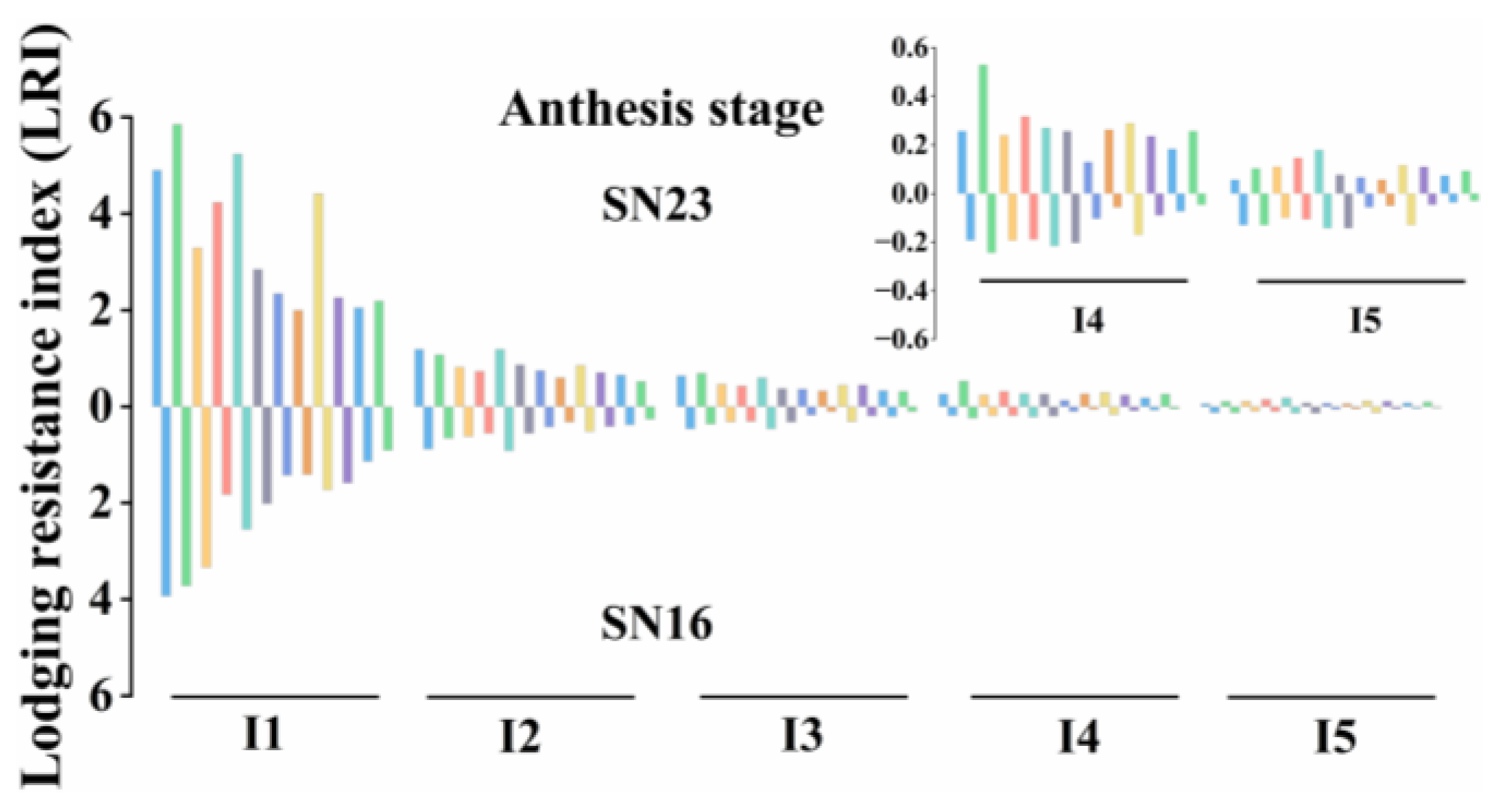
3.1. Lodging Resistance Index (LRI)
3.2. Yield and Yield Components
3.3. Bending Moment (WP) and Its Components
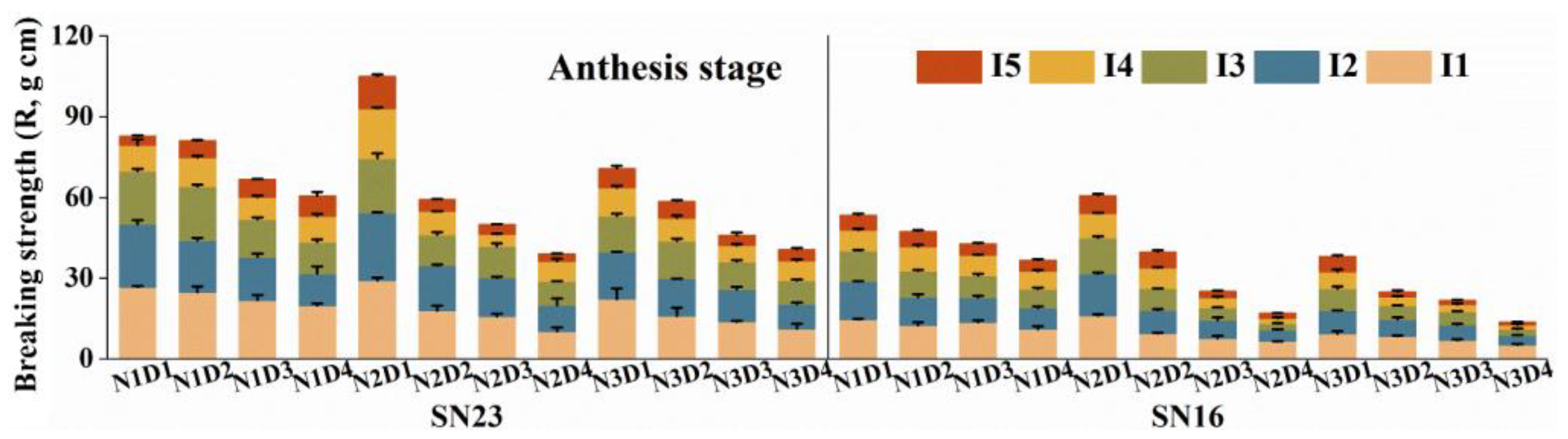
3.4. Breaking Strength (R)
3.5. Carbohydrates Contents
3.5.1. Cellulose
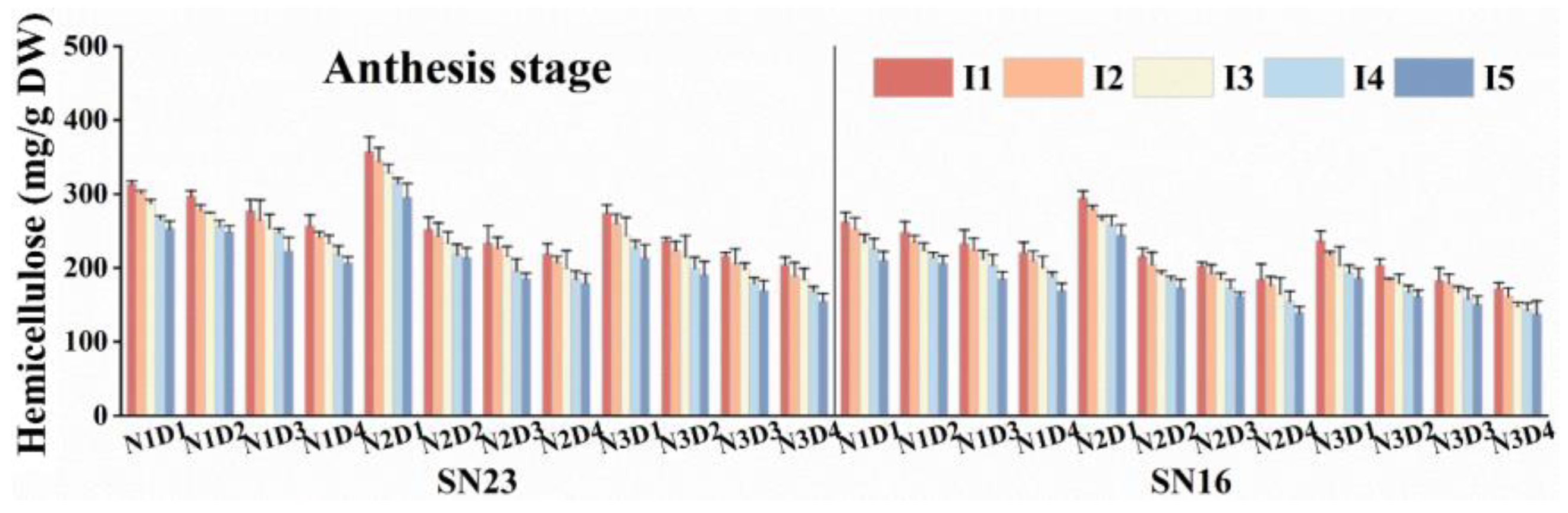
3.5.2. Hemicellulose
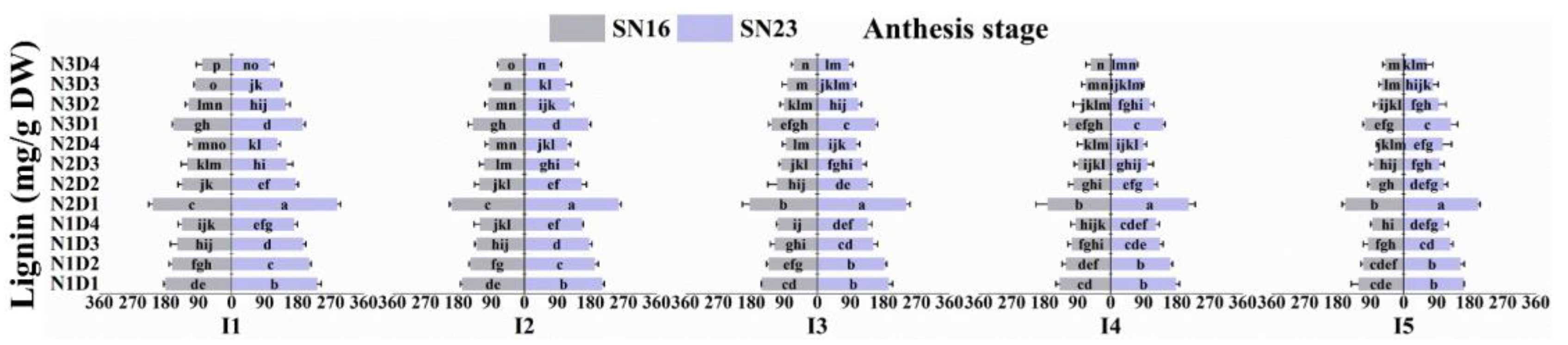
3.5.3. Lignin
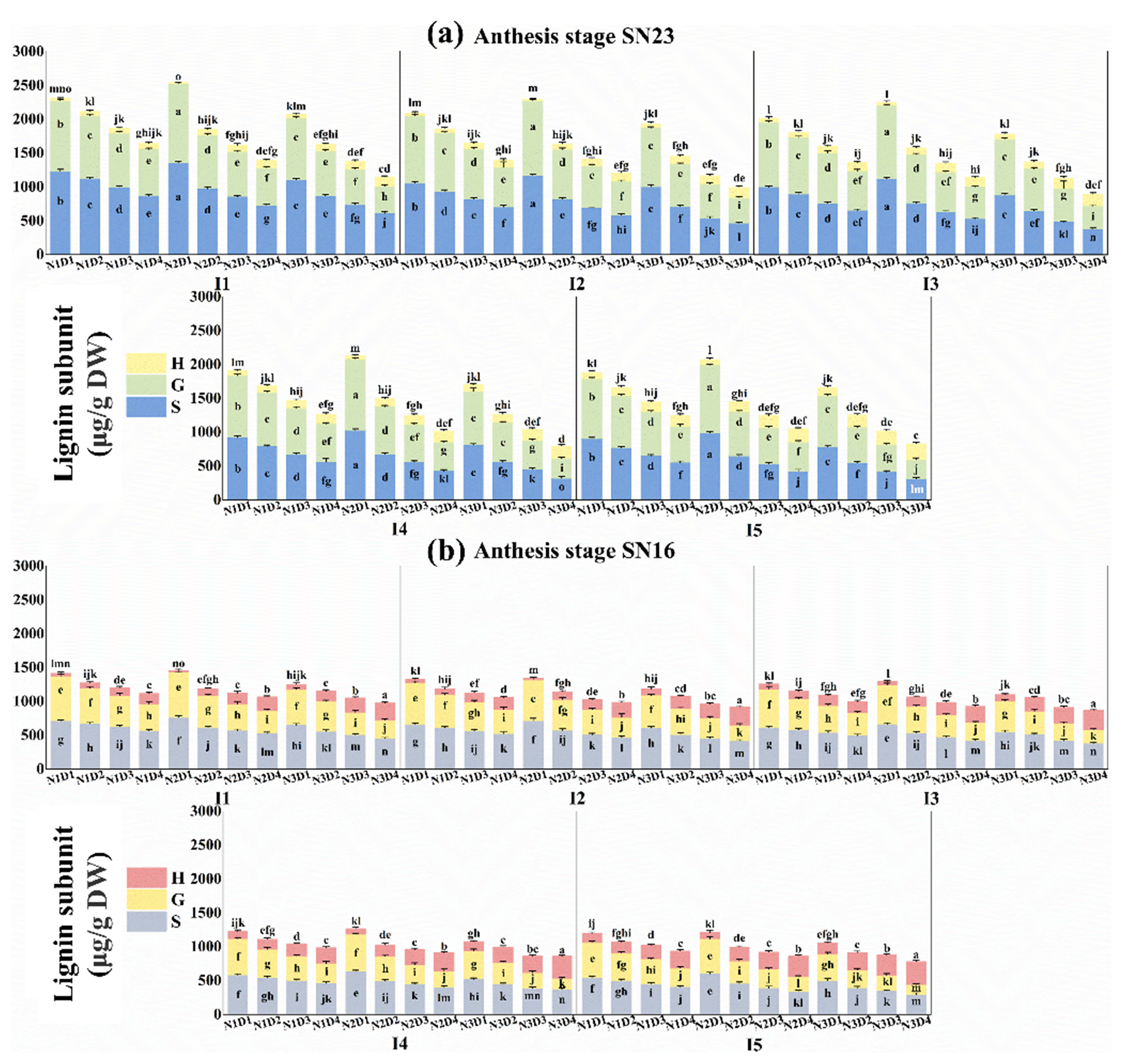
3.5.4. Lignin Subunit Content
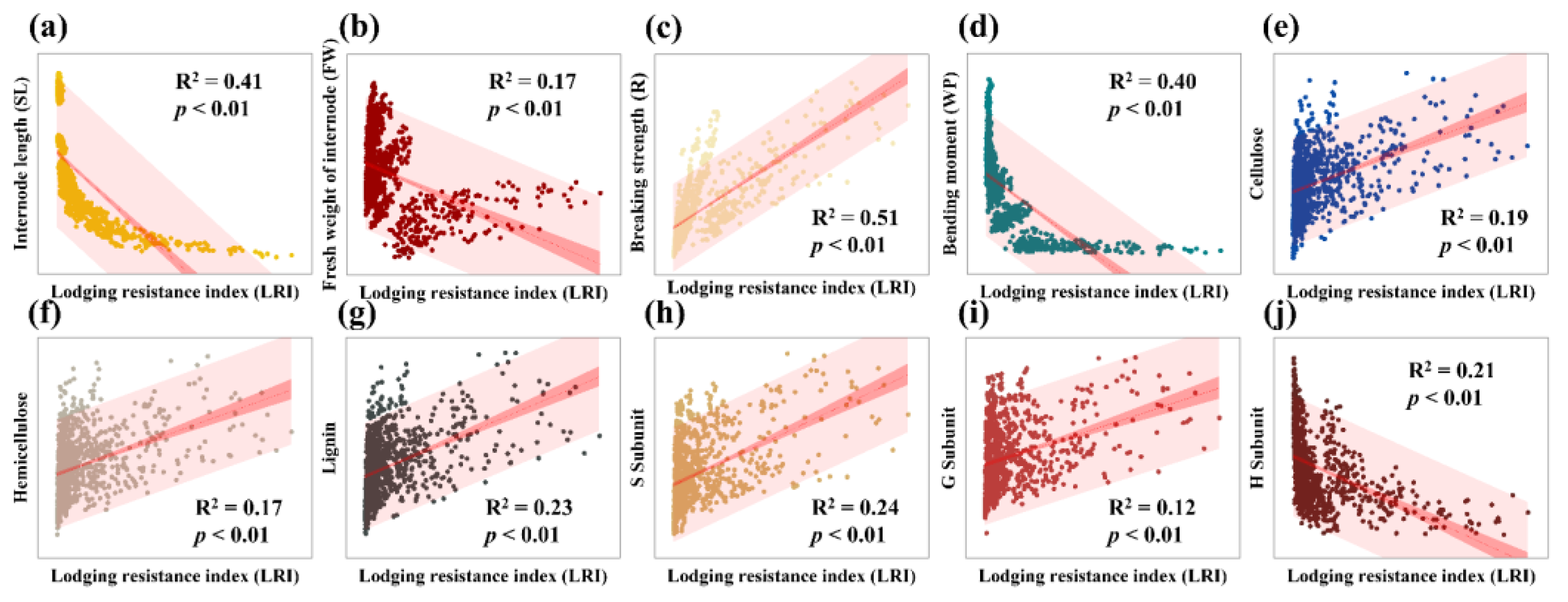
3.6. Correlation of Lodging Resistance Index (LRI) with Other Indicators
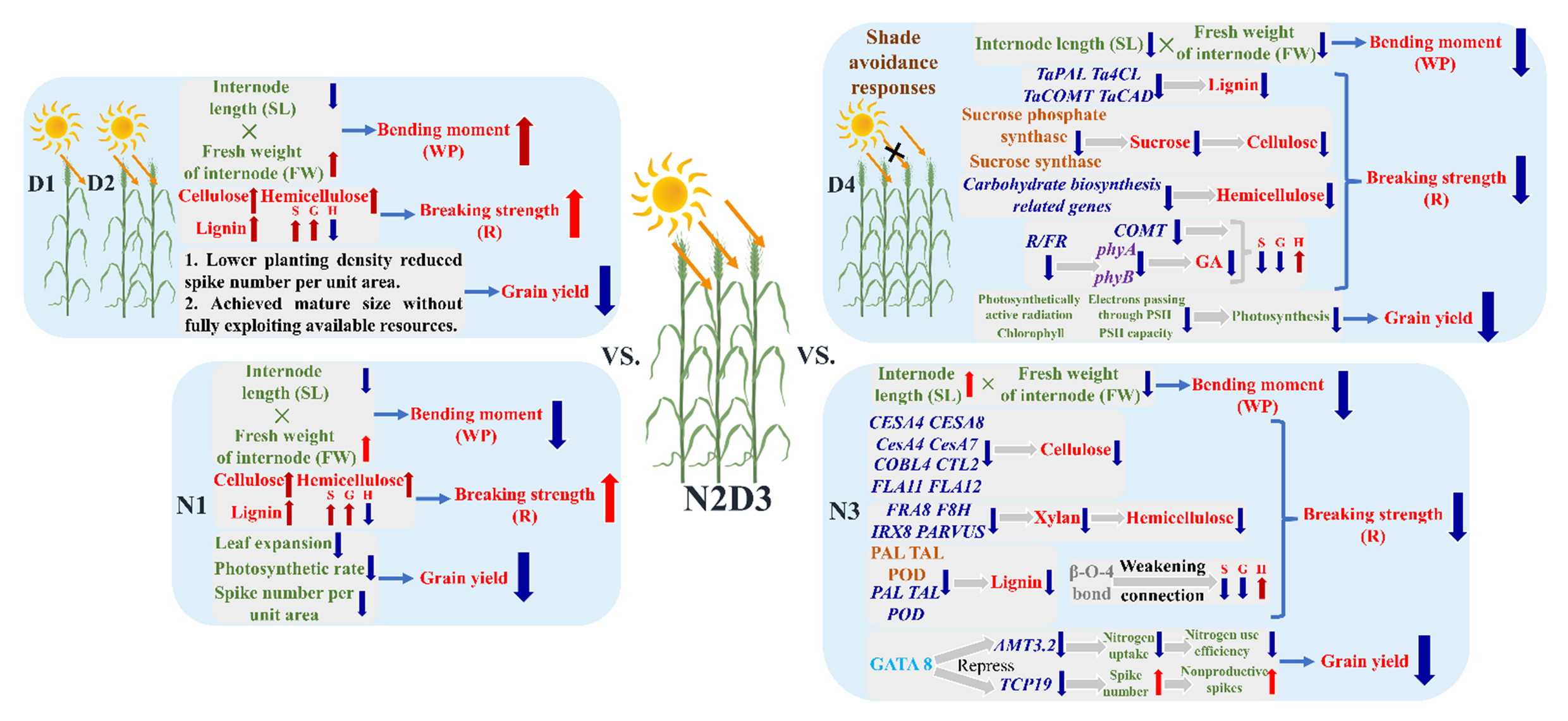
4. Discussion
4.1. Causes of Internode Lodging Resistance Variability
4.2. Causes of Differential Lodging Resistance at Different Stages
4.3. Lodging Resistance Variability Induced by Nitrogen Application
4.4. Lodging Resistance Variability Induced by Planting Density
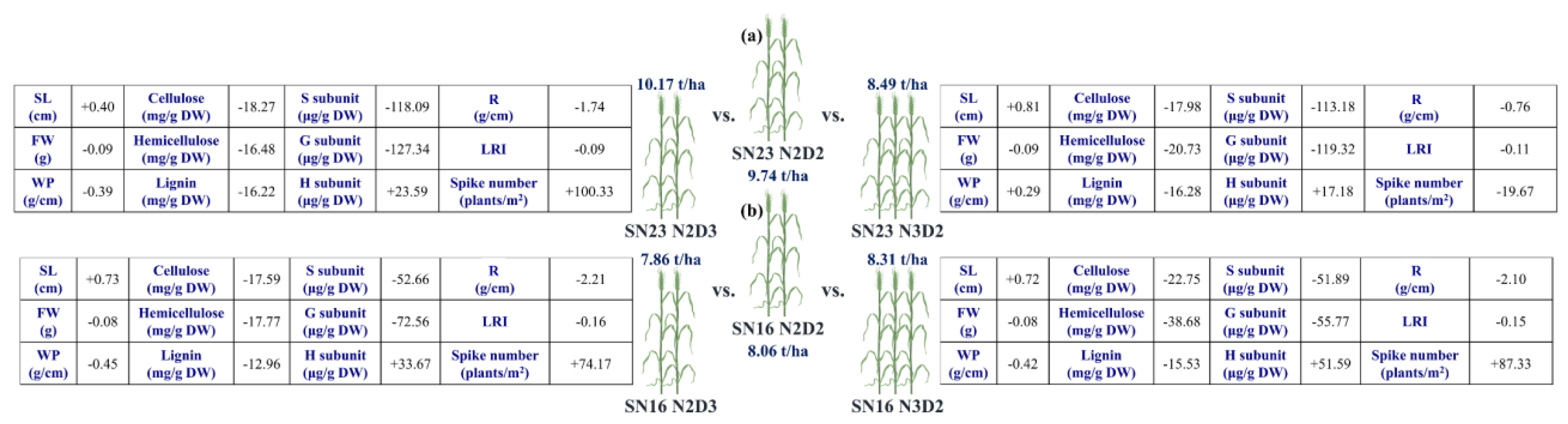
4.5. Trade−Off Between Yield and Lodging Resistance
4.6. Potential Trade−Off Mechanism Between Yield and Lodging Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gooding, M.J.; Shewry, P.R. Wheat and Humans. In Wheat; Gooding, M.J., Shewry, P.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Zuber, U.; Winzeler, H.; Messmer, M.M.; Keller, M.; Keller, B.; Schmid, J.E.; Stamp, P. Morphological traits associated with lodging resistance of spring wheat (Triticum aestivum L.). J. Agron. Crop Sci. 1999, 182, 17–24. [Google Scholar] [CrossRef]
- Piñera-Chavez, F.J.; Berry, P.M.; Foulkes, M.J.; Jesson, M.A.; Reynolds, M.P. Avoiding lodging in irrigated spring wheat. I. Stem and root structural requirements. Field Crops Res. 2016, 196, 325–336. [Google Scholar] [CrossRef]
- Mirabella, N.E.; Abbate, P.E.; Alonso, M.P.; Panelo, J.S.; Pontaroli, A.C. Identifying traits at crop maturity and models for estimation of lodging susceptibility in bread wheat. Crop Pasture Sci. 2019, 70, 95–106. [Google Scholar] [CrossRef]
- Berry, P.M.; Spink, J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 2012, 137, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Luo, Y.; Chang, Y.; Zhu, J.; Li, Y.; Wang, Z. Effects of irrigation on stem lignin and breaking strength of winter wheat with different planting densities. Field Crops Res. 2022, 282, 108518. [Google Scholar] [CrossRef]
- Martre, P.; Dueri, S.; Guarin, J.R.; Ewert, F.; Webber, H.; Calderini, D.; Molero, G.; Reynolds, M.; Miralles, D.; Garcia, G.; et al. Global needs for nitrogen fertilizer to improve wheat yield under climate change. Nat. Plants 2024, 10, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhao, G.; Sun, X.; Fu, Y. Coordinating lodging incidence and grain yield through wheat genetic diversity. Field Crops Res. 2024, 315, 109468. [Google Scholar] [CrossRef]
- McLellan, E.L.; Cassman, K.G.; Eagle, A.J.; Woodbury, P.B.; Sela, S.; Tonitto, C.; Marjerison, R.D.; van Es, H.M. The nitrogen balancing act: Tracking the environmental performance of food production. BioScience 2018, 68, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Plant biomass and nitrogen partitioning changes between silking and maturity in newer versus older maize hybrids. Field Crops Res. 2015, 183, 315–328. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Wu, D.; Xia, T.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.; Gao, H.; Yang, X.; Dou, Z.; Yuan, X.; Gao, W.; Zhang, H. Optimizing nitrogen management can improve stem lodging resistance and stabilize grain yield of japonica rice in rice-crayfish coculture systems. J. Integr. Agric. 2024, 23, 3983–3997. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Wu, X.; Ding, Y.; Li, G.; Li, J.; Weng, F.; Liu, Z.; Tang, S.; Ding, C.; et al. Lodging resistance of japonica rice (Oryza Sativa L.): Morphological and anatomical traits due to top-dressing nitrogen application rates. Rice 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Sun, Y.; Zhou, M.; Zhang, P.; Zuo, Q.; Wu, J.; Zhou, G. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crops Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, J.; Liu, Y.; Huang, N.; Tian, K.; Shah, F.; Liang, K.; Zhong, X.; Liu, B. Optimized nitrogen management enhances lodging resistance of rice and its morpho-anatomical, mechanical, and molecular mechanisms. Sci. Rep. 2019, 9, 20274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C.; et al. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, B.L.; Whalen, J.K. Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: Perspectives for stress adaptation from root system architecture. Adv. Agron. 2018, 151, 87–157. [Google Scholar] [CrossRef]
- Luo, Y.; Ni, J.; Pang, D.; Jin, M.; Chen, J.; Kong, X.; Li, W.; Chang, Y.; Li, Y.; Wang, Z. Regulation of lignin composition by nitrogen rate and density and its relationship with stem mechanical strength of wheat. Field Crops Res. 2019, 241, 107572. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Lodging prevention in cereals: Morphological, biochemical, anatomical traits and their molecular mechanisms, management and breeding strategies. Field Crops Res. 2022, 289, 108733. [Google Scholar] [CrossRef]
- Wu, W.; Shah, F.; Ma, B.L. Understanding of crop lodging and agronomic strategies to improve the resilience of rapeseed production to climate change. Crop Environ. 2022, 1, 133–144. [Google Scholar] [CrossRef]
- Djaman, K.; Allen, S.; Djaman, D.S.; Koudahe, K.; Irmak, S.; Puppala, N.; Darapuneni, M.K.; Angadi, S.V. Planting date and plant density effects on maize growth, yield and water use efficiency. Environ. Chall. 2022, 6, 100417. [Google Scholar] [CrossRef]
- Deng, J.; Ran, J.; Wang, Z.; Fan, Z.; Wang, G.; Ji, M.; Liu, J.; Wang, Y.; Liu, J.; Brown, J.H. Models and tests of optimal density and maximal yield for crop plants. Proc. Natl. Acad. Sci. USA 2012, 109, 15823–15828. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Peng, D.; Wang, R.; Jia, X.; Qiao, D.; Liu, T.; Jia, Z.; Wang, Z.; Ren, X. Can intercropping or mixed cropping of two genotypes enhance wheat lodging resistance? Field Crops Res. 2019, 239, 10–18. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, X.; Hou, P.; Xue, J.; Liu, G.; Liu, W.; Wang, Y.; Zhao, R.; Ming, B.; Xie, R.; et al. Quantitative effects of solar radiation on maize lodging resistance mechanical properties. Field Crops Res. 2020, 255, 107906. [Google Scholar] [CrossRef]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Ding, Y.; Zhang, J.; Cambula, E.D.; Weng, F.; Liu, Z.; Ding, C.; Tang, S.; Chen, L.; et al. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in japonica rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef]
- Li, Q.; Fu, C.; Liang, C.; Ni, X.; Zhao, X.; Chen, M.; Ou, L. Crop lodging and the roles of lignin, cellulose, and hemicellulose in lodging resistance. Agronomy 2022, 12, 1795. [Google Scholar] [CrossRef]
- Rathke, G.W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crops Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Muchow, R.C. Radiation use efficiency. Adv. Agron. 1999, 65, 215–265. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Oosterhuis, D.M. Canopy development and photosynthesis of cotton as influenced by nitrogen nutrition. J. Plant Nutr. 1990, 13, 1141–1154. [Google Scholar] [CrossRef]
- O’connell, M.G.; O’leary, G.J.; Whitfield, D.M.; Connor, D.J. Interception of photosynthetically active radiation and radiation-use efficiency of wheat, field pea and mustard in a semi-arid environment. Field Crops Res. 2004, 85, 111–124. [Google Scholar] [CrossRef]
- Rinehardt, J.M.; Edmisten, K.L.; Wells, R.; Faircloth, J.C. Response of ultra–narrow and conventional spaced cotton to variable nitrogen rates. J. Plant Nutr. 2004, 27, 743–755. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of multiple planting densities on lignin metabolism and lodging resistance of the strip intercropped soybean stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Z.; Shah, F.; Wu, W. Optimizing biomass allocation for optimum balance of seed yield and lodging resistance in rapeseed. Field Crops Res. 2024, 316, 109493. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Li, G.; Jiang, D.; Dong, S. Maize canopy apparent photosynthesis and 13C-photosynthate reallocation in response to different density and N rate combinations. Front. Plant Sci. 2019, 10, 1113. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Zhu, Q.; Jiang, D.; Dong, S. Optimising yield and resource utilisation of summer maize under the conditions of increasing density and reducing nitrogen fertilization. Sci. Nat. 2017, 104, 86. [Google Scholar] [CrossRef] [PubMed]
- Ookawa, T.; Ishihara, K. Varietal difference of physical characteristics of the culm related to lodging resistance in paddy rice [Oryza sativa]. Jpn. J. Crop Sci. 1992, 61, 419–425. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Luo, Y.; Jin, M.; Chang, Y.; Cui, H.; Sun, S.; Li, Y.; Wang, Z. Mixed cropping increases grain yield and lodging resistance by improving the canopy light environment of wheat populations. Eur. J. Agron. 2023, 147, 126849. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asgharr, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Shi, Y.; Li, Y.; Yin, Y.; Yang, D.; Luo, Y.; Pang, D.; Xu, X.; Li, W.; et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2017, 7, 41805. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Zhou, Q.; Wang, X.; Cai, J.; Huang, M.; Dai, T.; Jiang, D. Effects of planting density on stem morphological characteristics, chemical composition and lodging resistance of different wheat varieties. J. Nanjing Agric. Univ. 2018, 41, 808–816. [Google Scholar] [CrossRef]
- Dong, X.C.; Qian, T.F.; Chu, J.P.; Zhang, X.; Liu, Y.J.; Dai, X.L.; He, M.R. Late sowing enhances lodging resistance of wheat plants by improving the biosynthesis and accumulation of lignin and cellulose. J. Integr. Agric. 2023, 22, 1351–1365. [Google Scholar] [CrossRef]
- Handakumbura, P.P.; Hazen, S.P. Transcriptional regulation of grass secondary cell wall biosynthesis: Playing catch-up with Arabidopsis thaliana. Front. Plant Sci. 2012, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Gou, L.; Zhao, Y.; Yao, M.; Yao, H.; Tian, J.; Zhang, W. Effects of light intensity within the canopy on maize lodging. Field Crops Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Li, W.Q.; Han, M.M.; Pang, D.W.; Chen, J.; Wang, Y.Y.; Dong, H.H.; Chang, Y.; Jin, M.; Luo, Y.; Li, Y.; et al. Characteristics of lodging resistance of high-yield winter wheat as affected by nitrogen rate and irrigation managements. J. Integr. Agric. 2022, 21, 1290–1309. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Sayre, K.D.; Kaul, J.N.; Narang, R.S. Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: Effects of genotypes, N levels and ethephon. Field Crops Res. 2003, 84, 271–290. [Google Scholar] [CrossRef]
- Niu, L.; Feng, S.; Ding, W.; Li, G. Influence of speed and rainfall on large-scale wheat lodging from 2007 to 2014 in China. PLoS ONE 2016, 11, e0157677. [Google Scholar] [CrossRef] [PubMed]
- Mulsanti, I.W.; Yamamoto, T.; Ueda, T.; Samadi, A.F.; Kamahora, E.; Rumanti, I.A.; Thanh, V.C.; Adachi, S.; Suzuki, S.; Kanekatsu, M.; et al. Finding the superior allele of japonica-type for increasing stem lodging resistance in indica rice varieties using chromosome segment substitution lines. Rice 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Ru, Z.G.; Ding, W.H.; Hu, T.Z.; Li, G. Study of the relationship between field lodging and stem quality traits of winter wheat in the north China plain. Crop Pasture Sci. 2019, 70, 772–780. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Cui, Z.; Zhang, T.; Chen, S.; Xiang, S.; Jia, L.; Yan, B.; Wang, Y.; Guo, L.; et al. Optimized nitrogen and potassium fertilizers application increases stem lodging resistance and grain yield of oil flax by enhancing lignin biosynthesis. J. Integr. Agric. 2024, in press. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.; Fan, J.J.; Sun, M.; Yi, Y.; Guo, W.S.; Voldeng, H.D. Management of nitrogen fertilization to balance reducing lodging risk and increasing yield and protein content in spring wheat. Field Crops Res. 2019, 241, 107584. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Lin, J.; Wang, L.; Jia, Z.; Zhang, R. Optimal nitrogen management to achieve high wheat grain yield, grain protein content, and water productivity: A meta-analysis. Agric. Water Manag. 2023, 290, 108587. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wang, Z.; Li, W.; Wang, C.; Yan, S.; Li, H.; Zhang, A.; Tang, Z.; Wei, M. Optimized nitrogen fertilizer application mode increased culms lignin accumulation and lodging resistance in culms of winter wheat. Field Crops Res. 2018, 228, 31–38. [Google Scholar] [CrossRef]
- Raza, A.; Asghar, M.A.; Javed, H.H.; Ullah, A.; Cheng, B.; Xu, M.; Wang, W.; Liu, C.; Rahman, A.; Iqbal, T.; et al. Optimum nitrogen improved stem breaking resistance of intercropped soybean by modifying the stem anatomical structure and lignin metabolism. Plant Physiol. Biochem. 2024, 199, 107720. [Google Scholar] [CrossRef] [PubMed]
- Pitre, F.E.; Cooke, J.E.; Mackay, J.J. Short-term effects of nitrogen availability on wood formation and fibre properties in hybrid poplar. Trees 2007, 21, 249–259. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Huang, Q.; Liu, Z.; Ding, C.; Tang, S.; Chen, L.; Wang, S.; Ding, Y.; Zhang, W. Effects of culm carbohydrate partitioning on basal stem strength in a high-yielding rice population. Crop J. 2017, 5, 478–487. [Google Scholar] [CrossRef]
- Li, Z.; Gao, G.; Xu, L.; Wang, Z.; Wang, C.; Yang, T.; Kuai, J.; Wang, B.; Xu, Z.; Zhao, J.; et al. Reducing nitrogen application at high planting density enhances secondary cell wall formation and decreases stem lodging in rapeseed. Eur. J. Agron. 2024, 156, 127162. [Google Scholar] [CrossRef]
- Zhong, R.; Peña, M.J.; Zhou, G.K.; Nairn, C.J.; Wood-Jones, A.; Richardson, E.A.; Morrison, W.H.; Darvill, A.G.; York, W.S.; Ye, Z.H. Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell 2005, 17, 3390–3408. [Google Scholar] [CrossRef]
- Peña, M.J.; Zhong, R.; Zhou, G.K.; Richardson, E.A.; O’Neill, M.A.; Darvill, A.G.; York, W.S.; Ye, Z.H. Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 2007, 19, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zhong, R.; Richardson, E.A.; Himmelsbach, D.S.; McPhail, B.T.; Ye, Z.H. The PARVUS gene is expressed in cells undergoing secondary wall thickening and is essential for glucuronoxylan biosynthesis. Plant Cell Physiol. 2007, 48, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Teng, Q.; Huang, W.; Zhong, R.; Ye, Z.H. The F8H glycosyltransferase is a functional paralog of FRA8 involved in glucuronoxylan biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, F.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density. J. Integr. Agric. 2021, 20, 2077–2089. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, F.; Wang, X.L.; Zhan, X.X.; Guo, Z.X.; Liu, Q.L.; Wei, G.; Lan, T.Q.; Feng, D.J.; Kong, F.L.; et al. Optimizing nitrogen management enhances stalk lodging resistance and grain yield in dense planting maize by improving canopy light distribution. Eur. J. Agron. 2023, 148, 126871. [Google Scholar] [CrossRef]
- Qi, B.; Hu, J.; Zhu, L.; Duan, Y.; Zhang, W.; Gou, L. Response of maize stalk to plant density on cellulose accumulation by modulating enzymes activities. Field Crops Res. 2023, 304, 109152. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf angle: A target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef]
- Jin, R.; Li, Z.; Wang, X.L.; Liu, F.; Kong, F.L.; Liu, Q.L.; Lan, T.Q.; Feng, D.J.; Yuan, J.C. Optimizing row spacing increases stalk lodging resistance by improving light distribution in dense maize populations. Agronomy 2023, 13, 462. [Google Scholar] [CrossRef]
- Shi, D.Y.; Li, Y.H.; Zhang, J.W.; Liu, P.; Zhao, B.; Dong, S.T. Effects of plant density and nitrogen rate on lodging-related stalk traits of summer maize. Plant Soil Environ. 2016, 62, 299–306. [Google Scholar] [CrossRef]
- Li, C.Z.; Li, C.J. Ridge-furrow with plastic film mulching system decreases the lodging risk for summer maize plants under different nitrogen fertilization rates and varieties in dry semi-humid areas. Field Crops Res. 2021, 263, 108056. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.; Jin, M.; Sun, S.; Wang, Z.; Li, Y. Response of lignin metabolism to light quality in wheat population. Front. Plant Sci. 2021, 12, 729647. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gu, S.C.; Wang, Y.Y.; Xu, C.C.; Zhao, Y.T.; Liu, X.L.; Wang, P.; Huang, S.B. The relationships between maize (Zea mays L.) lodging resistance and yield formation depend on dry matter allocation to ear and stem. Crop J. 2022, 11, 258–268. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; Vries, W.D.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, L.; Van Dingenen, R.; Vieno, M.; Van Grinsven, H.J.; Zhang, X.; Zhang, S.; Chen, Y.; Wang, S.; Ren, C.; et al. Abating ammonia is more cost-effective than nitrogen oxides for mitigating PM2. 5 air pollution. Science 2021, 374, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Uebbing, L.F.; Beusen, A.H.; Bouwman, A.F.; De Vries, W. From planetary to regional boundaries for agricultural nitrogen pollution. Nature 2022, 610, 507–512. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, F.; Wu, Z.; Wang, X.; Ren, X.; Jia, Z.; Wang, Z.; Chen, X.; Cai, T. Heterogeneous population distribution enhances resistance to wheat lodging by optimizing the light environment. J. Integr. Agric. 2024, 23, 2211–2226. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Djakovic, T.; Stikic, R.; Prokic, L.; Sukalovic, V.H. Effect of N deficiency on leaf growth and cell wall peroxidase activity in contrasting maize genotypes. Plant Soil 2004, 265, 211–223. [Google Scholar] [CrossRef]
- Kavanov, A.M.; Lattanzi, F.A.; Schnyder, H. Nitrogen deficiency inhibits leaf blade growth in Lolium perenne by increasing cell cycle duration and decreasing mitotic and post-mitotic growth rates. Plant Cell Environ. 2008, 31, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Chandler, P.M.; Swain, S.M.; King, R.W.; Richards, R.A.; Spielmeyer, W. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol. 2012, 160, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, F.; Liu, Y.; Tian, X.; Zhang, Y. Can optimizing seeding rate and planting density alleviate the yield loss of double-season rice caused by prolonged seedling age? Crop Sci. 2021, 61, 2759–2774. [Google Scholar] [CrossRef]


| Treatment | Spike Number (Plants/m2) | Grain Number per Spike | 1000−Grain Weight (g) | Yield (t/ha) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| SN23 | ||||||||
| N1D1 | 292.67±16.17 m | 242.67 ± 19.73 o | 62.33 ± 3.79 a | 73.33 ± 0.58 a | 45.34 ± 0.30 cd | 48.51 ± 0.23 bcd | 6.33 ± 0.42 l | 6.94 ± 0.44 j |
| N1D2 | 328.00 ± 11.14 l | 298.67 ± 16.29 mn | 56.33 ± 1.15 c | 66.67 ± 1.53 c | 46.63 ± 0.73 ab | 50.17 ± 0.69 a | 6.98 ± 0.33 jk | 7.91 ± 0.13 efgh |
| N1D3 | 422.67 ± 8.08 j | 381.33 ± 10.07 l | 52.00 ± 1.00 de | 62.33 ± 1.53 de | 45.79 ± 0.70 bcd | 48.97 ± 1.21 bc | 7.78 ± 0.25 f | 9.24 ± 0.42 b |
| N1D4 | 466.00 ± 11.14 i | 429.33 ± 13.61 hij | 44.33 ± 1.15 f | 53.33 ± 0.58 g | 43.45 ± 0.28 fgh | 47.37 ± 0.25 ef | 7.30 ± 0.34 ghij | 8.83 ± 0.29 bc |
| N2D1 | 387.33 ± 14.05 k | 323.33 ± 32.33 m | 61.33 ± 1.53 ab | 69.33 ± 1.53 b | 46.84 ± 0.35 a | 50.83 ± 0.49 a | 8.61 ± 0.19 cd | 8.83 ± 0.31 bc |
| N2D2 | 480.00 ± 5.29 hi | 442.00 ± 20.88 ghi | 54.33 ± 0.58 cd | 61.33 ± 2.52 e | 45.48 ± 0.48 cd | 48.13 ± 0.77 bcde | 9.13 ± 0.44 b | 10.36 ± 0.76 a |
| N2D3 | 594.67 ± 22.03 f | 528.00 ± 14.42 de | 50.00 ± 2.65 e | 57.00 ± 3.00 f | 42.16 ± 1.09 ij | 46.14 ± 0.60 gh | 9.83 ± 0.28 a | 10.51 ± 1.44 a |
| N2D4 | 628.67 ± 10.07 de | 560.00 ± 25.06 cd | 41.67 ± 1.15 fg | 51.00 ± 1.73 gh | 41.64 ± 0.21 jk | 45.22 ± 0.31 hij | 8.96 ± 0.15 bc | 10.14 ± 0.79 a |
| N3D1 | 334.67 ± 26.03 l | 285.33 ± 12.06 n | 59.33 ± 1.15 b | 64.33 ± 2.08 cd | 45.77 ± 0.87 bcd | 49.07 ± 0.72 b | 7.54 ± 0.30 fgh | 7.56 ± 0.89 ghij |
| N3D2 | 464.00 ± 33.29 i | 418.67 ± 13.01 ijk | 50.67 ± 2.52 e | 57.33 ± 1.53 f | 44.25 ± 0.48 ef | 47.48 ± 0.05 def | 8.28 ± 0.21 de | 8.69 ± 0.23 bcde |
| N3D3 | 511.33 ± 11.37 g | 434.67 ± 10.26 ghi | 43.00 ± 1.00 f | 51.00 ± 1.00 gh | 44.99 ± 0.23 de | 47.92 ± 0.33 cde | 7.69 ± 0.16 fg | 8.24 ± 0.34 cdefg |
| N3D4 | 598.67 ± 21.39 ef | 512.00 ± 18.33 e | 39.00 ± 1.73 gh | 49.67 ± 2.52 h | 38.97 ± 0.58 l | 44.27 ± 0.46 jkl | 7.32 ± 0.49 ghij | 8.07 ± 0.37 cdefgh |
| SN16 | ||||||||
| N1D1 | 354.00 ± 13.86 l | 284.67 ± 19.43 n | 44.00 ± 0.00 f | 47.00 ± 1.00 ij | 44.80 ± 0.17 de | 47.55 ± 0.34 def | 5.52 ± 0.19 m | 5.14 ± 0.37 k |
| N1D2 | 493.33 ± 9.87 ghi | 389.33 ± 38.80 kl | 39.67 ± 1.15 gh | 49.33 ± 1.15 hi | 46.00 ± 0.51 abc | 50.28 ± 0.82 a | 6.88 ± 0.14 jk | 7.70 ± 0.15 fghij |
| N1D3 | 587.33 ± 18.90 f | 462.00 ± 9.17 gh | 37.00 ± 1.00 hi | 46.33 ± 2.08 jk | 45.19 ± 0.34 cde | 48.79 ± 1.14 bc | 7.46 ± 0.13 fghi | 8.22 ± 0.24 cdefg |
| N1D4 | 655.33 ± 9.45 cd | 558.67 ± 26.10 cd | 30.33 ± 3.21 k | 39.67 ± 1.53 l | 42.53 ± 0.31 hij | 45.53 ± 1.15 hi | 7.08 ± 0.25 ijk | 7.86 ± 0.13 fghi |
| N2D1 | 498.00 ± 24.58 gh | 397.33 ± 28.38 jkl | 43.00 ± 1.00 f | 46.33 ± 0.58 jk | 43.72 ± 0.28 fg | 46.82 ± 0.25 fg | 7.00 ± 0.29 jk | 7.09 ± 0.26 ij |
| N2D2 | 606.67 ± 31.64 ef | 501.33 ± 28.59 ef | 38.33 ± 0.58 hi | 46.00 ± 1.00 jk | 42.97 ± 1.45 ghi | 46.17 ± 1.11 gh | 7.61 ± 0.34 fgh | 8.50 ± 0.33 bcdef |
| N2D3 | 678.00 ± 16.37 bc | 578.33 ± 42.50 bc | 33.67 ± 0.58 j | 40.33 ± 0.58 l | 40.87 ± 0.59 k | 44.09 ± 0.82 kl | 7.55 ± 0.19 fgh | 8.16 ± 0.25 cdefg |
| N2D4 | 750.00 ± 32.92 a | 631.00 ± 10.54 a | 29.67 ± 1.53 k | 36.67 ± 2.08 m | 40.67 ± 0.71 k | 43.79 ± 0.27 l | 7.24 ± 0.16 hij | 8.00 ± 0.31 defgh |
| N3D1 | 598.67 ± 14.05 ef | 469.33 ± 22.75 fg | 39.33 ± 1.15 gh | 44.33 ± 1.53 k | 42.69 ± 0.39 hi | 45.88 ± 0.76 ghi | 7.46 ± 0.17 fghi | 7.89 ± 0.26 efghi |
| N3D2 | 691.33 ± 9.45 b | 591.33 ± 18.15 bc | 35.67 ± 2.08 ij | 41.33 ± 0.58 l | 41.63 ± 0.64 jk | 44.91 ± 0.47 ijk | 7.84 ± 0.26 ef | 8.78 ± 0.28 bcd |
| N3D3 | 648.00 ± 17.78 cd | 533.00 ± 17.52 de | 30.00 ± 1.00 k | 39.33 ± 1.15 l | 42.06 ± 0.54 ij | 45.43 ± 0.20 hi | 7.00 ± 0.26 jk | 7.77 ± 0.19 fghi |
| N3D4 | 702.67 ± 9.45 b | 608.33 ± 23.46 ab | 28.00 ± 1.00 k | 34.33 ± 1.53 m | 40.88 ± 0.61 k | 44.22 ± 0.09 jkl | 6.69 ± 0.18 kl | 7.29 ± 0.35 hij |
| Analysis of variance | F values | |||||||
| Y (Year) | 546.59 *** | 809.80 *** | 1045.18 *** | 109.01 *** | ||||
| C (Variety) | 1261.21 *** | 3636.61 *** | 222.38 *** | 215.03 *** | ||||
| N (Nitrogen) | 514.35 *** | 169.25 *** | 181.24 *** | 126.79 *** | ||||
| D (Density) | 746.33 *** | 540.56 *** | 194.07 *** | 60.84 *** | ||||
| Y × C | 54.95 *** | 9.50 ** | 1.73 ns | 2.51 ns | ||||
| Y × N | 2.54 ns | 5.84 ** | 0.14 ns | 1.25 ns | ||||
| Y × D | 1.22 ns | 5.34 ** | 0.87 ns | 7.21 *** | ||||
| C × N | 39.29 *** | 4.32 * | 18.07 *** | 51.35 *** | ||||
| C × D | 5.25 ** | 37.55 *** | 14.04 *** | 2.78 * | ||||
| N × D | 20.40 *** | 4.73 *** | 34.34 *** | 21.47 *** | ||||
| Y × C × N | 0.44 ns | 0.51 ns | 0.31 ns | 3.84 * | ||||
| Y × C × D | 0.68 ns | 3.22 * | 2.50 ns | 0.52 ns | ||||
| Y × N × D | 0.41 ns | 0.88 ns | 1.62 ns | 0.64 ns | ||||
| C × N × D | 18.73 *** | 1.22 ns | 11.02 *** | 2.92 * | ||||
| Y × C × N × D | 598.57 ns | 5.52 ns | 0.30 ns | 53,810.17 ns | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Sun, S.; Jin, M.; Li, C.; Wang, J.; Cui, H.; Li, Y.; Wang, Z. Optimum Nitrogen and Density Allocation for Trade−Off Between Yield and Lodging Resistance of Winter Wheat. Agronomy 2025, 15, 168. https://doi.org/10.3390/agronomy15010168
Liu H, Sun S, Jin M, Li C, Wang J, Cui H, Li Y, Wang Z. Optimum Nitrogen and Density Allocation for Trade−Off Between Yield and Lodging Resistance of Winter Wheat. Agronomy. 2025; 15(1):168. https://doi.org/10.3390/agronomy15010168
Chicago/Turabian StyleLiu, Haitao, Shufang Sun, Min Jin, Chunhui Li, Jiayu Wang, Haixing Cui, Yong Li, and Zhenlin Wang. 2025. "Optimum Nitrogen and Density Allocation for Trade−Off Between Yield and Lodging Resistance of Winter Wheat" Agronomy 15, no. 1: 168. https://doi.org/10.3390/agronomy15010168
APA StyleLiu, H., Sun, S., Jin, M., Li, C., Wang, J., Cui, H., Li, Y., & Wang, Z. (2025). Optimum Nitrogen and Density Allocation for Trade−Off Between Yield and Lodging Resistance of Winter Wheat. Agronomy, 15(1), 168. https://doi.org/10.3390/agronomy15010168




