Effects of Climatic, Chemical, and Cultural Control Strategies on Community Composition of Auchenorrhyncha and Population Dynamics of Two Major Green Leafhopper Pests in Peach Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location
2.2. Experimental Procedure
2.3. Morphological Identification
2.4. Data Analysis
3. Results
3.1. Auchenorrhyncha Community and Known Vectors or Potential Vectors of Phytopathogens
| Species | Pests/Vectors/Direct Injury | References |
|---|---|---|
| Neophilaenus campestris (Fallén) | Xylella fastidiosa | [55,56,57] |
| Philaenus spumarius (Linnaeus) | AY, Ca P. solani, Xylella fastidiosa | [52,58,59,60,61] |
| Anaceratagallia glabra Dmitriev | AY, BN | [62,63,64,65] |
| Austroagallia sinuata (Mulsant & Rey) | AY, Ca P. aurantifolia, Ca P. solani | [66,67,68] |
| Aphrodes makarovi Zachvatkin | FD, Ca P. solani | [22,69] |
| Cicadella viridis (Linnaeus) | Ca P. solani, Xylella fastidiosa | [61,70] |
| Balclutha puntacta (Fabricius) | MDP | [71] |
| Euscelidius variegatus (Kirschbaum) | AY, Ca P. solani, CY, FD, WXD | [59,61,72,73,74] |
| Euscelis incisus (Kirschbaum) | AY, Ca P. solani, CY, FD, XD | [59,61,69,75] |
| Fieberiella florii (Stål) | Ca P. mali, Ca P. pronorum, XD | [65,76,77] |
| Neoaliturus fenestratus (Herrich-Schäffer) | AY, Ca P. solani, GY, SP, LP | [64,78,79,80] |
| Psammotettix striatus (Linnaeus) | WWV, Ca P. solani (potential vector) | [61,63,81] |
| Eupelix cuspidata (Fabricius) | Ca P. solani (potencial vector) | [62] |
| Megophthalmus scabripennis Edwards | AY (potencial vector) | [64] |
| Asymmetrasca decedens (Paoli) | Ca P. phoenicium, Ca P. pronorum, direct injury, EFSY | [10,20,21,22,82] |
| Edwardsiana rosae (Linnaeus) | Direct injury | [83,84,85] |
| Fruticidia bisignata (Mulsant & Rey) | Direct injury | [83,87] |
| Hauptidia marocanna (Melichar) | Direct injury | [88] |
| Hauptidia provincialis (Ribaut) | Direct injury | [89] |
| Hebata decipiens Paoli | Alm WB, Ca P. asteris, Ca P. aurantifolia | [20,53,54,86] |
| Hebata solani (Curtis) | Direct injury | [14] |
| Jacobiasca lybica (Bergevin & Zanon) | Direct injury | [7,90,91,92] |
| Ribautiana tenerrima (Herrich-Schäffer) | Direct injury | [93] |
| Zyginidia scutellaris (Herrich-Schäffer) | Direct injury | [94] |
| Hyalesthes obsoletus Signoret | AY, BN | [64,95,96,97,98] |
| Laodelphax striatellus (Fallén) | AY, BN, BYSMV, Ca P. solani, MMV, MRDV, NCMV, RBSDV, RSV | [59,61,99,100,101,102] |
| Metadelphax propinqua (Fieber) | CCSV, MMV, MRDV | [38,68] |
| Dictyophara europaea (Linnaeus) | Ca P. solani, FD | [61,104,105,106] |
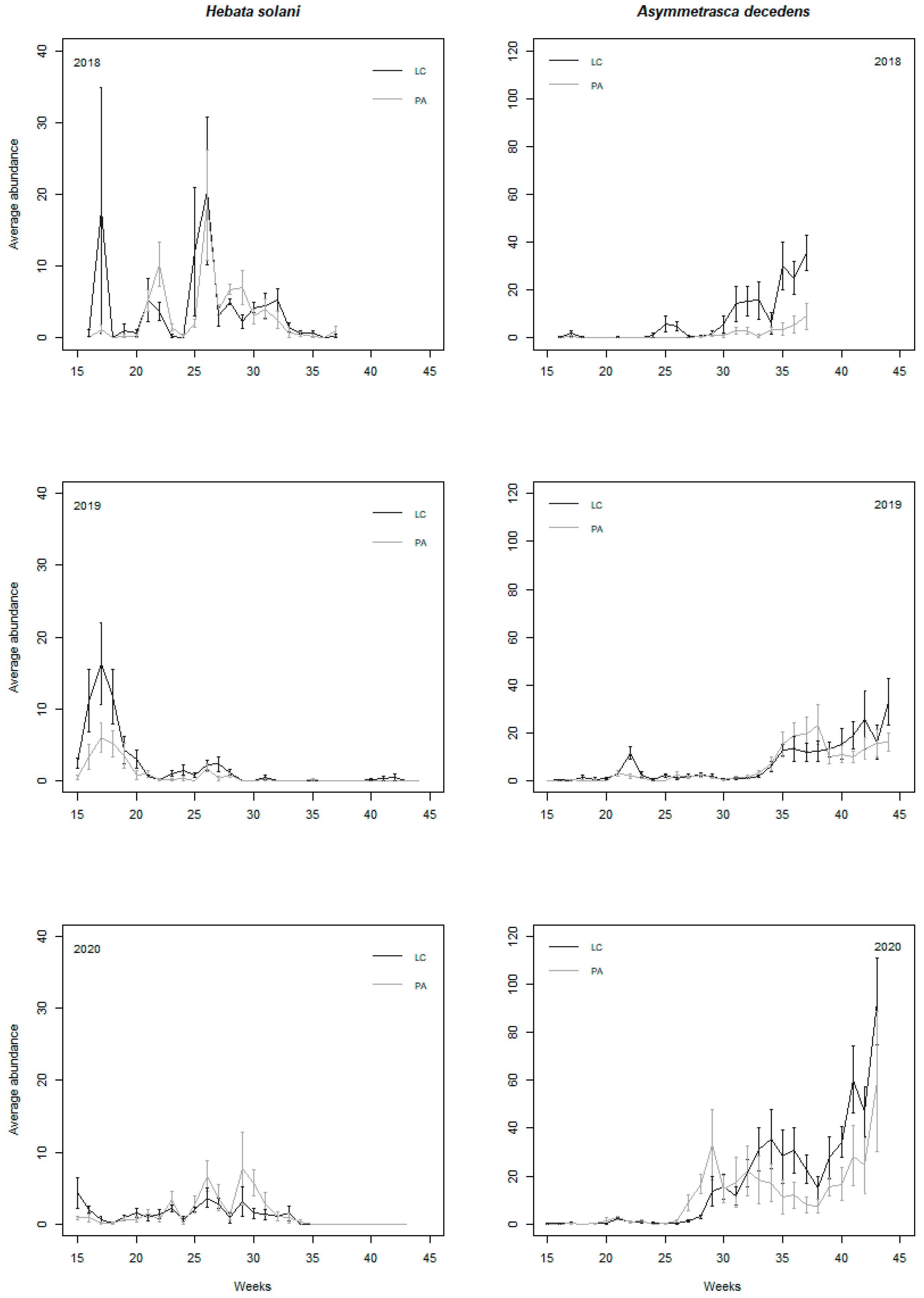
3.2. Population Dynamics of the Two Main Leafhopper Species, Asymmetrasca decedens and Hebata solani
3.3. Analysis and Modelling of Climate Conditions and Management Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Dietrich, C.H.; Zhang, Y.L.; Dmitriev, D.A.; Zhang, L.; Wang, Y.M.; Lu, S.H.; Qin, D.Z. Phylogeny of the tribe Empoascini (Hemiptera: Cicadellidae: Typhlocybinae) based on morphological characteristics, with reclassification of the Empoasca generic group. Syst. Entomol. 2021, 46, 266–286. [Google Scholar] [CrossRef]
- Wolfenbarger, D.; Heuberger, J. Potato yields from different potato leafhopper densities. Am. Pot. J. 1946, 23, 389–395. [Google Scholar] [CrossRef]
- Quartau, J.A.; Rebelo, M.T. Estudos preliminares sobre os cicadelídeos que constituem pragas das vinhas em Portugal (Homoptera, Cicadellidae). Bol. San. Veg. Plagas 1992, 18, 407–413. [Google Scholar]
- Quartau, J.A.; Rebelo, M.T. New data on the monitoring of leafhoppers infesting vineyards in Portugal (Homoptera, Cicadellidae). Bul. OILB/SROP 1993, 16, 36. [Google Scholar]
- Delrio, G.; Lentini, A.; Serra, G. Spatial distribution and sampling of Jacobiasca lybica on grapevine. IOBC WPRS Bull. 2001, 24, 211–216. [Google Scholar]
- Mazzoni, V.; Cosci, F.; Lucchi, A.; Santini, L. Occurrence of leafhoppers (Auchenorrhyncha, Cicadellidae) in three vineyards of the Pisa district. IOBC WPRS Bull. 2001, 24, 267–271. [Google Scholar]
- Alma, A. Auchenorrhyncha as pests on grapevine. Denisia 2002, 176, 541–548. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark, Part 1: Introduction, Infraorder Fulgoromorpha; Scandinavian Science Press: Klampenborg, Denmark, 1978; pp. 1–221. [Google Scholar]
- Raupach, K.; Borgemeister, C.; Hommes, M.; Poehling, H.; Sétamou, M. Effect of temperature and host plants on the bionomics of Empoasca decipiens (Homoptera: Cicadellidae). Crop Prot. 2002, 21, 113–119. [Google Scholar] [CrossRef]
- Alvarado, M.; Villagordo, E.; Berlanga, M.; González, E.; Serrano, A.; De La Rosa, A. Contribución al conocimiento del mosquito verde (Empoasca decedens Paoli) en melocotonero en el Valle del Guadalquivir. Bol. San. Veg. Plagas 1994, 20, 771–783. [Google Scholar]
- Pollini, A.; Bariselli, M. Diffuse infestazioni di cicaline sul pesco e orientamenti di difesa. Inf. Fitopatol. 1995, 1, 15–18. [Google Scholar]
- Backus, E.; Serrano, M.; Ranger, C. Mechanisms of hopperburn: An overview of insect taxonomy, behavior, and physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Jacas, J.; Mendoza, A.; Cambra, M.; Balduque, R. Asymmetrasca decedens: A new pest of almond in Spain. Bull. OEPP 1997, 27, 523–524. [Google Scholar] [CrossRef]
- Freitas, J.; Amaro, P. “Explosion” de cicadelle verte dans la région du Douro au Portugal en juillet/août 1998. IOBC WPRS Bull. 2001, 24, 217–219. [Google Scholar]
- Torres, J.; Mendoza, A.; Garrido, A.; Jacas, J. Dinámica de las poblaciones de cicadélidos (Homoptera: Cicadellidae) en almendros en el Alto Palancia (Prov. Castellón). Bol. San. Plagas 1998, 24, 279–292. [Google Scholar]
- Torres, J.; Mendoza, A.; Jacas, J. Influencia de la temperatura y el fotoperíodo sobre el desarrollo de Asymmetrasca decedens (Paoli) (Homoptera: Cicadellidae). Bol. San. Veg. Plagas 2002, 28, 263–272. [Google Scholar]
- Coutinho, J.; Amado, C.; Barateiro, A.; Quartau, J.; Rebelo, M.T. First record of the leafhopper Asymmetrasca decedens (Homoptera: Cidadellidae) in mainland Portugal. Rev. Ciênc. Agrár. 2015, 38, 213–219. [Google Scholar]
- Instituto Nacional de Estatística. Recenseamento Agrícola. Análise dos Principais Resultados. 2023. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000019&xlang=pt&contexto=bd&selTab=tab2 (accessed on 15 July 2024).
- Chaieb, I.; Bouhacgem-Boukhris, S.; Nusillard, B. Asymmetrasca decedens Paoli and Zygina flammigera Fourcroy (Hemiptera: Typhlocybinae), new pests in peach and almond orchards in Tunisia. Pest Tech. 2011, 5, 71–73. [Google Scholar]
- Dakhil, H.; Hammad, E.; El-Mohtar, C.; Abou-Jawdah, Y. Survey of leafhopper species in almond orchards infected with almond witches’-broom phytoplasma in Lebanon. J. Insect Sci. 2011, 11, 60. [Google Scholar] [CrossRef]
- Abou-Jawdah, Y.; Sater, A.; Jawhari, M.; Sobh, H.; Abdul-Nour, H.; Bianco, P.; Lova, M.; Alma, A. Asymmetrasca decedens (Cicadellidae, Typhlocybinae), a natural vector of ‘Candidatus phytoplasma phoenicium’. Ann. Appl. Biol. 2014, 165, 395–403. [Google Scholar] [CrossRef]
- Pastore, M.; Raffone, E.; Paltrinieri, S.; Bertaccini, A.; Priore, R.; Simeone, A. Phytoplasma detection in Empoasca decedens and Empoasca spp. and their possible role as vectors of European stone fruit yellows (16SrXB) phytoplasma. Acta Hort. 2004, 657, 507–511. [Google Scholar] [CrossRef]
- Decker, G.; Cunningham, H. The mortality rate of the potato leafhopper and some related species when subjected to prolonged exposure at various temperatures. J. Econ. Entomol. 1967, 60, 373–379. [Google Scholar] [CrossRef]
- Habib, A.; Badawi, A.; Herakly, F. Biological studies on certain species of leafhoppers (Hemiptera—Cicadellidae) in Egypt. Z. Ang. Ent. 1972, 71, 172–178. [Google Scholar] [CrossRef]
- Polgar, A.; Kuroli, G.; Orosz, A. Individual number change of Empoasca spp. cicadas species in potato. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 2002, 67, 547–556. [Google Scholar]
- Meisner, J.; Klein, M.; Ben-Moshe, E. Effect of Margosan-O on the development of the leafhopper Asymmetrasca decedens. Phytoparasitica 1992, 20, 15–23. [Google Scholar] [CrossRef]
- Grassi, A.; Maines, R.; Saviane, A. Efficacy of 3 neonicotinoid insecticides for the control of the green leafhopper Asymmetrasca (Empoasca) decedens Paoli, a new pest on cultivated red raspberry in Trentino, Italy. IOBC-WPRS Bull. 2008, 39, 107–113. [Google Scholar]
- Jervis, M. Life history studies on Aphelopus species (Hymenoptera, Dryinidae) and Chalarus species (Diptera, Pipunculidae) primary parasites of Typhlocybinae leafhoppers (Homoptera, Cicadellidae). J. Nat. Hist. 1980, 14, 769–780. [Google Scholar] [CrossRef]
- Waloff, N.; Jervis, M. Communities of parasitoids associated with leafhoppers and planthoppers in Europe. Adv. Ecol. Res. 1987, 17, 281–402. [Google Scholar]
- Agboka, K.; Tounou, A.; Poehling, H.; Raupach, K.; Borgemeister, C. Searching and oviposition behavior of Anagrus atomus L. (Hymenoptera: Mymaridae) on four host plants of its host, the green leafhopper Empoasca decipiens Paoli (Homoptera: Cicadellidae). J. Insect Behav. 2003, 16, 667–678. [Google Scholar] [CrossRef]
- Hesami, S.; Seyedoleslami, H.; Ebadi, R. Biology of Anagrus atomus (Hymenoptera: Mymaridae), an egg parasitoid of the grape leafhopper Arboridia kermanshah (Homoptera: Cicadellidae). Entomol. Sci. 2004, 7, 271–276. [Google Scholar] [CrossRef]
- Triapitsyn, S.; Rugman-Jones, P.; Jeong, G.; Morse, J.; Stouthamer, R. Morphological and molecular differentiation of the Anagrus epos species complex (Hymenoptera: Mymaridae), egg parasitoids of leafhoppers (Hemiptera: Cicadellidae) in North America. Zootaxa 2010, 2428, 1–21. [Google Scholar] [CrossRef]
- Ribaut, H. Homopteres Auchenorhynques (I. Typhlocybidae); Federation Française des Sociétés de Sciences Naturelles: Paris, France, 1936; pp. 1–231. [Google Scholar]
- Ribaut, H. Homopteres Auchenorhynques II (Jassidae); Federation Française des Sociétés de Sciences Naturelles: Paris, France, 1952; pp. 1–474. [Google Scholar]
- Le Quesne, W. Handbooks for the Identification of British Insects—Hemiptera, Fulgoromorpha; Royal Entomological Society of London: London, UK, 1960; pp. 1–68. [Google Scholar]
- Le Quesne, W. Handbooks for the Identification of British Insects—Hemiptera, Cicadomorpha (Excluding Deltocephalinae and Typhlocybinae); Royal Entomological Society of London: London, UK, 1965; pp. 1–64. [Google Scholar]
- Le Quesne, W. Handbooks for the Identification of British Insects—Hemiptera, Deltocephalinae; Royal Entomological Society of London: London, UK, 1969; pp. 1–83. [Google Scholar]
- Nickel, H. Second addendum to the leafhoppers and planthoppers of Germany (Hemiptera: Auchenorrhyncha). Cicadina 2022, 21, 19–54. [Google Scholar]
- Le Quesne, W.; Payne, K. Handbooks for the Identification of British Insects—Cicadellidae (Typhlocybinae) with a Check List of the British Auchenorrhyncha (Hemiptera, Homoptera); Royal Entomological Society of London: London, UK, 1981; pp. 1–95. [Google Scholar]
- Della Giustina, W. Homopteres Cicadellidae III Compléments aux Ouvrages d’Henri Ribaut; Federation Française des Sociétés de Sciences Naturelles: Paris, France, 1989; pp. 1–350. [Google Scholar]
- Dmitriev, D. 3I Interactive Keys and Taxonomic Databases. Available online: http://dmitriev.speciesfile.org/ (accessed on 12 October 2022).
- Dietrich, C. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Zenner, G.; Stocckmann, M.; Niedringhaus, R. Preliminary key to the nymphs of the families and subfamilies of the German Auchenorrhyncha fauna (Hemiptera, Fulgoromorpha et Cicadomorpha). Beitr. Zikadenk. 2005, 8, 59–78. [Google Scholar]
- Biedermann, R.; Niedrighaus, R. The Plant- and Leafhoppers of Germany—Identification Key to all Species; Wissenschaftlich Akademischer Buchvertrieb-Frund: Scheeßel, Germany, 2009; pp. 1–409. [Google Scholar]
- Bluemel, J.; Derlink, M.; Pavlovcic, P.; Russo, I.; King, R.; Corbett, E.; Sherrard-Smith, E.; Blejec, A.; Wilson, M.; Stewart, A.; et al. Integrating vibrational signals mitochondrial DNA and morphology for species determination in the genus Aphrodes (Hemiptera: Cicadellidae). Syst. Entomol. 2014, 39, 304–324. [Google Scholar] [CrossRef]
- Dmitriev, D.A.; Anufriev, G.A.; Bartlett, C.R.; Blanco-Rodríguez, E.; Borodin, O.I.; Cao, Y.-H.; Deitz, L.L.; Dietrich, C.H.; Dmitrieva, M.O.; El-Sonbati, S.A.; et al. World Auchenorrhyncha Database. TaxonPages. Available online: https://hoppers.speciesfile.org/ (accessed on 15 June 2024).
- Evangelou, V.; Lytra, I.; Krokida, A.; Antonatos, S.; Georgopoulou, I.; Milonas, P.; Papachristos, D.P. Insights into the diversity and population structure of predominant Typhlocybinae species existing in vineyards in Greece. Insects 2023, 14, 894. [Google Scholar] [CrossRef]
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data, 3rd ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 1–432. [Google Scholar]
- Brooks, M.E.; Kristensenk, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; SJ, M.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 July 2024).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 10 July 2024).
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.; Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Galleto, L.; Marzachi, C.; Demichelis, S.; Bosco, D. Host plant determines the phytoplasma transmission competence of Empoasca decipiens (Hemiptera: Cicadellidae). J. Econ. Entomol. 2011, 104, 360–366. [Google Scholar] [CrossRef]
- Safarova, D.; Lauterer, P.; Stary, M.; Valova, P.; Navratil, M. Insight onto epidemiological importance of phytoplasma vector on vineyards in South Moravia, Czech Republic. Plant Protect. Sci. 2018, 54, 234–239. [Google Scholar] [CrossRef]
- Cornara, D.; Saponari, M.; Zeilinger, A.; Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.; Almeida, R.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Yaseen, T.; Valentini, F.; Moussa, I.; Mazzoni, V.; D’onghia, M. Identification of three potential insect vectors of Xylella fastidiosa in southern Italy. Phytopathol. Mediterr. 2014, 53, 328–332. [Google Scholar]
- EFSA panel on plant health. Scientific opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989. [Google Scholar] [CrossRef]
- Orságová, H.; Březíková, M.; Schlesingerova, G. Presence of phytoplasmas in hemipterans in Czech vineyards. Bull. Insectol. 2011, 64, S119–S120. [Google Scholar]
- Rosa, C.; McCarthy, E.; Duong, K.; Hoover, G.; Moorman, G. First Report of the spittlebug Lepyronia quadrangularis and the leafhopper Latalus sp. as vectors of the elm yellows associated phytoplasma, Candidatus phytoplasma ulmi in North America. Plant Dis. 2014, 98, 154. [Google Scholar] [CrossRef]
- Quaglino, F.; Sanna, F.; Moussa, A.; Faccincani, M.; Passera, A.; Casati, P.; Mori, N. Identification and ecology of alternative insect vectors of ‘Candidatus phytoplasma solani’ to grapevine. Sci. Rep. 2019, 9, 19522. [Google Scholar] [CrossRef]
- Battle, A.; Martínez, M.; Laviña, A. Occurrence, distribution and epidemiology of grapevine yellows in Spain. Eur. J. Plant Pathol. 2000, 106, 811–816. [Google Scholar] [CrossRef]
- Drobnjakovic, T.; Peric, P.; Marcic, D.; Picciau, L.; Alma, A.; Motrovic, J.; Duduk, B.; Betraccini, A. Leafhoppers and cixiids in phytoplasma-infected carrots fields: Species composition and potential phytoplasma vectors. Pestic. Phytomed. 2010, 25, 311–318. [Google Scholar] [CrossRef]
- Orenstein, S.; Zahavi, T.; Nestel, D.; Sharon, R.; Barkalifa, M.; Weintraub, P. Spatial dispersion patterns of potential leafhopper and planthopper (Homoptera) vectors of phytoplasma in wine vineyards. Ann. Appl. Biol. 2003, 142, 341–348. [Google Scholar] [CrossRef]
- Tanne, E.; Boudon-Padieu, E.; Clair, D.; Davidovich, M.; Melamed, S.; Klein, M. Detection of phytoplasma by polymerase chain reaction of insect feeding medium and its use in determining vectoring ability. Phytopathology 2001, 91, 741–746. [Google Scholar] [CrossRef]
- Hemmati, C.; Nikooei, M.; Bertaccini, A. Identification and transmission of phytoplasmas and their impact on essential oil composition in Aerva javanica. Biotech 2019, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, C.; Nikooei, M. Austroagallia sinuata transmission of “Candidatus phytoplasma aurantifolia” to Zinnia elegans. J. Plant Pathol. 2019, 101, 1223. [Google Scholar] [CrossRef]
- Nahdi, S.; Bouhachem, S.B.; Mahfoudhi, N.; Paltrinieri, S.; Bertaccini, A. Identification of phytoplasmas and Auchenorryncha in Tunisian vineyards. Phytopathogenic Mollicutes 2020, 10, 25–35. [Google Scholar] [CrossRef]
- Bressan, A.; Clair, D.; Semetey, O.; Boudon-Padieu, E. Insect injection and artificial feeding bioassays to test the vector specificity of Flavescence Dorée phytoplasma. Phytopathology 2006, 96, 790–797. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Saponari, M.; Dongiovanni, C.; Altamura, G.; Bosco, D. Transmission of Xylella fastidiosa subsp. pauca ST53 by the sharpshooter Cicadella viridis from different source plants and artificial diets. J. Econ. Entomol. 2022, 115, 1852–1858. [Google Scholar]
- Han, S. Transmission of mulberry dwarf phytoplasma by Balclutha punctata. J. Korean For. Soc. 2012, 101, 635–639. [Google Scholar]
- Bosco, D.; Minucci, C.; Boccardo, G.; Maurizio, C. Differential acquisition of chrysanthemum yellows phytoplasma by three leafhopper species. Entomol. Exp. Appl. 1997, 83, 219–224. [Google Scholar] [CrossRef]
- Jensen, D. Comparative transmission of Western X-Disease virus by Colladonus montanus, C. geminatus, and a new leafhopper vector, Euscelidius variegatus. J. Econ. Entomol. 1969, 62, 1147–1150. [Google Scholar] [CrossRef]
- Lefol, C.; Lherminier, J.; Boudon-Padieu, E.; Larrue, J.; Louis, C.; Caudwell, A. Propagation of Flavescence dorée MLO (mycoplasma-like organism) in leafhopper vector Euscelidius variegatus Kbm. J. Invertebr. Pathol. 1994, 63, 293–295. [Google Scholar] [CrossRef]
- Marzachi, C.; Veratti, F.; Bosco, D. Direct PCR detection of phytoplasmas in experimentally infected insects. Ann. Appl. Biol. 1998, 133, 45–54. [Google Scholar] [CrossRef]
- Landi, L.; Isidoro, N.; Riolo, P. Natural phytoplasma infection of four phloem-feeding Auchenorrhyncha across vineyard agroecosystems in Central-Eastern Italy. J. Econ. Entomol. 2013, 106, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Alma, A. Fieberiella florii (Homoptera: Auchenorrhyncha) as a vector of “Candidatus phytoplasma mali”. Plant Dis. 2006, 90, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, G.; Jamshidi, E.; Lo Verde, G.; Bella, P.; Mondello, V.; Giambra, S.; D’Urso, V.; Tsolakis, H.; Murolo, S.; Burruano, S.; et al. Epidemiological investigations and molecular characterization of ‘Candidatus phytoplasma solani’ in grapevines, weeds vectors and putative vectors in Western Sicily (Southern Italy). Pathogens 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Raccah, B. Transmission of the safflower phyllody mollicute by Neoaliturus fenestratus. Phytopathology 1982, 71, 230–232. [Google Scholar]
- Salehi, M.; Izadpanah, K.; Nejat, N.; Siampour, M. Partial characterization of phytoplasmas associated with lettuce and wild lettuce phyllodies in Iran. Plant Pathol. 2007, 56, 669–676. [Google Scholar] [CrossRef]
- Zazhurilo, V.; Sitnikova, G. Interrelations between mosaic disease virus of winter wheat and its vector, Deltocephalus striates. Proc. Lenin Acad. Agr. Sci. USSR 1941, 11, 27–29. [Google Scholar]
- Atakan, E. Damage assessment of the leafhopper complex [Asymmetrasca decedens (Paoli) and Empoasca decipiens Paoli] (Homoptera: Cicadellidae) in cotton. J. Pest Sci. 2009, 82, 227–234. [Google Scholar] [CrossRef]
- Bobzuga, R.; Elekcioglu, Z. Pests and natural enemies determined in olive orchards in Turkey. Turk Bilimsel Derlemeler Derg. 2008, 1, 87–97. [Google Scholar]
- Bodingius, P. Cicaden, onbekend, maar niet onschadelijk. Fuitteelt 1990, 80, 21–22. [Google Scholar]
- Straub, R.; Jentsch, J. Relationship of the white apple leafhopper, Typhlocyba pomeria McAtee, and the rose leafhopper, Edwardsiana rosae (L.), on apple in the Hudson Valley Region of New York. J. Agric. Entomol. 1994, 11, 301–309. [Google Scholar]
- Mozaffarian, F. An identification key to the species of Auchenorrhyncha of Iranian fauna recorded as pests in orchards and review on the pest status of the species. Zootaxa 2018, 4420, 475–501. [Google Scholar] [CrossRef] [PubMed]
- Snare, L. Pest and Disease Analysis in Hazelnuts; Horticultural Australia Ltd.: Sydney, Australia, 2006; pp. 1–68. [Google Scholar]
- Gillespie, A. The Potential of Entomogenous Fungi to Control Glasshouse Pests and Brown Planthopper of Rice. Ph.D. Thesis, University of Southampton, Southampton, UK, 1984. [Google Scholar]
- Seljak, G. Contribution to the knowledge of planthoppers and leafhoppers of Slovenia (Hemiptera: Auchenorrhyncha). Acta Entomol. Slov. 2004, 12, 189–216. [Google Scholar]
- Bissaad, F.Z.; Razi, S.; Bounaceur, F. Influence of grapevine vigor on the dynamic and the installation of the invasive pest Jacobiasca lybica in Mitidja, Algeria. Tunis. J. Plant Prot. 2018, 13, 139–145. [Google Scholar]
- Lentini, A.; Delrio, G.; Serra, G. Observations on the infestation of Jacobiasca lybica on grapevine in Sardinia. IOBC WPRS Bul. 2000, 23, 127–129. [Google Scholar]
- Tsolakis, H.; Ernesto, R. Grapevine pests in Sicily. IOBC WPRS Bul. 2008, 36, 355–361. [Google Scholar]
- Andison, H. The bramble leafhopper, Typhlocyba tenerrina H-S (Homoptera: Cicadellidae), a destructive European insect new to the Pacific Northwest. Can. Entomol. 1950, 82, 68–70. [Google Scholar] [CrossRef]
- Marion-Poll, F.; Della Giustina, W.; Mauchamp, B. Changes of electric patterns related to feeding in a mesophyll feeding leafhopper Zyginidia scutellaris. Entomol. Exp. Appl. 1987, 43, 115–124. [Google Scholar] [CrossRef]
- Bressan, A.; Turata, R.; Maixner, M.; Spiazzi, S.; Boudon-Padieu, E.; Girolami, V. Vector activity of Hyalesthes obsoletus living in nettles and transmitting a stolbur phytoplasma to grapevines: A case study. Ann Appl Biol 2007, 150, 331–339. [Google Scholar] [CrossRef]
- Johannesen, J.; Lux, B.; Michel, K.; Seitz, A.; Maixner, M. Invasion biology and host specificity of the grapevine yellows disease vector Hyalesthes obsoletus in Europe. Entomol. Exp. Appl. 2008, 126, 217–227. [Google Scholar] [CrossRef]
- Maixner, M. Transmission of German grapevine yellows (Verilbungskrankheit) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae). Vitis 1994, 33, 103–104. [Google Scholar]
- Sforza, R.; Clair, D.; Daire, X.; Larrue, J.; Boudon-Padieu, E. The role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the occurrence of bois noir of grapevines in France. J. Phytopathol. 1998, 146, 549–556. [Google Scholar] [CrossRef]
- Achon, M.; Subira, J.; Sin, E. Seasonal occurrence of Laodelphax striatellus in Spain: Effect on the incidence of maize rough dwarf virus. J. Crop Prot. 2013, 47, 1–5. [Google Scholar] [CrossRef]
- Hsieh, C. Transmission of rice stripe virus by Laodelphax striatellus Fallen in Taiwan. Plant Prot. Bull. Taiwan 1973, 15, 153–162. [Google Scholar]
- Ruan, Y.; Chiang, W.; Lin, R. Studies on the rice virus vector small brown planthopper Laodelphax striatella Fallen. Acta Entomol. Sin. 1981, 24, 283–290. [Google Scholar]
- Zhang, F.; Guo, H.; Zheng, H.; Zhou, T.; Zhou, Y.; Wang, S.; Fang, R.; Qian, W.; Chen, X. Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV). BMC Genom. 2010, 11, 303. [Google Scholar] [CrossRef]
- Vidano, C. Phases of maize rough dwarf virus multiplication in the vector Laodelphax striatellus (Fallén). Virology 1970, 41, 218–232. [Google Scholar] [CrossRef]
- Cvrkovic, T.; Jovic, J.; Mitrovic, M.; Krstic, O.; Krnjajic, S.; Tosevki, I. Potential new hemipteran vectors of stolbur phytoplasma in Serbian vineyard. Bull. Insectology 2011, 64, 129–130. [Google Scholar]
- Filippin, L.; Jovic, J.; Cvrkovic, T.; Forte, V.; Clair, D.; Tosevski, I.; Boudon-Padieu, E.; Borgo, M.; Angelini, E. Molecular characteristics of phytoplasmas associated with Flavescence doreé in clematis and grapevine and preliminary results on the role of Dictyophara europaea as vector. Plant Pathol. 2009, 58, 826–837. [Google Scholar] [CrossRef]
- Krstic, O.; Cvrkovic, T.; Mitrovic, M.; Radonijc, S.; Hrncic, S.; Tosevski, I.; Jovic, J. Wolbachia infection in natural populations of Dictyophara europaea, an alternative vector of grapevine Flavescence doreé phytoplasma: Effects and interactions. Ann Appl Biol. 2018, 172, 47–64. [Google Scholar] [CrossRef]
- Neto, A.C.; Mateus, C.; Andrade, E.; Barateiro, A.; Bigolin, M.; Chaves, M.; Guerreiro, V.; Pereira, F.; Soares, C.; Tomé, D.; et al. First record of the invasive leafhopper Sophonia orientalis in mainland Portugal. J. Pest Sci. 2020, 94, 241–249. [Google Scholar] [CrossRef]
- Kersting, U.; Baspinar, H.; Uygun, N.; Satar, S. Comparison of two sampling methods for leafhoppers (Homoptera, Cicadellidae) associated with sesame in the east Mediterranean region of Turkey. Anz. Schidlingskde. 1997, 70, 131–135. [Google Scholar] [CrossRef]
- Villaescusa, F.; Sanjuan, S.; Cebrian, M.; Alfaro-Fernández, A.; Font, M.; Ferrándiz, J.; Mendoza, A. Prospección de posibles vectores (Hemiptera: Cicadellidae, Aphididae y Psylloidea de patógenos en apio y zanahoria. Bol. San. Plagas 2011, 37, 163–171. [Google Scholar]
- Atakan, E. Development of a sampling strategy for the leafhopper complex [Asymmetrasca decedens (Paoli) and Empoasca decipiens Paoli] (Hemiptera: Cicadellidae) in cotton. J. Pest Sci. 2011, 84, 143–152. [Google Scholar] [CrossRef]
- Lamparski, R.; Rolbiecki, R.; Piesik, D. Wpływ nawadniania kroplowego na występowanie owadów w uprawie dwóch odmian dyni zwyczajnej (Cucurbita pepo L.). Infrastrukt. I Ekol. Teren. Wiej. 2009, 3, 159–166. [Google Scholar]
- Margus, A.; Saifullah, S.; Kankare, M.; Lindström, L. Fungicides modify pest insect fitness depending on their genotype and population. Sci Rep. 2023, 13, 17879. [Google Scholar] [CrossRef]
- Bayo, S.F. Indirect effect of pesticides on insects and other arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef]
- Elskus, A. Toxicity, Sublethal Effects, and Potential Modes of Action of Select Fungicides on Freshwater Fish and Invertebrates (ver. 1.1, November 2014); U.S. Geological Survey Open-File Report 2012-1213; U.S. Geological Survey: Reston, VA, USA, 2014; pp. 1–42.
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
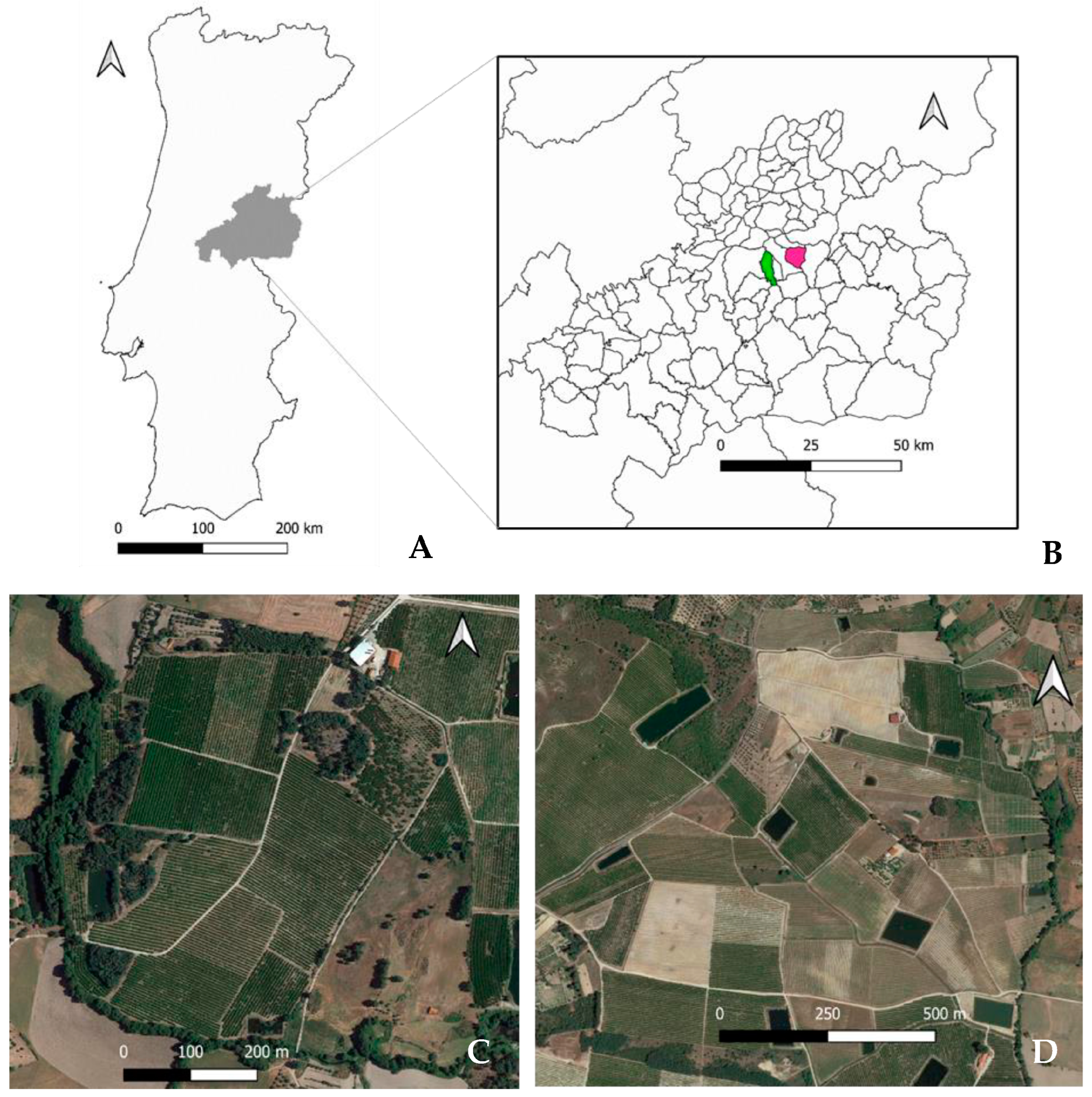


| Date | Orchard |
|---|---|
| 14 August 2018 | Both |
| 21 June 2019 | LC |
| 21 June 2019 | PA |
| 24 June2019 | PA |
| 24–25 June 2019 | PA |
| 27 June 2019 | LC |
| 1 July 2019 | LC |
| 28–31 July 2019 | LC |
| Active Ingredient | Pest | Application Date |
|---|---|---|
| acrinathrin | Red spider mite | 2019 (29 May) |
| chlorpyrifos-methyl | San Jose scale | 2018 (6 June) |
| cyprodinil | Moniliosis | 2018 (20 March, 4–5 April) |
| copper oxychloride | Peach leaf curl, Gumspot of stone fruit | 2018 (17–24 February), 2019 (7–14 February), 2020 (15–18 February) |
| deltamethrin + thiacloprid | Mediterranean fruit fly | 2019 (29 July and 15 August) |
| difenoconazole | Powdery mildew, Brown rot | 2019 (12–14 April, 30 July–1 August), 2020 (20 July) |
| flonicamid | Aphids | 2019 (21, 23 March), 2020 (18, 20 March) |
| fluopyram and tebuconazole | Moniliosis | 2018 (16 April, 20, 28 June, 15 July) |
| fluopyram and tebuconazole | Brown rot | 2019 (8, 18 July, 1, 24 August), 2020 (8, 23 June, 8, 18, 26 July, 4, 14 August) |
| imidacloprid | Aphids | 2018 (26, 27 March) |
| lambda-cyhalothrin | Mediterranean fruit fly | 2018 (20, 28 June, 15 July), 2019 (8, 18 July, 1, 24 August), 2020 (8, 23 June, 8, 18, 26 July, 4,14 August) |
| penconazole | Powdery mildew | 2018 (26–27 April), 2019 (22–26 June), 2020 (2–3 June) |
| spinetoram | Thrips | 2018 (27 March) |
| spirodiclofen | Red spider mite | 2018 (13 June) |
| sulfur | Powdery mildew | 2018 (13 June), 2019 (16, 21–23 May) 2020 (3–4 May) |
| thiram | Peach leaf curl, Gumspot of stone fruit | 2018 (9–12 March, 16 April), 2019 (2–3, 21–23 March) |
| ziram | Peach leaf curl, Gumspot of stone fruit | 2018 (4–5 April), 2020 (27–28 February, 13, 24 March) |
| Infraorder | Family | Subfamily | Genus/Species | 2018 | 2019 | 2020 | |||
|---|---|---|---|---|---|---|---|---|---|
| LC | PA | LC | PA | LC | PA | ||||
| Cicadomorpha | Aphrophoridae | Aphrophorinae | Neophilaenus campestris (Fallén) | 0 | 0 | 1 | 3 | 3 | 0 |
| Philaenus spumarius (Linnaeus) | 2 | 3 | 3 | 1 | 3 | 5 | |||
| Cercopidae | Cercopinae | Cercopis intermedia Kirschbaum | 1 | 0 | 0 | 0 | 0 | 0 | |
| Cicadellidae | Aphrodinae | Aphrodes makarovi Zachvatkin | 23 | 20 | 12 | 15 | 16 | 39 | |
| Cicadellinae | Cicadella viridis (Linnaeus) | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Deltocephalinae | Balclutha puntacta (Fabricius) | 0 | 0 | 0 | 0 | 2 | 2 | ||
| Cicadula persimilis (Edwards) | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Euscelidius variegatus (Kirschbaum) | 5 | 22 | 2 | 6 | 10 | 11 | |||
| Euscelis incisus (Kirschbaum) | 0 | 0 | 0 | 0 | 0 | 2 | |||
| Fieberiella florii (Stål) | 0 | 0 | 1 | 0 | 0 | 6 | |||
| Macrosteles sordidipennis (Stål) | 0 | 0 | 0 | 0 | 2 | 1 | |||
| Neoaliturus fenestratus (Herrich-Schäffer) | 4 | 3 | 4 | 0 | 2 | 0 | |||
| Phlepsius intricatus (Herrich-Schäffer) | 1 * | 1 * | 0 | 0 | 0 | 0 | |||
| Platymetopius gutattus Fieber | 0 | 0 | 2 | 0 | 1 | 0 | |||
| Psammotettix striatus (Linnaeus) | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Sardius argus (Marshall) | 1 | 1 | 0 | 0 | 2 | 1 | |||
| Dorycephalinae | Eupelix cuspidata (Fabricius) | 0 | 0 | 4 | 0 | 1 | 2 | ||
| Evacanthinae | Sophonia orientalis (Matsumura) | 1 * | 1 * | 0 | 0 | 0 | 0 | ||
| Macropsinae | Macropsis cerea (Germar) | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Macropsis scutellata (Boheman) | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Megophthalminae | Agallia consobrina Curtis | 225 | 113 | 93 | 70 | 289 | 264 | ||
| Anaceratagallia glabra Dmitriev | 18 | 16 | 42 | 35 | 25 | 28 | |||
| Austroagallia sinuata (Mulsant & Rey) | 0 | 0 | 10 | 4 | 2 | 0 | |||
| Megophthalmus scabripennis Edwards | 3 | 1 | 1 | 0 | 0 | 0 | |||
| Typhlocybinae | Alebra coryli Le Quesne | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Alnetoidia alneti (Dahlbom) | 4 | 0 | 0 | 0 | 1 | 0 | |||
| Arboridia parvula (Boheman) | 0 | 4 | 0 | 0 | 2 | 6 | |||
| Asymmetrasca decedens (Paoli) | 1913 | 603 | 3716 | 2754 | 6930 | 4775 | |||
| Edwardsiana gratiosa (Boheman) | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Edwardsiana rosae (Linnaeus) | 0 | 0 | 0 | 0 | 2 | 1 | |||
| Eupteryx filicum (Newman) | 0 | 0 | 0 | 1 | 0 | 0 | |||
| Fruticidia bisignata (Mulsant & Rey) | 1 | 1 | 1 | 0 | 3 | 2 | |||
| Fruticidia sanguinosa (Rey) | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Hauptidia marocanna (Melichar) | 2 | 6 | 0 | 0 | 29 | 10 | |||
| Hauptidia provincialis (Ribaut) | 0 | 0 | 0 | 0 | 4 | 15 | |||
| Hebata decipiens Paoli | 22 | 26 | 1 | 2 | 2 | 0 | |||
| Hebata solani (Curtis) | 796 | 622 | 710 | 398 | 621 | 875 | |||
| Jacobiasca lybica (Bergevin & Zanon) | 0 | 5 | 0 | 0 | 0 | 0 | |||
| Lindbergina aurovittata (Douglas) | 0 | 1 | 0 | 0 | 0 | 0 | |||
| Ribautiana cruciata (Ribaut) | 1 | 1 | 1 | 1 | 1 | 0 | |||
| Ribautiana debilis (Douglas) | 0 | 1 | 3 | 1 | 6 | 2 | |||
| Ribautiana tenerrima (Herrich-Schäffer) | 0 | 0 | 0 | 0 | 5 | 3 | |||
| Zygina lunaris (Mulsant & Rey) | 14 | 1 | 5 | 1 | 8 | 1 | |||
| Zygina nivea (Mulsant & Rey) | 10 | 7 | 2 | 2 | 1 | 0 | |||
| Zygina ordinaria (Ribaut) | 115 | 84 | 61 | 45 | 3 | 22 | |||
| Zygina schneideri (Gunthart) | 0 | 0 | 0 | 0 | 1 | 3 | |||
| Zyginidia scutellaris (Herrich-Schäffer) | 15 | 17 | 26 | 52 | 17 | 12 | |||
| Fulgoromorpha | Cixiidae | Cixiinae | Hyalesthes obsoletus Signoret | 0 | 2 | 0 | 0 | 0 | 0 |
| Delphacidae | - | Conomelus lorifer Ribaut | 0 | 0 | 0 | 0 | 0 | 1 | |
| Laodelphax striatella (Fallén) | 8 | 13 | 1 | 3 | 26 | 18 | |||
| Metadelphax propinqua (Fieber) | 14 | 7 | 6 | 0 | 1 | 14 | |||
| Dictyopharidae | Dictyopharinae | Dictyophara europaea (Linnaeus) | 0 | 0 | 0 | 1 | 0 | 0 | |
| Tettigometridae | - | Tettigometra griseola (Fieber) | 0 | 0 | 0 | 0 | 1 | 1 | |
| - | Tettigometra impressopunctata (Dufour) | 0 | 0 | 0 | 0 | 1 | 1 | ||
| - | Tettigometra virescens (Panzer) | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Structural Zeros Component | Estimate | OR | OR 95% CI | p-Value |
| (Intercept) | 5.263 | -- | -- | 0.0012 |
| DayOfYear | −0.029 | 0.972 | (0.956, 0.987) | 0.0003 |
| MaxTemp | −0.114 | 0.892 | (0.809, 0.984) | 0.0231 |
| NrRainyDays | 0.406 | 1.500 | (1.071, 2.101) | 0.0181 |
| Count Component | Estimate | IRR | IRR 95% CI | p-Value |
| (Intercept) | −1.970 | -- | -- | <0.001 |
| DayOfYear | 0.018 | 1.018 | (1.016, 1.021) | <0.001 |
| NrRainyDays | −0.107 | 0.989 | (0.847, 0.953) | <0.001 |
| FungProp | −0.502 | 0.605 | (0.400, 0.916) | 0.0175 |
| Insecticide = yes | −0.024 | 0.977 | (0.798, 1.195) | 0.8192 |
| NrRainyDays × FungProp | 0.327 | 1.387 | (0.992, 1.940) | 0.0558 |
| Structural Zeros Component | Estimate | OR | OR 95% CI | p-Value |
| (Intercept) | 7.192 | -- | -- | 0.0010 |
| DayOfYear | −0.035 | 0.966 | (0.942, 0.990) | 0.0055 |
| MaxRelHum | −0.071 | 0.932 | (0.899, 0.966) | <0.001 |
| Count Component | Estimate | IRR | IRR 95% CI | p-Value |
| (Intercept) | 1.154 | -- | -- | 0.3242 |
| DayOfYear | −0.005 | 0.995 | (0.983, 1.007) | 0.4222 |
| AvgTemp | 0.405 | 1.499 | (1.294, 1.737) | <0.001 |
| MinRelHum | 0.018 | 1.018 | (1.002, 1.034) | 0.0287 |
| MaxRelHum | −0.021 | 0.980 | (0.970, 0.990) | <0.001 |
| NrDaysInsecticide | 0.031 | 1.031 | (0.980, 1.085) | 0.2342 |
| DayOfYear×AvgTemp | −0.002 | 0.998 | (0.998, 0.999) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, P.M.; Neto, A.C.; Guerreiro, V.; Barateiro, A.; Anjos, H.; Coutinho, J.P.; Antunes, M.; Rebelo, M.T. Effects of Climatic, Chemical, and Cultural Control Strategies on Community Composition of Auchenorrhyncha and Population Dynamics of Two Major Green Leafhopper Pests in Peach Orchards. Agronomy 2025, 15, 163. https://doi.org/10.3390/agronomy15010163
Nascimento PM, Neto AC, Guerreiro V, Barateiro A, Anjos H, Coutinho JP, Antunes M, Rebelo MT. Effects of Climatic, Chemical, and Cultural Control Strategies on Community Composition of Auchenorrhyncha and Population Dynamics of Two Major Green Leafhopper Pests in Peach Orchards. Agronomy. 2025; 15(1):163. https://doi.org/10.3390/agronomy15010163
Chicago/Turabian StyleNascimento, Patrícia Monteiro, Ana Carina Neto, Vera Guerreiro, Anabela Barateiro, Hugo Anjos, José Pereira Coutinho, Marília Antunes, and Maria Teresa Rebelo. 2025. "Effects of Climatic, Chemical, and Cultural Control Strategies on Community Composition of Auchenorrhyncha and Population Dynamics of Two Major Green Leafhopper Pests in Peach Orchards" Agronomy 15, no. 1: 163. https://doi.org/10.3390/agronomy15010163
APA StyleNascimento, P. M., Neto, A. C., Guerreiro, V., Barateiro, A., Anjos, H., Coutinho, J. P., Antunes, M., & Rebelo, M. T. (2025). Effects of Climatic, Chemical, and Cultural Control Strategies on Community Composition of Auchenorrhyncha and Population Dynamics of Two Major Green Leafhopper Pests in Peach Orchards. Agronomy, 15(1), 163. https://doi.org/10.3390/agronomy15010163






