Effects of Grazing Intensity on Microbial Diversity at Different Soil Depths in Desert Steppe Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement of Soil Physicochemical Properties
- Soil pH measurement: Soil pH was measured using a pHS-3G digital pH meter (Starter3100, OHAUS, Parsippany, NJ, USA). Five grams of air-dried soil were placed in a 50 mL conical flask, mixed with 25 mL of ultrapure water (soil-to-water ratio of 1:5), shaken thoroughly, and left to stand for 30 min before measurement.
- Soil moisture (SM) and bulk density (SBD): Soil moisture content was determined by the drying method, and soil bulk density was measured using the ring knife method, each with three replicates.
- Total soil phosphorus (TP) measurement: Total phosphorus was measured using the HClO4-H2SO4 digestion method. Five grams of air-dried soil were weighed with an analytical balance (CP224C, Sartorius, Germany) accurate to 0.0001 g and placed in a digestion tube. Five milliliters of concentrated sulfuric acid and ten drops of perchloric acid were added. The mixture was digested at 150 °C for 1 h in a temperature-controlled far-infrared digestion furnace (LWY84B) and then at 360 °C for an additional hour. The digest was transferred to a 100 mL volumetric flask, diluted to volume with distilled water, and filtered through phosphorus-free filter paper into a 10 mL centrifuge tube. Total phosphorus content was determined using a flow analyzer (AUTO ANALYZER3-AA3, SEAL Analytical, Germany).
- Soil organic carbon (SOC) measurement: Soil organic carbon was determined using the external heating potassium dichromate oxidation method. A 0.2500 g sample of air-dried soil was weighed into a digestion tube. Five milliliters each of concentrated sulfuric acid and 0.8 mol·L⁻¹ potassium dichromate solution (prepared after drying the reagents at 130 °C for 1 h) were added. The mixture was heated at 180 °C for 10 min in a digital constant-temperature oil bath (HH-S8). The digest was transferred to a conical flask, diluted with distilled water to 60–70 mL, and 1,10-phenanthroline indicator was added. The solution was titrated with 0.2 mol·L⁻1 ferrous sulfate standard solution until it turned brick-red, and the reading was recorded.
- Soil total nitrogen (TN) and available phosphorus (AP) measurement: Soil total nitrogen (TN) was determined using an elemental analyzer (Vario TOC, Elemental Inc., Germany). Specifically, air-dried soil samples were ground, passed through a 100-mesh sieve, and precisely weighed (105 mg) on a high-precision balance. Each subsample was then wrapped in a tin capsule and analyzed for total nitrogen content. Available phosphorus (AP) was measured using a spectrophotometer following standard colorimetric procedures.
2.3. Soil DNA Extraction, PCR Amplification, and Bacterial 16S rRNA Gene Sequencing
2.4. Statistical Analysis
3. Results
3.1. Soil Properties and Vegetation Traits
3.2. Microbial Community Richness and Biodiversity
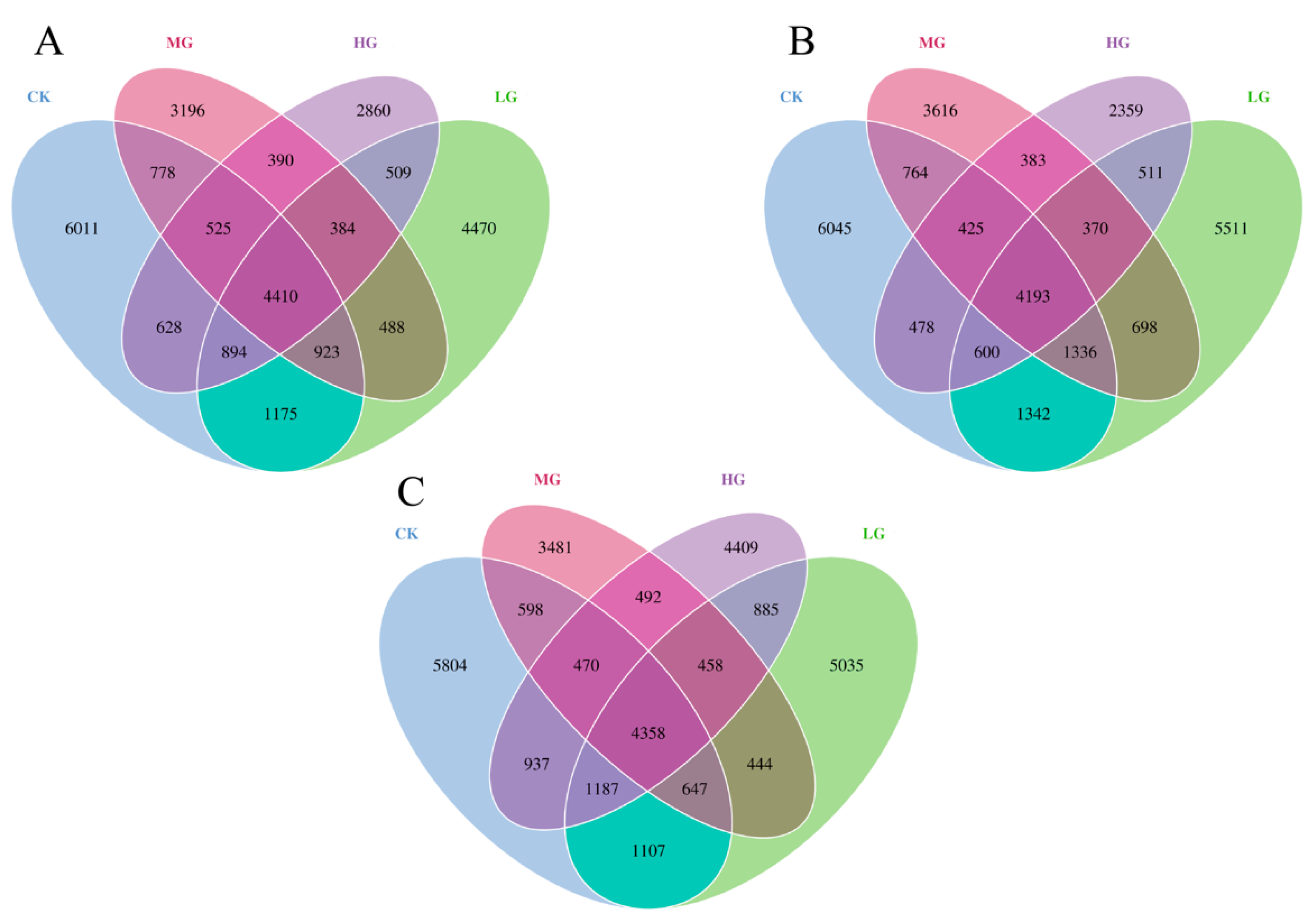
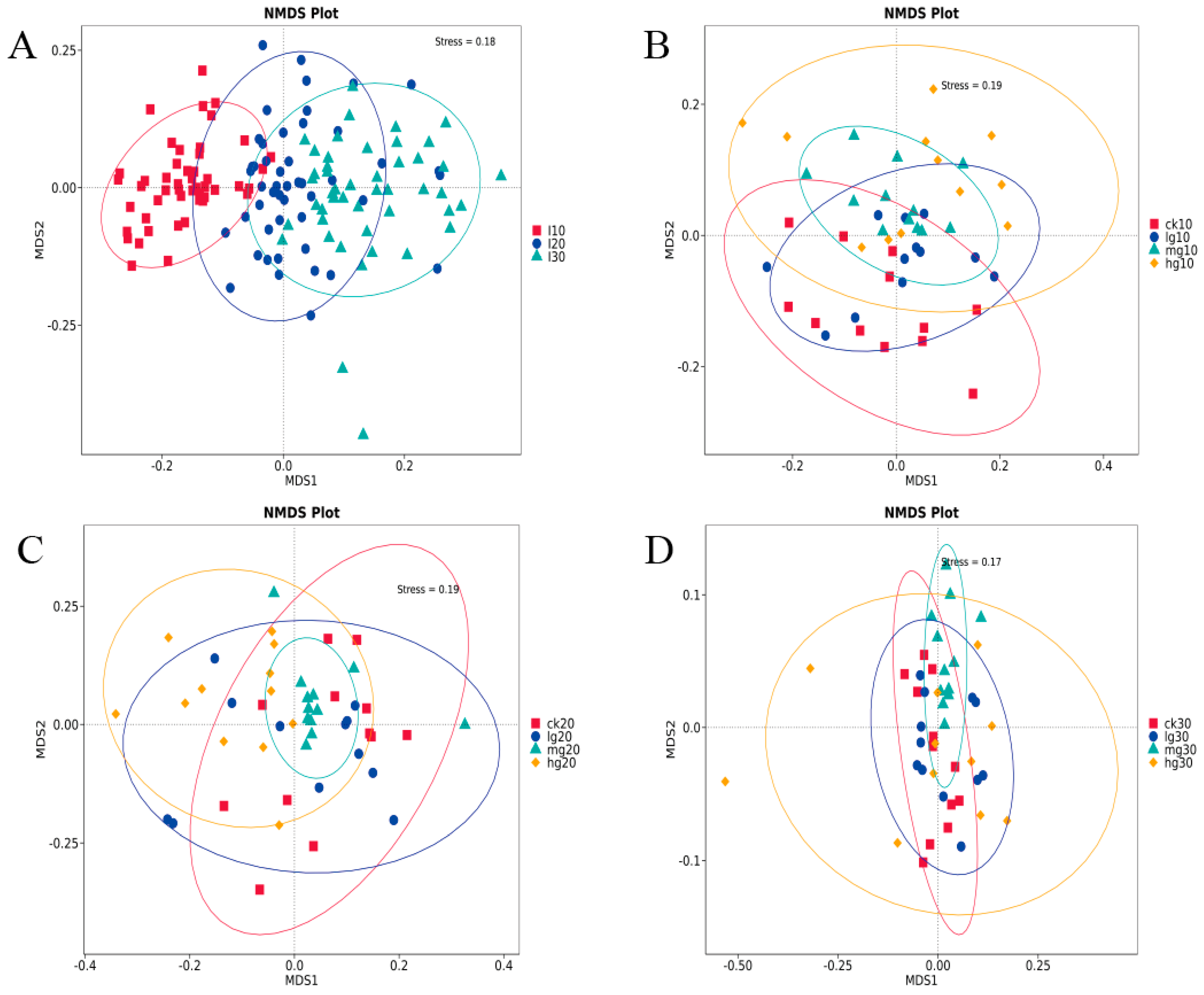
3.3. Bacterial Beta Diversity
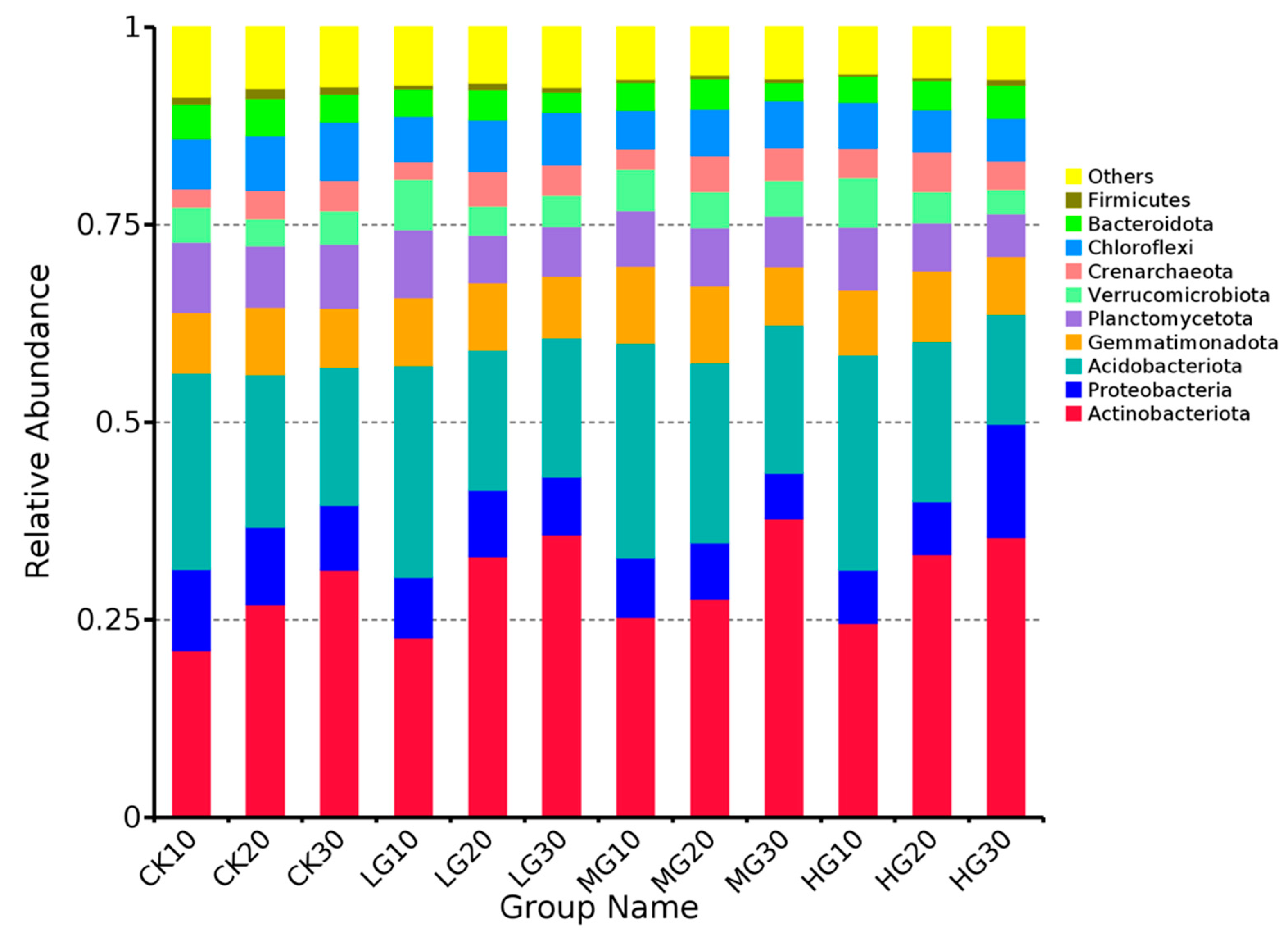
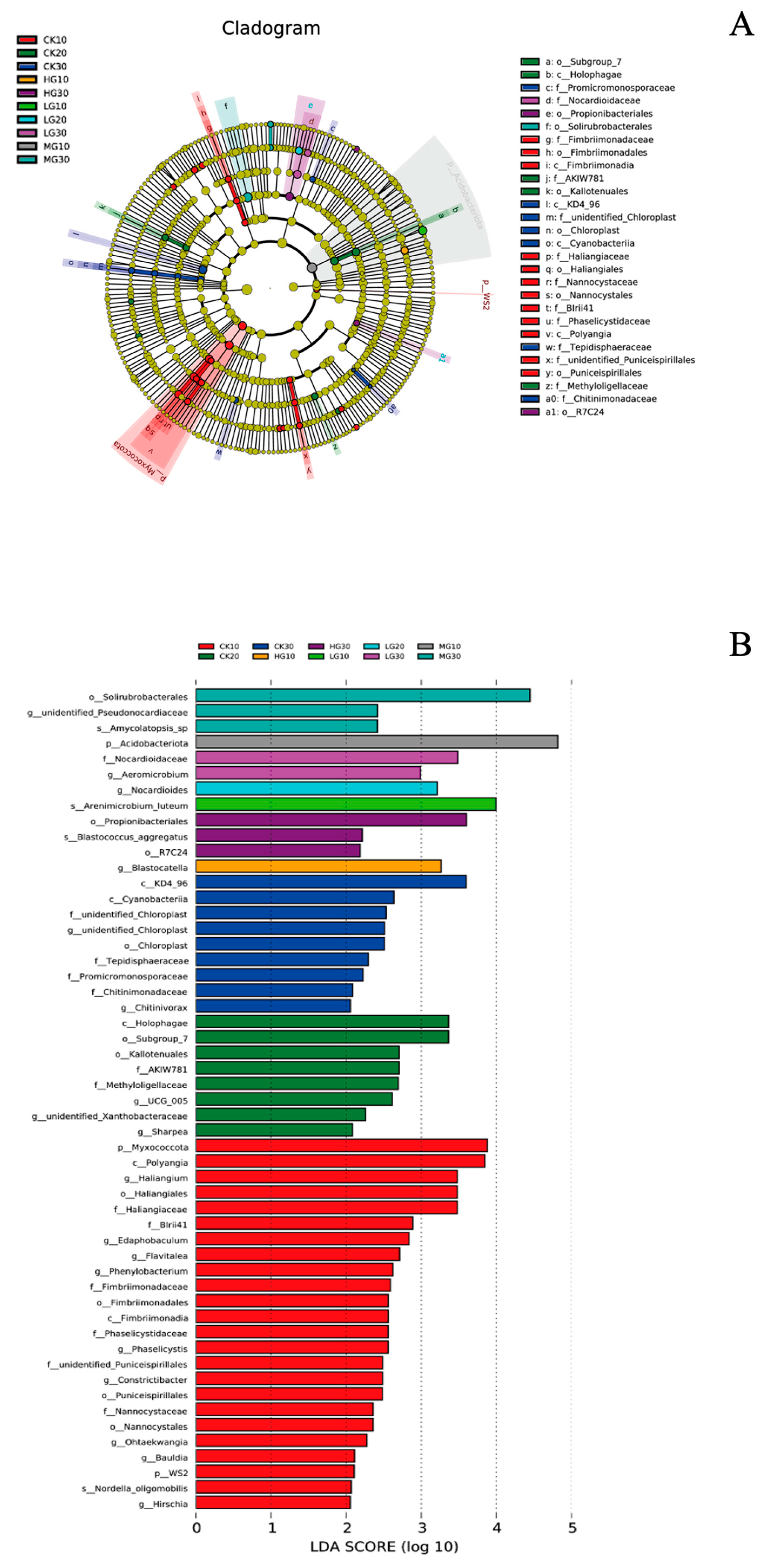
3.4. Community Composition
3.5. Soil Properties and Relative Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, P.P.; Huang, Y.T.; Liu, B.Y.; Chen, J.Y.; Lei, Z.Y.; Zhang, Y.H.; Cheng, B.H.; Zhou, T.; Peng, S.L. Effects of daytime and nighttime warming on soil microbial diversity. Geoderma 2024, 447, 116909. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Lin, Q.Y.; Wang, S.P.; Li, X.Z.; Liu, W.T.; Luo, C.Y.; Zhang, Z.H.; Zhu, X.X.; Jiang, L.L.; Li, X.N. Soil bacterial community responses to warming and grazing in a Tibetan alpine meadow. FEMS Microbiol. Ecol. 2016, 92, fiv152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil microorganisms: Their role in enhancing crop nutrition and health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Miller, C. Handbook of Biodiversity Methods. Survey, Evaluation and Monitoring. Austral Ecol. 2007, 32, 956. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, C.; Zuo, S.; Ji, Y.; Liu, W.; Huang, D. Heavy grazing reduces soil bacterial diversity by increasing soil pH in a semi-arid steppe. PeerJ 2024, 12, e17031. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, X.; Bardy, M.; Loiseau, P.; Louault, F. Stimulation of soil nitrification and denitrification by grazing in grasslands: Do changes in plant species composition matter? Oecologia 2003, 137, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, S.; Struik, P.C.; Wang, H.; Jin, K.; Wu, R.; Na, R.; Mu, H.; Ta, N. Plant and soil responses to grazing intensity drive changes in the soil microbiome in a desert steppe. Plant Soil 2023, 491, 219–237. [Google Scholar] [CrossRef]
- Eldridge, D.; Greene, R. Microbiotic soil crusts: A review of their roles in soil and ecological processes in the rangelands of Australia. Aust. J. Soil. Res. 1994, 32, 389–415. [Google Scholar] [CrossRef]
- Hernández-Álvarez, C.; García-Oliva, F.; Cruz-Ortega, R.; Romero, M.F.; Barajas, H.R.; Piñero, D.; Alcaraz, L.D. Squash root microbiome transplants and metagenomic inspection for in situ arid adaptations. Sci. Total Environ. 2021, 801, 150136. [Google Scholar] [CrossRef]
- Shu, X.; Ye, Q.; Huang, H.; Xia, L.; Tang, H.; Liu, X.; Wu, J.; Li, Y.; Zhang, Y.; Deng, L.; et al. Effects of grazing exclusion on soil microbial diversity and its functionality in grasslands: A meta-analysis. Front. Plant Sci. 2024, 15, 1366821. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Zhang, H.; Luo, H.; Li, Z. Characteristics of soil bacterial and fungal communities on interval seawater covering Linchang Island, China. Arch. Microbiol. 2021, 203, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, M.; Peay, K.G. Does Microbial Diversity Confound General Predictions? Trends Plant Sci. 2015, 20, 695–697. [Google Scholar] [CrossRef]
- Louisson, Z.; Gutiérrez-Ginés, M.J.; Taylor, M.; Buckley, H.L.; Hermans, S.M.; Lear, G. Soil conditions are a more important determinant of microbial community composition and functional potential than neighboring plant diversity. iScience 2024, 27, 110056. [Google Scholar] [CrossRef]
- Kemp, D.R.; Han, G.; Hou, X.; Michalk, D.L.; Hou, F.; Wu, J.; Zhang, Y. Innovative grassland management systems for environmental and livelihood benefits. Proc. Natl. Acad. Sci. USA 2013, 110, 8369–8374. [Google Scholar] [CrossRef]
- Wang, Z.W.; Jiao, S.Y.; Han, G.D.; Zhao, M.L.; Havstad, K.M. Effects of stocking rate on the variability of peak standing crop in a desert steppe of Eurasia grassland. Environ. Manag. 2014, 53, 266–273. [Google Scholar] [CrossRef]
- Wei, Z.J.; Han, G.D.; Yang, J.; Li, W.J.; Wang, G.L.; Zhou, X.F. The response of Stipa breviflora community to stocking rate. Grassl. China 2000, 6, 1–5. [Google Scholar]
- de Vries, F.T.; Brown, C.; Stevens, C.J. Grassland species root response to drought: Consequences for soil carbon and nitrogen availability. Plant Soil 2016, 409, 297–312. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Staley, C.; Gao, H.; Ishii, S.; Wei, X.; Liu, J.; Cheng, J.; Hao, M.; Sadowsky, M.J. Impact of long-term grazing exclusion on soil microbial community composition and nutrient availability. Biol. Fertil. Soils 2019, 55, 121–134. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Jiang, M.; Tian, Y.; Guo, R.; Li, S.; Guo, J.; Zhang, T. Effects of warming and nitrogen addition on soil fungal and bacterial community structures in a temperate meadow. Front. Microbiol. 2023, 14, 1231442. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 3. [Google Scholar] [CrossRef]
- Xu, T.; Chen, X.; Hou, Y.; Zhu, B. Changes in microbial biomass, community composition and diversity, and functioning with soil depth in two alpine ecosystems on the Tibetan plateau. Plant Soil 2021, 459, 137–153. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.; Bell, S.M.; Soromotin, A.V.; Peng, C.; An, S.; Wu, X.; Xu, X.; Wang, K.; Li, J.; et al. Carbon in Chinese grasslands: Meta-analysis and theory of grazing effects. Carbon Res. 2023, 2, 19. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, M.J.; Jung, J.Y.; Hwang, C.Y.; Kim, M.; Ro, H.M.; Chun, J.; Lee, Y.K. Vertical distribution of bacterial community is associated with the degree of soil organic matter decomposition in the active layer of moist acidic tundra. J. Microbiol. 2016, 54, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, F.; Yang, W.; Zhang, M.; He, S.; Li, Z. Fertilizers and Manures Enhance the Bioavailability of Soil Phosphorus Fractions in Karst Grassland. Agronomy 2024, 14, 1429. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, R.; Qin, J.; Wang, Y.; Lu, X.; Yang, P.; Bai, Y. Grassland management regimes regulate soil phosphorus fractions and conversion between phosphorus pools in semiarid steppe ecosystems. Biogeochemistry 2023, 163, 33–48. [Google Scholar] [CrossRef]
- Fang, M.; Lu, G.; Zhang, S.; Liang, W. Overgrazing on Unmanaged Grassland Interfered with the Restoration of Adjacent Grazing-Banned Grassland by Affecting Soil Properties and Microbial Community. Front. Microbiol. 2024, 14, 1327056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Li, X.; Wang, Y.; Zhang, Y.; Zhang, Z. Grazing Regulates Changes in Soil Microbial Communities in Plant-Soil Systems. Agronomy 2023, 13, 708. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, X.; Wang, Y.; Zhang, Z.; Yang, W. Effects of Grazing Intensity on Diversity and Composition of Rhizosphere and Non-Rhizosphere Microbial Communities in a Desert Grassland. Ecol. Evol. 2023, 13, e10300. [Google Scholar] [CrossRef]
- He, N.P.; Zhao, L.; Liu, L.; Cheng, J.; Song, J.; Li, J.; Hu, S.; Zhou, H. Grazing Intensity Impacts Soil Carbon and Nitrogen Storage of Continental Steppe. Ecosphere 2011, 2, art8. [Google Scholar] [CrossRef]
- Jiao, F.; Shi, X.R.; Han, F.P.; Yuan, Z.Y. Increasing Aridity, Temperature, and Soil pH Induce Soil C-N-P Imbalance in Grasslands. Sci. Rep. 2016, 6, 19601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, L.; Yuan, H.; Li, E.; Yang, X. The Impact of Artificial Afforestation on the Soil Microbial Community and Function in Desertified Areas of NW China. Forests 2024, 15, 1140. [Google Scholar] [CrossRef]
- Li, J.; Benti, G.; Wang, D.; Yang, Z.; Xiao, R. Effect of Alteration in Precipitation Amount on Soil Microbial Community in a Semi-Arid Grassland. Front. Microbiol. 2022, 13, 842446. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; van Der Putten, W.H.; Roggero, P.P.; et al. Shifts in Microbial Diversity through Land Use Intensity as Drivers of Carbon Mineralization in Soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Fan, J.; Jin, H.; Zhang, C.; Zheng, J.; Zhang, J.; Han, G. Grazing Intensity Induced Alternations of Soil Microbial Community Composition in Aggregates Drive Soil Organic Carbon Turnover in a Desert Steppe. Agric. Ecosyst. Environ. 2021, 313, 107387. [Google Scholar] [CrossRef]
- Olivera, N.L.; Prieto, L.; Bertiller, M.B.; Ferrero, M.A. Sheep Grazing and Soil Bacterial Diversity in Shrublands of the Patagonian Monte, Argentina. J. Arid Environ. 2016, 125, 16–20. [Google Scholar] [CrossRef]
- Tapia-Torres, Y.; López-Lozano, N.E.; Souza, V.; García-Oliva, F. Vegetation-Soil System Controls Soil Mechanisms for Nitrogen Transformations in an Oligotrophic Mexican Desert. J. Arid Environ. 2015, 114, 62–69. [Google Scholar] [CrossRef]


| Functional Groups | CK | LG | MG | HG | ||||
|---|---|---|---|---|---|---|---|---|
| Density (Individuals/m²) | Relative Abundance (%) | Density (Individuals/m²) | Relative Abundance (%) | Density (Individuals/m²) | Relative Abundance (%) | Density (Individuals/m²) | Relative Abundance (%) | |
| Perennial bunch grasses | 106.4 ± 8.59 a | 17% | 146.4 ± 11.01 ab | 22% | 169.6 ± 11.95 b | 24% | 238.0 ± 20.8 c | 37% |
| Perennial forbs | 44.8 ± 19.8 | 48% | 15.9 ± 5.47 | 19% | 13.7 ± 3.89 | 18% | 25.3 ± 3.87 | 15% |
| Perennial rhizome grasses | 15.9 ± 9.06 a | 33% | 2.00 ± 1.87 a | 17% | 16.5 ± 13.7 a | 46% | 1.07 ± 0.77 a | 4% |
| Shrubs and semi-shrubs | 18.7 ± 4.07 a | 45% | 3.60 ± 1.12 b | 25% | 2.13 ± 0.71 b | 13% | 1.87 ± 0.68 b | 18% |
| Annual and biennials | 12.6 ± 3.28 a | 49% | 2.80 ± 1.22 b | 18% | 1.33 ± 0.62 b | 15% | 0.66 ± 0.33 b | 17% |
| Soil Properties | Vegetation Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | SM (%) | SBD (g/cm3) | pH | SOC (g/kg) | TN (g/kg) | TP (g/kg) | AP (mg/kg) | BGB (g/m2) | Coverage (%) |
| CK | 2.68 ± 0.10 ab | 91.5 ± 3.20 a | 7.73 ± 0.20 a | 15.9 ± 0.20 a | 1.43 ± 0.10 ab | 0.27 ± 0.01 a | 0.49 ± 0.010 b | 665.7 ± 41.1 a | 17.3 ± 0.30 a |
| LG | 2.94 ± 0.10 a | 94.3 ± 0.60 a | 8.23 ± 0.20 a | 17.0 ± 0.20 a | 1.55 ± 0.10 a | 0.27 ± 0.01 a | 0.51 ± 0.003 a | 511.2 ± 30.1 b | 14.6 ± 0.40 b |
| MG | 2.77 ± 0.20 a | 96.0 ± 1.00 a | 8.20 ± 0.20 a | 14.4 ± 0.50 b | 1.31 ± 0.10 ab | 0.26 ± 0.01 ab | 0.48 ± 0.003 b | 403.8 ± 19.6 c | 9.57 ± 0.60 c |
| HG | 2.25 ± 0.40 b | 96.4 ± 2.10 a | 8.06 ± 0.10 a | 14.3 ± 0.50 b | 1.18 ± 0.10 b | 0.23 ± 0.01 b | 0.46 ± 0.003 c | 279.6 ± 24.2 d | 8.73 ± 0.60 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ju, X.; Wu, Q.; Han, G. Effects of Grazing Intensity on Microbial Diversity at Different Soil Depths in Desert Steppe Soils. Agronomy 2025, 15, 124. https://doi.org/10.3390/agronomy15010124
Wang Y, Ju X, Wu Q, Han G. Effects of Grazing Intensity on Microbial Diversity at Different Soil Depths in Desert Steppe Soils. Agronomy. 2025; 15(1):124. https://doi.org/10.3390/agronomy15010124
Chicago/Turabian StyleWang, Yuxin, Xin Ju, Qian Wu, and Guodong Han. 2025. "Effects of Grazing Intensity on Microbial Diversity at Different Soil Depths in Desert Steppe Soils" Agronomy 15, no. 1: 124. https://doi.org/10.3390/agronomy15010124
APA StyleWang, Y., Ju, X., Wu, Q., & Han, G. (2025). Effects of Grazing Intensity on Microbial Diversity at Different Soil Depths in Desert Steppe Soils. Agronomy, 15(1), 124. https://doi.org/10.3390/agronomy15010124





