Effects of Irrigation with Treated Slaughterhouse Effluent and Bradyrhizobium spp. Inoculation on Soybean Development and Productivity: Strategies for Sustainable Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Soybean Cultivation
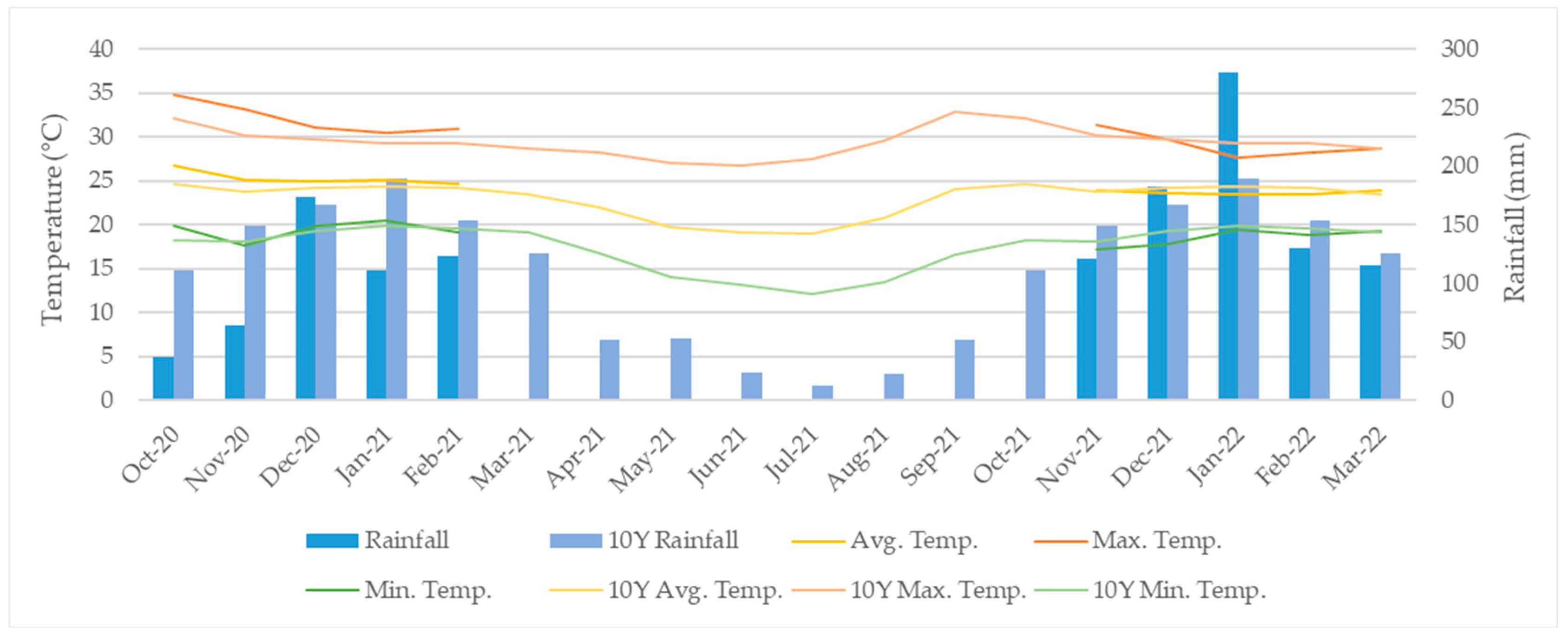
2.3. Irrigation Management
2.4. Biometric Analyses
2.4.1. Foliar Diagnosis
2.4.2. Instrumentation: Chlorophyll Index and Normalized Difference Vegetation Index
2.4.3. Yield and Bromatological Analysis of Grains
2.5. Statistical Analysis
2.6. Generative AI Assistance
3. Results
3.1. Irrigation Management and Nitrogen Incoming
3.2. Biometric Analyses
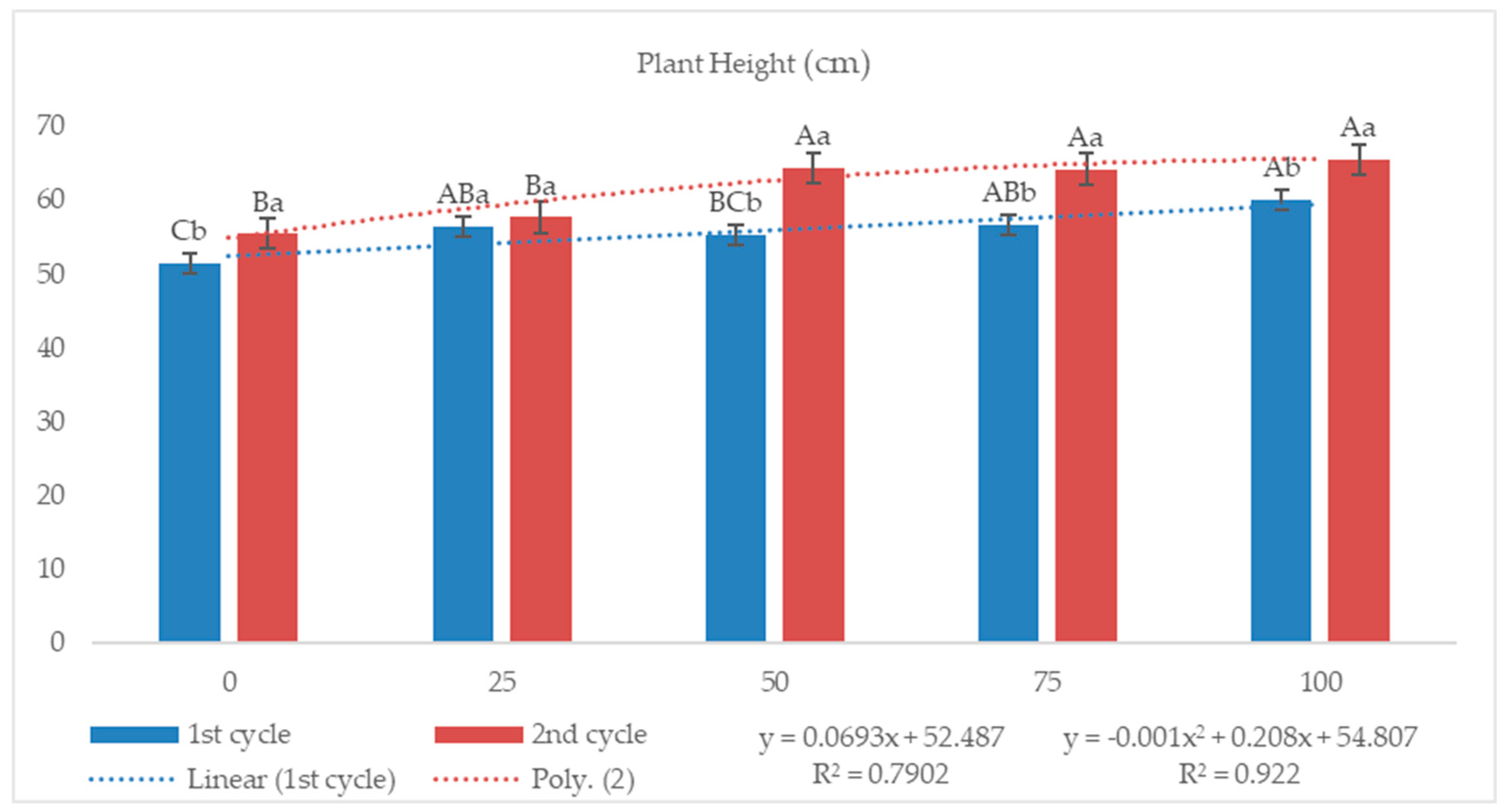
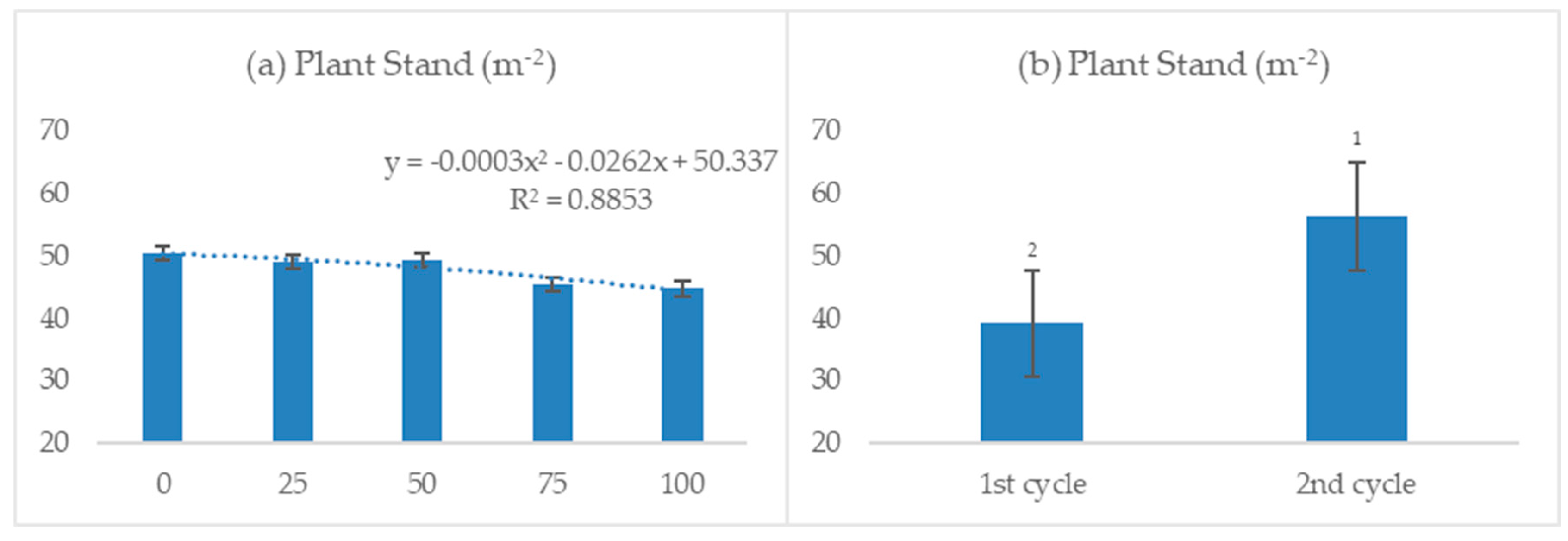
3.2.1. Plant Height and Stand
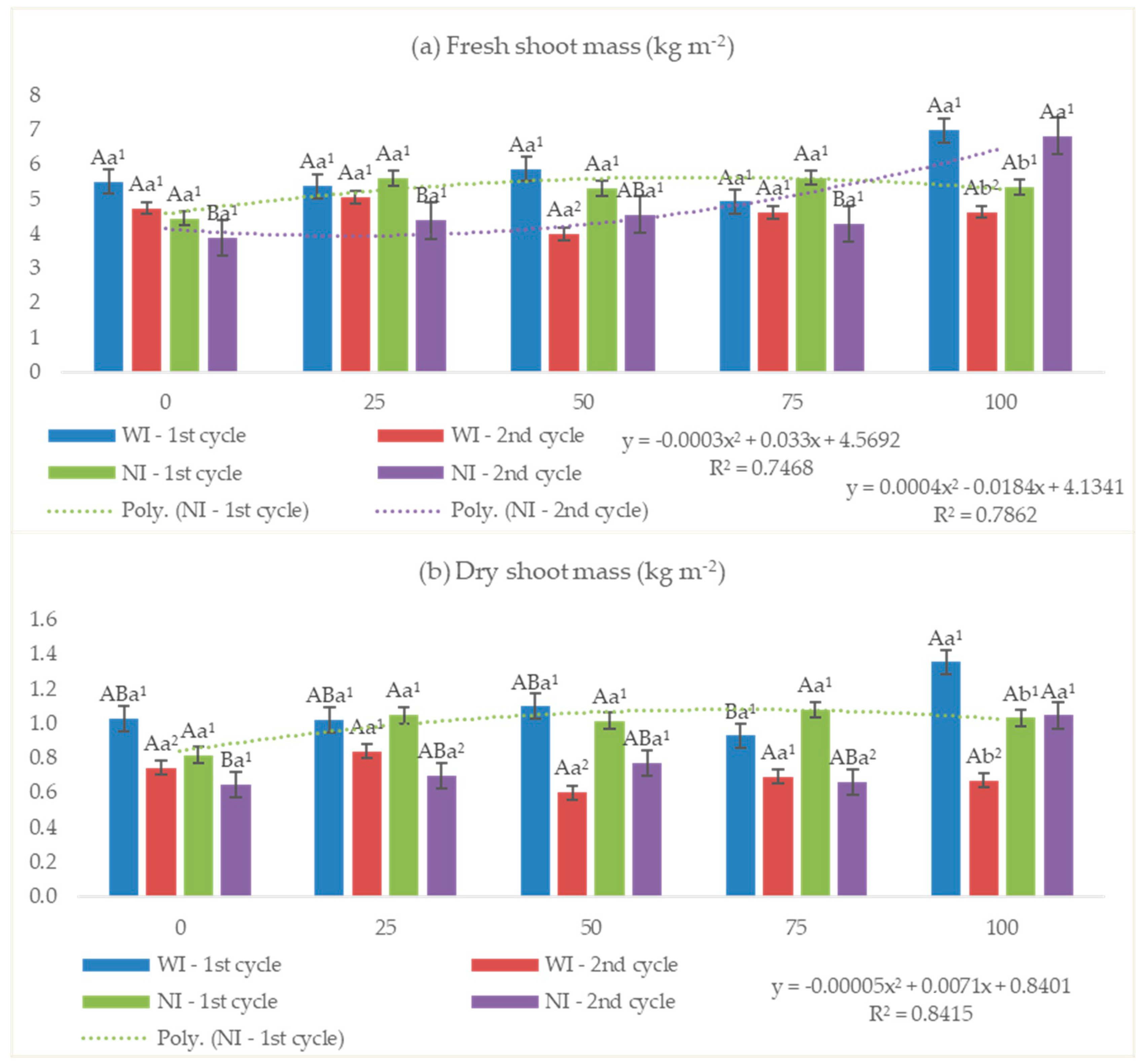
3.2.2. Fresh and Dry Shoot Mass
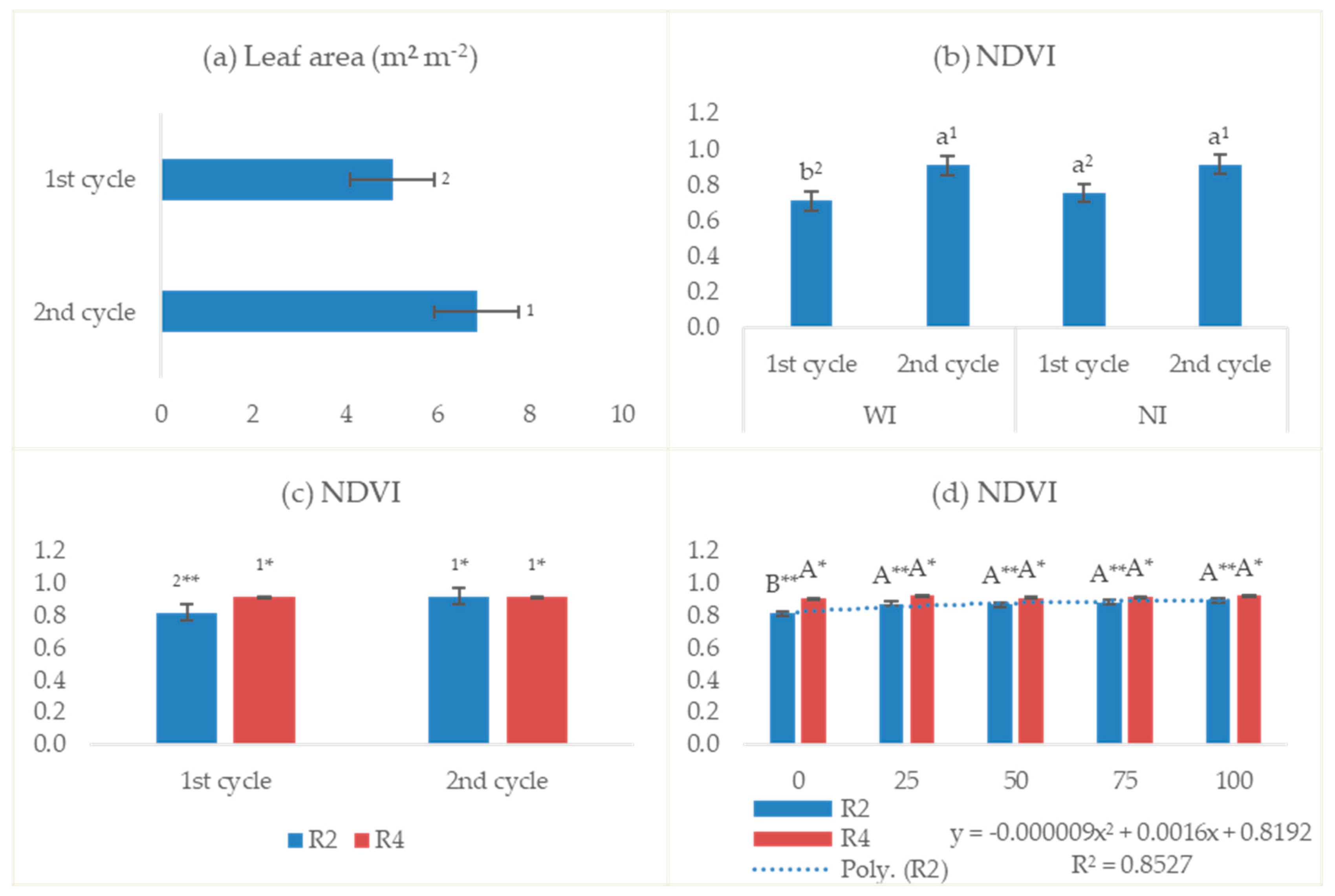
3.2.3. Leaf Area and Normalized Difference Vegetation Index
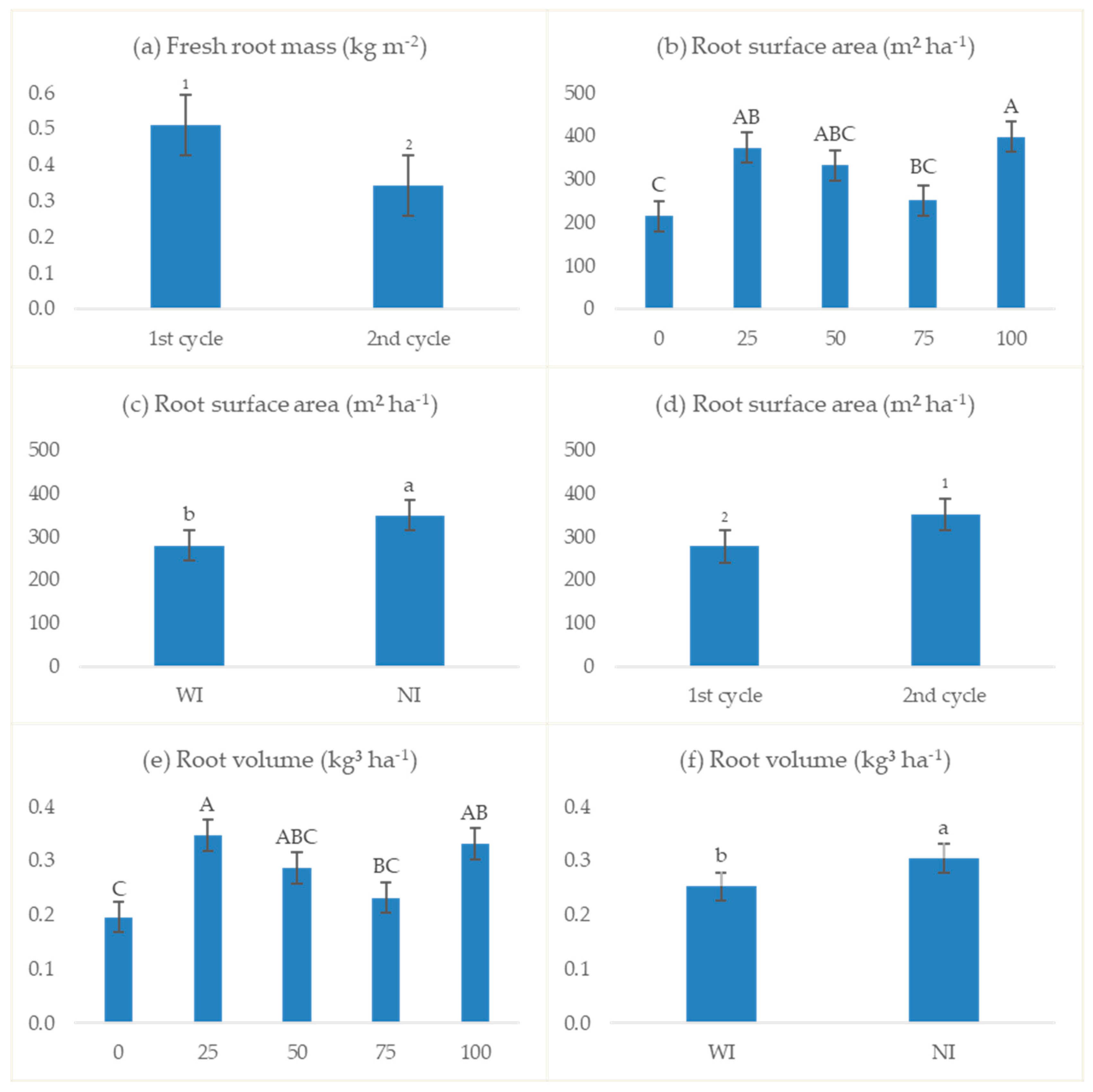
3.2.4. Root Parameters
3.2.5. Foliar Diagnosis
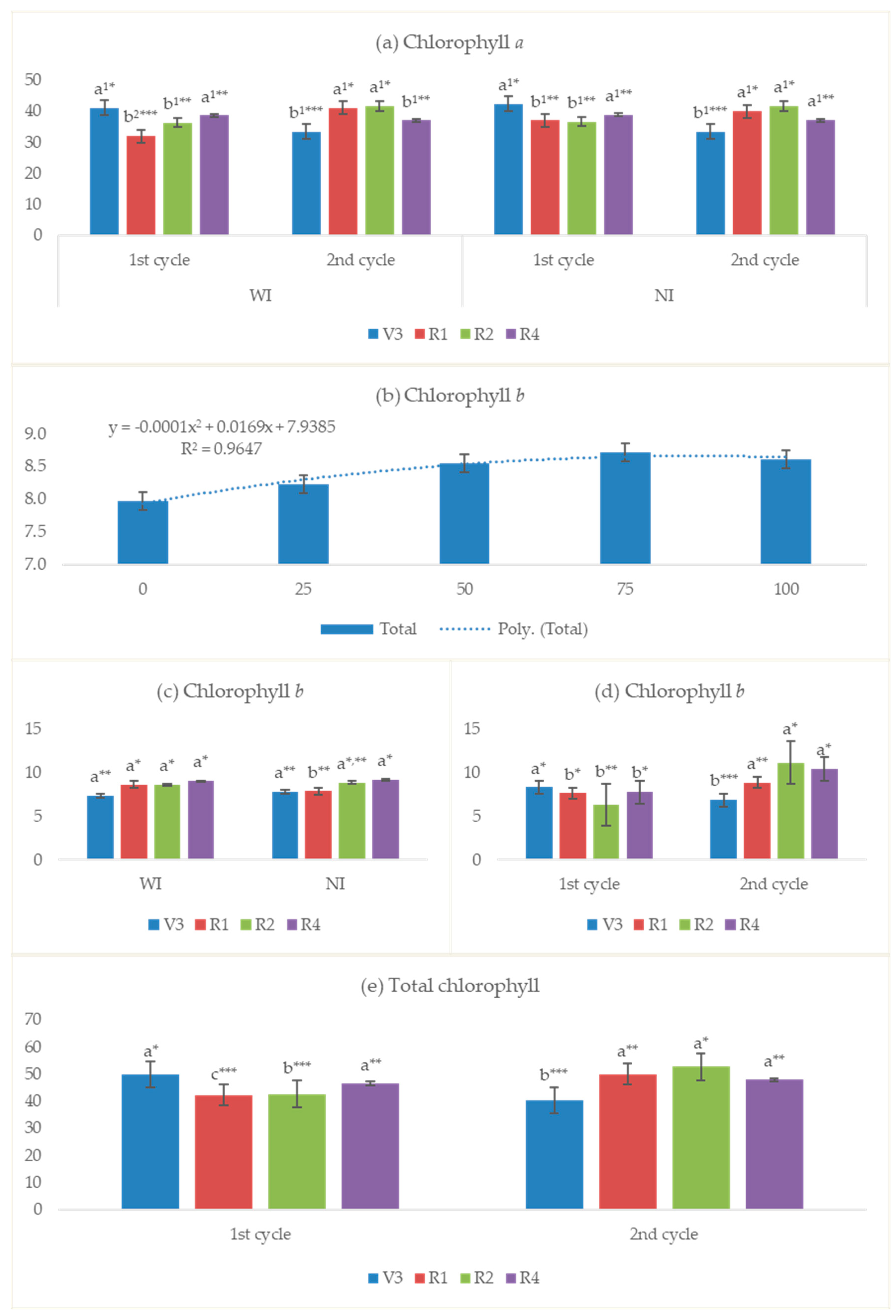
3.2.6. Chlorophyll Index
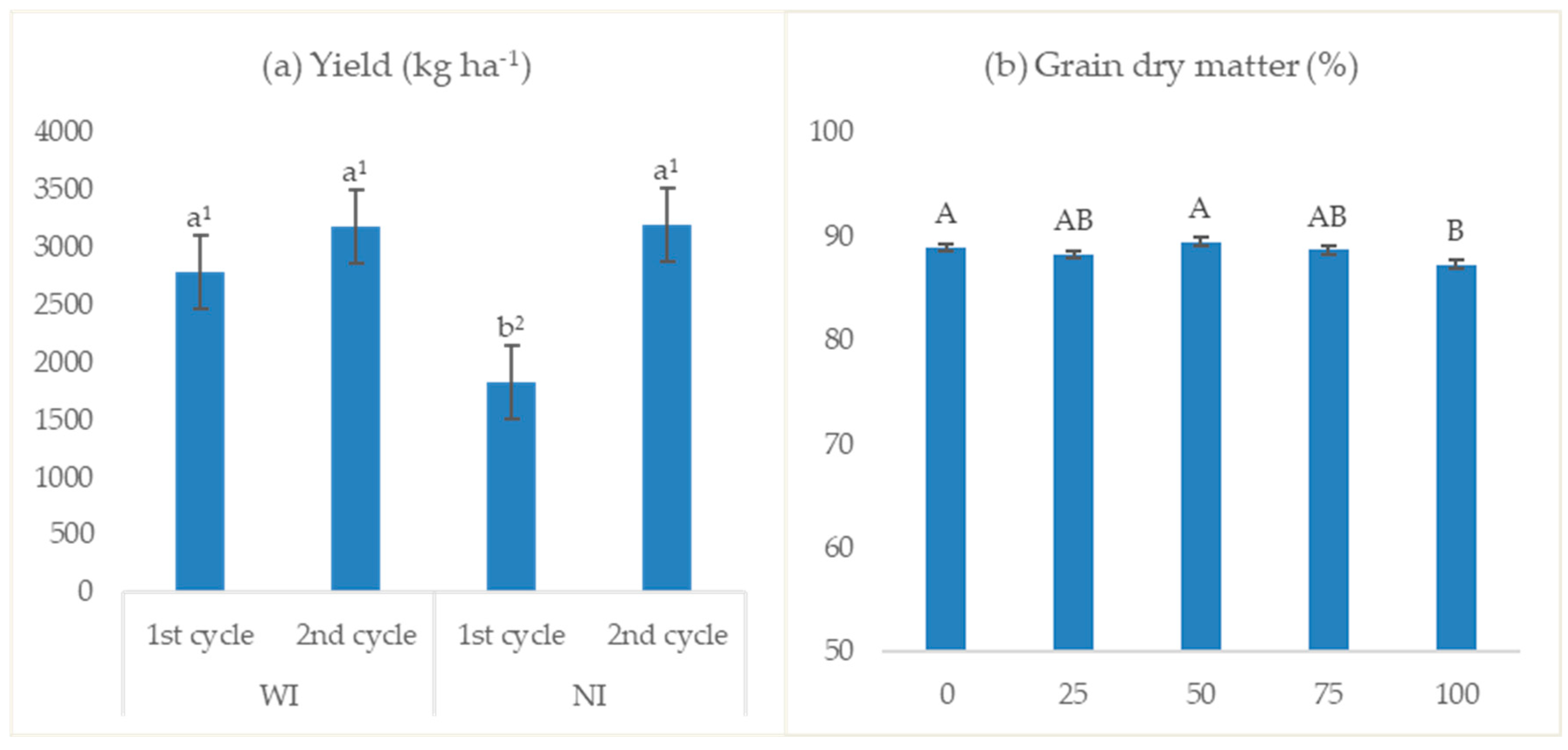
3.2.7. Yield and Grain Quality
4. Discussion
4.1. Plant Height and Stand
4.2. Fresh and Dry Shoot Mass
4.3. Leaf Area and Normalized Difference Vegetation Index
4.4. Root Parameters
4.5. Foliar Diagnosis
4.6. Chlorophyll Index
4.7. Yield and Grain Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, W.C.; Conferência da Água da ONU: A Contribuição da USP. Jornal da USP, São Paulo, 12 April 2023. Available online: https://jornal.usp.br/?p=626794 (accessed on 20 October 2024).
- Bruyninckx, H.; Hatfield-Dodds, S.; Hellweg, S.; Schandl, H.; Vidal, B.; Razian, H.; Nohl, R.; Marcos-Martinez, R.; West, J.; Lu, Y.; et al. Global Resources Outlook 2024: Bend the Trend—Pathways to a Liveable Planet as Resource Use Spikes; International Resource Panel: Nairobi, Kenya, 2024; Available online: https://wedocs.unep.org/20.500.11822/44901 (accessed on 20 October 2024).
- Agência Nacional de Águas e Saneamento Básico (ANA). Conjuntura dos Recursos Hídricos no Brasil 2023: Informe Anual; ANA: Brasília, Brazil, 2024; 118p. [Google Scholar]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Almeida, I.C.C.; Fernandes, R.B.A.; Neves, J.C.L.; Ruiz, H.A.; De Lima, T.L.B.; Hoogmoed, W. Soil quality after six years of paper mill industrial wastewater application. Rev. Bras. Ciênc. Solo 2017, 41, e0160400. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yu, X. Wastewater irrigation and crop yield: A meta-analysis. J. Integr. Agric. 2022, 21, 1215–1224. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT: Crops. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 29 September 2024).
- Companhia Nacional de Abastecimento (CONAB). Boletim da Safra de Grãos. 12° Levantamento—Safra 2023/24. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 14 October 2024).
- Ramlal, A.; Nautiyal, A.; Lal, S.K.; Chigeza, G. A wonder legume, soybean: Prospects for improvement. Front. Plant Sci. 2023, 14, 1294185. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Pikuła, W.; Jańczak-Pieniążek, M. Effect of nitrogen fertilization and inoculation with Bradyrhizobium japonicum on nodulation and yielding of soybean. Agronomy 2023, 13, 1341. [Google Scholar] [CrossRef]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J.L. Does biological nitrogen fixation modify soybean nitrogen dilution curves? Field Crops Res. 2018, 223, 171–178. [Google Scholar] [CrossRef]
- Gai, Z.; Zhang, J.; Li, C. Effects of starter nitrogen fertilizer on soybean root activity, leaf photosynthesis and grain yield. PLoS ONE 2017, 12, e0174841. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Xiao, J.; Tang, L.; Zheng, Y. Interactive influences of intercropping by nitrogen on flavonoid exudation and nodulation in faba bean. Sci. Rep. 2019, 9, 4818. [Google Scholar] [CrossRef]
- Serafin-Andrzejewska, M.; Jama-Rodzeńska, A.; Helios, W.; Kozak, M.; Lewandowska, S.; Zalewski, D.; Kotecki, A. Influence of nitrogen fertilization, seed inoculation and the synergistic effect of these treatments on soybean yields under conditions in south-western Poland. Sci. Rep. 2024, 14, 6672. [Google Scholar] [CrossRef]
- Da Silva, G.F.; Calonego, J.C.; Luperini, B.C.O.; Chamma, L.; Zanetti, W.A.L.; Borghi, E.; Parrella, R.A.C.; Putti, F.F. Nitrogen fertilization in biomass sorghum improves the yield and quality of soybeans grains grown in succession in long-term management systems. Eur. J. Agron. 2024, 158, 127206. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012; 1496p. [Google Scholar]
- Farias, J.R.B.; Nepomuceno, A.L.; Neumaier, N. Ecofisiologia da Soja; Circular Técnica No. 48; Embrapa Soja: Londrina, Brazil, 2007; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/470308 (accessed on 20 August 2024).
- Braga, N.R.; Reco, P.C.; Galo, P.B.; de Miranda, M.A.C.; Mascarenhas, H.A.A. Soybean: Glycine max (L.) Merrill. In Boletim 200: Instruções agrícolas para as principais culturas econômicas, 7th ed.; da Eira Aguiar, A.T., Gonçalves, C., Zagatto Paterniani, M.E.A.G., Tucci, M.L.S.A., de Castro, C.E.F., Eds.; Instituto Agronômico: Campinas, Brazil, 2014; p. 452. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Oliveira, J.F.D.; Rodrigues, F.N.; Fia, R.; Vilela, H.S.; Landim, D.V. Chemical properties of soil fertirrigated with dairy and slaughterhouse wastewater. Eng. Agríc. 2017, 37, 1244–1253. [Google Scholar] [CrossRef]
- Araujo, I.R.; Sampaio, S.C.; Paz-Gonzalez, A.; Vilas-Boas, M.A.; Gonçalves, A.C.; Szekut, F.D. Reforested soil under drip irrigation with treated wastewater from poultry slaughterhouse. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 439–445. [Google Scholar] [CrossRef]
- Emerick, T.; Vieira, J.L.; Silveira, M.H.L.; João, J.J. Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J. Environ. Chem. Eng. 2020, 8, 104308. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Meiramkulova, K.; Poulopoulos, S.G. Treatment of a poultry slaughterhouse wastewater using advanced oxidation processes. J. Water Process Eng. 2022, 47, 102694. [Google Scholar] [CrossRef]
- Madeira, L.; Carvalho, F.; Almeida, A.; Teixeira, M.R. Optimization of atmospheric carbonation in the integrated treatment immediate one-step lime precipitation and atmospheric carbonation: The case study of slaughterhouse effluents. Results Eng. 2023, 17, 100807. [Google Scholar] [CrossRef]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Boletim Técnico 100; Instituto Agronômico de Campinas: Campinas, Brasil, 1997; 285p. [Google Scholar]
- Etiosa, O.R.; Chika, N.B.; Benedicta, A. Mineral and proximate composition of soya bean. Asian J. Phys. Chem. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Qin, Y.; Park, S.Y.; Oh, S.W.; Lim, M.H.; Shin, K.S.; Cho, H.S.; Lee, S.K.; Woo, H.J. Nutritional composition analysis for beta-carotene-enhanced transgenic soybeans (Glycine max L.). Appl. Biol. Chem. 2017, 60, 299–309. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Khan, M.A.; El-Harty, E.H.; Ammar, M.H.; Farooq, M.; Migdadi, H.M. Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi J. Biol. Sci. 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Revised Consensus Document on Compositional Considerations for New Varieties of Soybean [Glycine max (L.) Merr.]: Key Food and Feed Nutrients, Anti-Nutrients, Toxicants and Allergens; Series on the Safety of Novel Foods and Feeds; No. 25; OECD: Paris, France, 2012; Available online: https://www.oecd.org (accessed on 26 October 2024).
- Samudin, S.; Kuswantoro, H. Effect of rhizobium inoculation to nodulation and growth of soybean [Glycine max (L.) Merrill] germplasm. Legume Res. 2018, 41, 303–310. [Google Scholar] [CrossRef]
- Kakabouki, I.; Zisi, C.; Mavroeidis, A.; Kouneli, V.; Karydogianni, S.; Folina, A.; Triantafyllidis, V.; Efthimiadou, A.; Roussis, I.; Zotos, A.; et al. Effects of nitrogen fertilization on weed flora and productivity of soybean [Glycine max (L.) Merr.] crop. Nitrogen 2022, 3, 284–297. [Google Scholar] [CrossRef]
- Do Carmo, E.L.; Rocha, A.G.C.; Simon, G.A.; Da Silva, A.G.; Braz, G.B.P. Adensamento de plantas e épocas de cultivo de soja no cerrado. Colloq. Agrar. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J.L. Nutritional and environmental effects on biological nitrogen fixation in soybean: A meta-analysis. Field Crops Res. 2019, 240, 106–115. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Gurpreet Kaur, G.; Orlowski, J.M.; Shapiro, C.A.; Lee, C.D.; Wortmann, C.; Holshouser, D.; Nafziger, E.D.; Kandel, H.; Niekamp, J.; et al. Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crops Res. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Buczek, J.; Bobrecka-Jamro, D.; Szpunar-Krok, E.; Tobiasz-Salach, R.; Jarecki, W. Morphophysiology, productivity and quality of soybean (Glycine max (L.) Merr.) cv. Merlin in response to row spacing and seeding systems. Agronomy 2021, 11, 403. [Google Scholar] [CrossRef]
- Montoya, F.; García, C.; Pintos, F.; Otero, A. Effects of irrigation regime on the growth and yield of irrigated soybean in temperate humid climatic conditions. Agric. Water Manag. 2017, 193, 30–45. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A. The combined effect of drought stress and nitrogen fertilization on soybean. Agronomy 2020, 10, 384. [Google Scholar] [CrossRef]
- Da Silva, G.F.; Calonego, J.C.; Luperini, B.C.O.; Chamma, L.; Alves, E.R.; Rodrigues, S.A.; Putti, F.F.; Silva, V.M.; Silva, M.A. Soil–Plant relationships in soybean cultivated under conventional tillage and long-term no-tillage. Agronomy 2022, 12, 697. [Google Scholar] [CrossRef]
- McCoy, J.M.; Kaur, G.; Golden, B.R.; Orlowski, J.M.; Cook, D.R.; Bond, J.A.; Cox, M.S. Nitrogen fertilization of soybean affects root growth and nodulation on two soil types in Mississippi. Commun. Soil Sci. Plant Anal. 2018, 49, 181–187. [Google Scholar] [CrossRef]
- Bi, Y.; Fan, C.; Liang, W.; Liao, Y.; Han, D.; Li, W.; Chen, X.; Liu, M.; Liu, J.; Di, S.; et al. Combination effects of sulfur fertilizer and rhizobium inoculant on photosynthesis dynamics and yield components of soybean. Agronomy 2024, 14, 794. [Google Scholar] [CrossRef]
- Allen Jr, L.H.; Zhang, L.; Boote, K.J.; Hauser, B.A. Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant. Crop J. 2018, 6, 148–161. [Google Scholar] [CrossRef]
- Kebede, E. Competency of rhizobial inoculation in sustainable agricultural production and biocontrol of plant diseases. Front. Sustain. Food Syst. 2021, 5, 728014. [Google Scholar] [CrossRef]
- Fu, X.; Bian, D.; Gu, X.; Cao, J.; Liu, J. Combination of graphene oxide and rhizobium improved soybean tolerance in saline-alkali stress. Agronomy 2023, 13, 1637. [Google Scholar] [CrossRef]
- Flajšman, M.; Mihelič, R.; Kolmanič, A.; Ačko, D.K. Influence of soil amended with zeolite and/or mineral N on agronomic performance and soil mineral N dynamics in a soybean–winter triticale crop rotation field experiment. Cereal Res. Commun. 2020, 48, 239–246. [Google Scholar] [CrossRef]
- La Menza, N.C.; Monzon, J.P.; Specht, J.E.; Lindquist, J.L.; Arkebauer, T.J.; Graef, G.; Grassini, P. Nitrogen limitation in high-yield soybean: Seed yield, N accumulation, and N-use efficiency. Field Crops Res. 2019, 237, 74–81. [Google Scholar] [CrossRef]
- Hoffmann, B.; Varga, B.; Nagy, E.; Hoffmann, S.; Darkó, É.; Tajti, J.; Janda, T. Effects of nitrogen and water deficiency on agronomic properties, root characteristics and expression of related genes in soybean. Agronomy 2021, 11, 1329. [Google Scholar] [CrossRef]
- Shin, C.; Sharif, M.O.; Lee, H. Evaluating the effect of bacterial inoculation and fertilization on the soil nutrient status of coal mine soil by growing soybean (Glycine max) and shrub lespedeza (Lespedeza bicolor). Sustainability 2018, 10, 4793. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; de Borja Reis, A.F.; Córdova, S.C.; Castellano, M.J.; Archontoulis, S.V.; Correndo, A.A.; De Almeida, L.F.A.; Rosso, L.H.M. Revisiting biological nitrogen fixation dynamics in soybeans. Front. Plant Sci. 2021, 12, 727021. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil. 2017, 418, 191–203. [Google Scholar] [CrossRef]
- La Menza, N.C.; Monzon, J.P.; Specht, J.E.; Grassini, P. Is soybean yield limited by nitrogen supply? Field Crops Res. 2017, 213, 204–212. [Google Scholar] [CrossRef]
| Parameter | This Study | Oliveira et al. [20] | Araújo et al. [21] | Emerick et al. [22] | Kanafin et al. [23] | Madeira et al. [24] | ||
|---|---|---|---|---|---|---|---|---|
| 2020–2021 | 2021–2022 | Average | ||||||
| Slaughterhouse Type | General | NP | Poultry | Swine | Poultry | General | ||
| Treatment Type | Anaerobic | Physical | Aerobic | Physical Chemist | Photochemical | Physical Chemist | ||
| pH | 8.12 ± 0.75 | 7.45 ± 0.12 | 7.79 ± 0.63 | 7.70 ± 0.30 | 7.00 ± 0.10 | 8.20 ± 0.71 | 7.53 ± 0.22 | 7.50 ± 0.10 |
| EC (dS m−1) | 0.4 ± 0.07 | 0.77 ± 0.09 | 0.58 ± 0.20 | 1.20 ± 0.10 | 0.01 ± 0.00 | 2.82 ± 0.53 | - | 3.00 ± 0.10 |
| COD (mg L−1) | 96.04 ± 42.25 | 178.46 ± 80.67 | 279.62 ± 243.00 | 5.81 ± 3007.00 | 80.90 ± 14.70 | 405.00 ± 8.20 | 155.00 ± 15.00 | 226.00 ± 41.00 |
| TKN (mg L−1) | 34.03 ± 9.27 | 77.49 ± 7.74 | 53.34 ± 23.25 | 157.50 ± 27.00 | 48.70 ± 11.50 | 9.20 ± 2.52 | - | 58.00 ± 0.00 |
| N-NH4+ (mg L−1) | 22.19 ± 8.88 | 59.71 ± 9 | 40.95 ± 20.79 | - | - | - | 0.84 | 52.00 ± 0.00 |
| Nitrate (mg L−1) | 0.32 ± 0.18 | ND | 0.32 ± 0.18 | - | - | - | 2.98 | - |
| Nitrite (mg L−1) | ND | ND | ND | - | - | - | - | - |
| Soluble Phosphoru (mg L−1) | 3.57 ± 0.4 | 5.46 ± 0.99 | 4.62 ± 1.22 | - | - | - | - | - |
| Total Phosphorus (mg L−1) | 7.16 ± 2.9 | 7.98 ± 0.87 | 7.61 ± 2.08 | 11.90 ± 9.60 | 20.20 ± 5.50 | 0.26 ± 0.06 | - | - |
| Potassium (mg L−1) | 14.88 ± 3.31 | NA | 14.88 ± 3.31 | 12.60 ± 5.00 | - | - | 14.86 | - |
| Calcium (mg L−1) | 17.97 ± 0.95 | 23.25 ± 1.45 | 17.97 ± 0.95 | 65.00 ± 38.10 | - | - | 132.43 | 190.50 ± 0.00 |
| Magnesium (mg L−1) | 1.58 ± 0.29 | 2.44 ± 0.19 | 1.58 ± 0.29 | 89.00 ± 52.20 | - | - | 67.27 | 60.20 ± 4.20 |
| Sulfur (mg L−1) | 2.12 ± 2.35 | NA | 2.12 ± 2.35 | - | - | - | - | - |
| Iron (mg L−1) | 2.61 ± 2.94 | NA | 2.61 ± 2.94 | - | - | - | - | - |
| Manganese (mg L−1) | 0.06 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.02 | - | - | - | - | - |
| Sodium (mg L−1) | 35.7 ± 9.59 | NA | 35.70 ± 9.59 | 21.50 ± 3.60 | - | - | 141.87 | - |
| SAR (mmol L−1)−0.5 | 3.34 ± 0.89 | - | - | - | - | - | - | - |
| Treatment | Precipitation | Tap Water Irrigation | Effluent Irrigation | Total Irrigation | Total Depth | Nitrogen Input |
|---|---|---|---|---|---|---|
| 2020–2021 | (mm) | (kg ha−1) | ||||
| 0% | 468.54 | 395.79 | 0.00 | 395.79 | 864.33 | 0.00 |
| 25% | 468.54 | 338.91 | 76.37 | 415.28 | 883.82 | 25.96 |
| 50% | 468.54 | 277.82 | 161.82 | 439.64 | 908.18 | 55.02 |
| 75% | 468.54 | 181.89 | 223.65 | 405.54 | 874.08 | 76.04 |
| 100% | 468.54 | 107.34 | 298.20 | 405.54 | 874.08 | 101.39 |
| 2021–2022 | (mm) | (kg ha−1) | ||||
| 0% | 756.85 | 287.93 | 0.00 | 287.93 | 1044.78 | 0.00 |
| 25% | 756.85 | 248.43 | 54.40 | 302.84 | 1059.69 | 41.89 |
| 50% | 756.85 | 205.94 | 115.54 | 321.47 | 1078.32 | 88.96 |
| 75% | 756.85 | 136.21 | 159.18 | 295.38 | 1052.23 | 122.56 |
| 100% | 756.85 | 83.15 | 212.23 | 295.38 | 1052.23 | 163.42 |
| N | P | Ca | Mg | S | Na | P | K | Ca | Mg | Na | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | ||||||||||||
| Min. | 40.00 | 2.50 | 4.00 | 3.00 | 2.10 | - | 2.50 | 17.00 | 4.00 | 3.00 | - | |
| Max. | 54.00 | 5.00 | 20.00 | 10.00 | 4.00 | - | 5.00 | 25.00 | 20.00 | 10.00 | - | |
| 2020–2021 | 49.04 | 2.17 1 | 8.95 2 | 6.32 | 1.26 | 0.68 | ||||||
| 0 | 46.37 B1 | 2.10 | 8.40 | 6.52 A2 | 1.03 | 0.19 | 0 | 2.03 | 16.15 A | 12.69 B | 6.91 | 0.29 |
| WI | 46.16 | 2.09 | 7.93 | 6.38 | 1.04 Aa2 | 0.00 | ||||||
| NI | 46.57 | 2.11 | 8.87 | 6.66 | 1.03 Aa2 | 0.38 | WI | 2.09 Aa | 15.83 | 12.37 | 6.83 | 0.17 Ba |
| 25 | 46.64 B1 | 2.19 | 8.49 | 6.84 A2 | 1.32 | 0.45 | ||||||
| WI | 47.26 | 2.08 | 8.67 | 6.88 | 1.33 Aa2 | 0.47 | NI | 1.97 Ba | 16.46 | 13.02 | 6.99 | 0.40 BCa |
| NI | 46.01 | 2.30 | 8.31 | 6.80 | 1.30 Aa2 | 0.42 | ||||||
| 50 | 46.90 B1 | 2.17 | 8.66 | 6.63 A2 | 1.21 | 0.58 | 25 | 2.06 | 14.82 AB | 13.67 AB | 7.51 | 0.40 |
| WI | 45.48 | 2.15 | 8.77 | 6.68 | 1.13 Aa2 | 0.35 | ||||||
| NI | 48.32 | 2.20 | 8.55 | 6.58 | 1.28 Aa2 | 0.80 | WI | 1.96 Ab | 14.39 | 13.81 | 7.46 | 0.41 Ba |
| 75 | 51.11 AB1 | 2.10 | 8.77 | 6.00 AB2 | 1.34 | 0.53 | ||||||
| WI | 52.69 | 2.04 | 9.00 | 5.87 | 1.31 Aa2 | 0.48 | NI | 2.17 ABa | 15.24 | 13.53 | 7.57 | 0.39 Ca |
| NI | 49.53 | 2.17 | 8.54 | 6.14 | 1.37 Aa2 | 0.57 | ||||||
| 100 | 54.19 A1 | 2.26 | 10.45 | 5.61 B2 | 1.39 | 1.65 | 50 | 2.11 | 15.59 AB | 13.61 AB | 7.39 | 0.51 |
| WI | 54.10 | 2.17 | 10.31 | 5.44 | 1.37 Aa2 | 1.72 | ||||||
| NI | 54.28 | 2.35 | 10.59 | 5.78 | 1.41 Aa2 | 1.58 | WI | 2.11 Aa | 15.73 | 13.65 | 7.43 | 0.20 Bb |
| 2021–2022 | 39.10 | 2.01 2 | 19.81 2 | 8.18 | 3.68 | 0.31 | ||||||
| 0 | 38.71 A2 | 1.94 | 18.42 | 7.43 B1 | 3.29 | 0.42 | NI | 2.10 ABa | 15.44 | 13.58 | 7.36 | 0.81 ABa |
| WI | 37.54 | 2.09 | 18.29 | 7.43 | 4.38 Aa1 | 0.40 | ||||||
| NI | 39.88 | 1.80 | 18.55 | 7.44 | 2.19 Bb1 | 0.43 | 75 | 2.10 | 14.33 B | 13.69 AB | 7.10 | 0.37 |
| 25 | 38.01 A2 | 1.89 | 20.57 | 8.40 A1 | 4.29 | 0.33 | ||||||
| WI | 38.86 | 1.80 | 20.66 | 8.22 | 4.55 Aa1 | 0.33 | WI | 2.00 Ab | 14.29 | 13.75 | 6.89 | 0.31 Ba |
| NI | 37.16 | 1.99 | 20.48 | 8.58 | 4.03 Aa1 | 0.33 | ||||||
| 50 | 39.95 A2 | 2.02 | 20.21 | 8.41 A1 | 3.31 | 0.42 | NI | 2.19 Aa | 14.37 | 13.63 | 7.31 | 0.43 BCa |
| WI | 41.45 | 2.06 | 20.14 | 8.43 | 4.10 ABa1 | 0.00 | ||||||
| NI | 38.44 | 1.98 | 20.28 | 8.39 | 2.52 Bb1 | 0.83 | 100 | 2.20 | 14.70 AB | 14.36 A | 6.68 | 1.04 |
| 75 | 38.30 A2 | 2.08 | 20.24 | 8.55 A1 | 4.04 | 0.17 | ||||||
| WI | 36.86 | 1.94 | 20.07 | 8.24 | 3.56 ABb1 | 0.10 | WI | 2.13 Aa | 14.76 | 13.86 | 6.38 | 1.07 Aa |
| NI | 39.74 | 2.23 | 20.41 | 8.86 | 4.53 Aa1 | 0.23 | ||||||
| 100 | 40.52 A2 | 2.11 | 19.58 | 8.09 AB1 | 3.49 | 0.23 | NI | 2.27 Aa | 14.64 | 14.86 | 6.97 | 1.01 Aa |
| WI | 39.83 | 2.08 | 18.61 | 7.63 | 3.19 Ba1 | 0.20 | WI | 2.06 | 15.00 | 13.49 | 7.00 a | 0.43 |
| NI | 41.22 | 2.15 | 20.56 | 8.56 | 3.79 Aa1 | 0.27 | NI | 2.14 | 15.23 | 13.72 | 7.24 b | 0.61 |
| This Study | Etiosa et al. [26] | Qin et al. [27] | Alghamdi et al. [28] | OECD [29] | |||
|---|---|---|---|---|---|---|---|
| Average | Average | cv Poongsan | Average | Average | Min. | Max. | |
| Dry Matter | 88.52 | 91.93 | - | 95.1 | 89.9 | 65.6 | 95.3 |
| Mineral Matter | 4.62 | 4.29 | 5.01 | 5.44 | 5.3 | 3.9 | 7 |
| Crude Protein | 45.03 | 37.69 | 35.05 | 39.02 | 39.5 | 33.2 | 45.5 |
| Crude Fiber | 11.8 | 5.44 | 4.75 | - | 7.8 | - | - |
| Ether Extract | 22.55 | 28.2 | 17.27 | 21.16 | 16.7 | 8.1 | 23.6 |
| Nitrogen-Free Extract | 15.97 | - | - | - | - | - | - |
| Acid Detergent Fiber | 10.5 | - | - | - | 12 | 7.8 | 18.6 |
| Neutral Detergent Fiber | 17.19 | - | - | - | 12.3 | 8.5 | 21.3 |
| This Study | Santachiara et al. [11] | Flajšman et al. [44] | Kakabouki et al. [31] | Szpunar-Krok et al. [10] | |||||
|---|---|---|---|---|---|---|---|---|---|
| SP-Brasil | Argentina | Slovenia | Greece | Poland | |||||
| Treatment | kg ha−1 | Treatment | kg ha−1 | Treatment | kg ha−1 | Treatment | kg ha−1 | Treatment | kg ha−1 |
| WI Cycle 1 | 2782 Aa | 0 kg N ha−1 | 4968 | WI | 3093 | 0 kg N ha−1 | 4066–4095 c | 0 kg N ha−1 | 3740 |
| NI Cycle 1 | 1823 Bb | 600 kg N ha−1 | 5081 | NI | 3162 | 80 kg N ha−1 | 4208–4225 bc | 30 kg N ha−1 | 3860 |
| WI Cycle 2 | 3180 Aa | Control | 3044 | 100 kg N ha−1 | 4327–4343 ab | 60 kg N ha−1 | 3950 | ||
| NI Cycle 2 | 3190 Aa | Zeolite 600 kg ha−1 + | 3161 | 120 kg N ha−1 | 4441–4462 a | NI | 3610 b | ||
| 30 kg N ha−1 + 72–90 kg N ha−1 | 3174 | WI HiStick® | 4010 a | ||||||
| Zeolite 600 kg ha−1 + 30 kg N ha−1 + 72–90 kg N ha−1 | 3132 | WI Nitragina | 3940 a | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad Lima, A.C.; Brichi, L.; Trevisan, L.R.; Leão de Souza Dominguez, A.; Nocera Santiago, G.; Gomes, T.M.; Rossi, F. Effects of Irrigation with Treated Slaughterhouse Effluent and Bradyrhizobium spp. Inoculation on Soybean Development and Productivity: Strategies for Sustainable Management. Agronomy 2025, 15, 167. https://doi.org/10.3390/agronomy15010167
Murad Lima AC, Brichi L, Trevisan LR, Leão de Souza Dominguez A, Nocera Santiago G, Gomes TM, Rossi F. Effects of Irrigation with Treated Slaughterhouse Effluent and Bradyrhizobium spp. Inoculation on Soybean Development and Productivity: Strategies for Sustainable Management. Agronomy. 2025; 15(1):167. https://doi.org/10.3390/agronomy15010167
Chicago/Turabian StyleMurad Lima, Ana Carolina, Lisiane Brichi, Lucas Renato Trevisan, André Leão de Souza Dominguez, Gustavo Nocera Santiago, Tamara Maria Gomes, and Fabrício Rossi. 2025. "Effects of Irrigation with Treated Slaughterhouse Effluent and Bradyrhizobium spp. Inoculation on Soybean Development and Productivity: Strategies for Sustainable Management" Agronomy 15, no. 1: 167. https://doi.org/10.3390/agronomy15010167
APA StyleMurad Lima, A. C., Brichi, L., Trevisan, L. R., Leão de Souza Dominguez, A., Nocera Santiago, G., Gomes, T. M., & Rossi, F. (2025). Effects of Irrigation with Treated Slaughterhouse Effluent and Bradyrhizobium spp. Inoculation on Soybean Development and Productivity: Strategies for Sustainable Management. Agronomy, 15(1), 167. https://doi.org/10.3390/agronomy15010167






