Effects of Ecotypes and Reduced N Fertilization on Root Growth and Aboveground Development of Ratooning Sorghum × Sudangrass Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Field Management
2.2. Summer Harvest Management and N Treatments
2.3. Ratooning Chlorophyll Content and LAI
2.4. Ratooning Root Sampling
2.5. Statistical Methods
3. Results
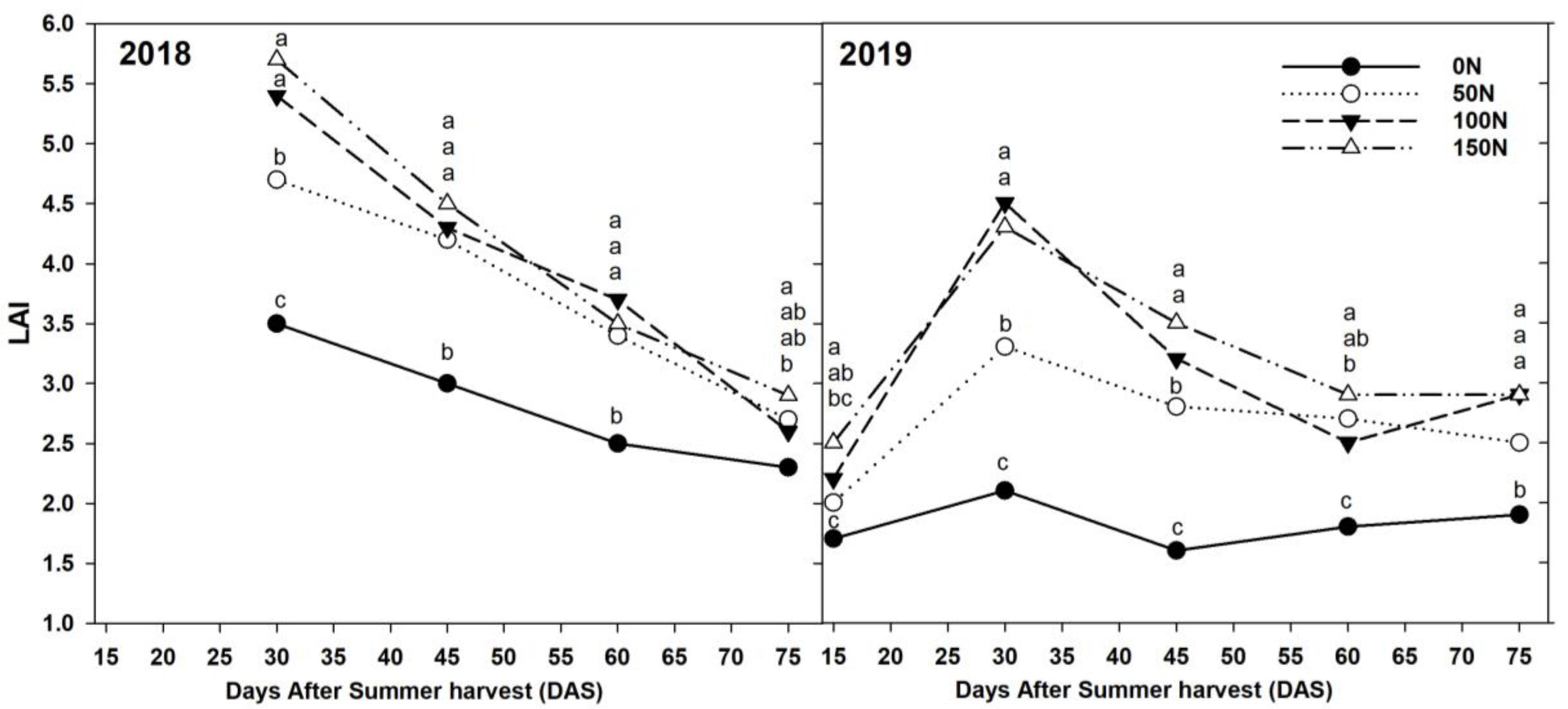
3.1. Chlorophyll Content and LAI
3.2. Root Morphology and Dry Matter
4. Discussion
4.1. Chlorophyll Content and LAI Responses
4.2. Root Morphology and Dry Matter Responses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, B.V.; Ramesh, S.; Reddy, P.S.; Ramaiah, B.; Salimath, M.; Kachapur, R. Sweet sorghum-a potential alternate raw material for bio-ethanol and bio-energy. Int. Sorghum Millets Newsl. 2005, 46, 79–86. [Google Scholar]
- Erickson, J.E.; Woodard, K.R.; Sollenberger, L.E. Optimizing sweet sorghum production for biofuel in the southeastern USA through nitrogen fertilization and top removal. Bioenergy Res. 2012, 5, 86–94. [Google Scholar] [CrossRef]
- Barnes, R.F.; Miller, D.A.; Nelson, C.J. Forages, 5th ed.; Iowa State University Press: Ames, IA, USA, 1995; pp. 121–135. [Google Scholar]
- Lauriault, L.M.; Marsalis, M.A.; VanLeeuwen, D.M. Planting date affects rainfed sorghum forage yields in semiarid, subtropical environments. Forage Grazinglands 2012, 10, 1–7. [Google Scholar] [CrossRef]
- Venuto, B.; Kindiger, B. Forage and biomass feedstock production from hybrid forage sorghum and sorghum–sudangrass hybrids. Grassland Sci. 2008, 54, 189–196. [Google Scholar] [CrossRef]
- Choi, N.; Kim, G.; Park, W.; Jeong, Y.; Kim, Y.-H.; Na, C.-I. Additional N application and ecotype affect yield and quality of ratoon harvested sorghum x sudangrass hybrid for temperate regions. Biomass Bioenergy 2022, 160, 106423. [Google Scholar] [CrossRef]
- Blum, A.; Ramaiah, S.; Kanemasu, E.; Paulsen, G. The physiology of heterosis in sorghum with respect to environmental stress. Ann. Bot. 1990, 65, 149–158. [Google Scholar] [CrossRef]
- Plucknett, D.L.; Evenson, J.; Sanford, W.G. Ratoon cropping. Adv. Agron. 1970, 22, 285–330. [Google Scholar] [CrossRef]
- Tarumoto, I. Studies on breeding forage sorghum by utilizing heterosis. Bul. Chugoku Nat. Agr. Exp. Sta. A 1971, 19, 21–138. [Google Scholar]
- Escalada, R.G.; Plucknett, D.L. Ratoon Cropping of Sorghum: II. Effect of Daylength and Temperature on Tillering and Plant Development 1. Agron. J. 1975, 67, 479–484. [Google Scholar] [CrossRef]
- Quinby, J. The maturity genes of sorghum. Adv. Agron. 1967, 19, 267–305. [Google Scholar] [CrossRef]
- Miller, F.; Barnes, D.; Cruzado, H. Effect of Tropical Photoperiods on the Growth of Sorghum When Grown in 12 Monthly Plantings 1. Crop Sci. 1968, 8, 499–509. [Google Scholar] [CrossRef]
- Rooney, W.L.; Aydin, S. Genetic control of a photoperiod-sensitive response in Sorghum bicolor (L.) Moench. Crop Sci. 1999, 39, 397–400. [Google Scholar] [CrossRef]
- Tarumoto, I.; Yanase, M.; Kadowaki, H.; Yamada, T.; Kasuga, S. Inheritance of photoperiod-sensitivity genes controlling flower initiation in sorghum, Sorghum bicolor Moench. Grassl. Sci. 2005, 51, 55–61. [Google Scholar] [CrossRef]
- Na, C.-I.; Sollenberger, L.E.; Erickson, J.E.; Woodard, K.R.; Vendramini, J.M.; Silveira, M.L. Management of perennial warm-season bioenergy grasses. I. Biomass harvested, nutrient removal, and persistence responses of elephantgrass and energycane to harvest frequency and timing. Bioenergy Res. 2015, 8, 581–589. [Google Scholar] [CrossRef]
- Wang, Z.; Jot Smyth, T.; Crozier, C.R.; Gehl, R.J.; Heitman, A.J. Yield and nitrogen removal of bioenergy grasses as influenced by nitrogen rate and harvest management in the Coastal Plain Region of North Carolina. BioEnergy Res. 2018, 11, 44–53. [Google Scholar] [CrossRef]
- Knoll, J.E.; Johnson, J.M.; Lee, R.D.; Anderson, W.F. Harvest Management of ‘Tifton 85’ Bermudagrass for Cellulosic Ethanol Production. BioEnergy Res. 2014, 7, 1112–1119. [Google Scholar] [CrossRef][Green Version]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S. Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front. Plant Sci. 2023, 13, 1067498. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef]
- Adams, C.B.; Reyes-Cabrera, J.; Nielsen, J.; Erickson, J.E. Root system architecture in genetically diverse populations of grain sorghum compared with shallow and steeply rooted monocultures. Crop Sci. 2020, 60, 2709–2719. [Google Scholar] [CrossRef]
- Reyes-Cabrera, J.; Adams, C.B.; Nielsen, J.; Erickson, J.E. Yield, nitrogen, and water-use efficiency of grain sorghum with diverse crown root angle. Field Crops Res. 2023, 294, 108878. [Google Scholar] [CrossRef]
- Slota, M.; Maluszynski, M.; Szarejko, I. An automated, cost-effective and scalable, flood-and-drain based root phenotyping system for cereals. Plant Methods 2016, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.-H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- Choi, M.; Choi, N.; Lee, J.; Lee, S.; Kim, Y.; Na, C. Effects of Italian Ryegrass (Lolium multiflorum) Cultivation for Green Manure and Forage on Subsequent Above-and Below-Ground Growth and Yield of Soybean (Glycine max). Agriculture 2023, 13, 2038. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramırez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Rich, S.M.; Watt, M. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.; Rebetzke, G.; Richards, R. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2013, 112, 447–455. [Google Scholar] [CrossRef]
- Heinze, J.; Sitte, M.; Schindhelm, A.; Wright, J.; Joshi, J. Plant-soil feedbacks: A comparative study on the relative importance of soil feedbacks in the greenhouse versus the field. Oecologia 2016, 181, 559–569. [Google Scholar] [CrossRef]
- Schittko, C.; Runge, C.; Strupp, M.; Wolff, S.; Wurst, S. No evidence that plant-soil feedback effects of native and invasive plant species under glasshouse conditions are reflected in the field. J. Ecol. 2016, 104, 1243–1249. [Google Scholar] [CrossRef]
- Rich, S.M.; Christopher, J.; Richards, R.; Watt, M. Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J. Exp. Bot. 2020, 71, 4751–4762. [Google Scholar] [CrossRef]
- Thornley, J. Acclimation of photosynthesis to light and canopy nitrogen distribution: An interpretation. Ann. Bot. 2004, 93, 473–475. [Google Scholar] [CrossRef]
- Gong, X.; Liu, C.; Ferdinand, U.; Dang, K.; Zhao, G.; Yang, P.; Feng, B. Effect of intercropping on leaf senescence related to physiological metabolism in proso millet (Panicum miliaceum L.). Photosynthetica 2019, 57, 993–1006. [Google Scholar] [CrossRef]
- Moi, P.E.; Kitonyo, O.M.; Chemining’wa, G.N.; Kinama, J.M. Intercropping and Nitrogen Fertilization Altered the Patterns of Leaf Senescence in Sorghum. Int. J. Agron. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Tollenaar, M. Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 2007, 47, S-202–S-215. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, T.; Lu, D.; Zhang, P.; Li, J.; Li, F.; Zhang, Z.; Chen, P.; Jiang, L.; Dai, C. Effects of Different Irrigation Management and Nitrogen Rate on Sorghum (Sorghum bicolor L.) Growth, Yield and Soil Nitrogen Accumulation with Drip Irrigation. Agronomy 2024, 14, 215. [Google Scholar] [CrossRef]
- Chumphu, S.; Jongrungklang, N.; Songsri, P. Association of physiological responses and root distribution patterns of ratooning ability and yield of the second ratoon cane in sugarcane elite clones. Agronomy 2019, 9, 200. [Google Scholar] [CrossRef]
- Jongrungklang, N.; Toomsan, B.; Vorasoot, N.; Jogloy, S.; Boote, K.; Hoogenboom, G.; Patanothai, A. Drought tolerance mechanisms for yield responses to pre-flowering drought stress of peanut genotypes with different drought tolerant levels. Field Crops Res. 2013, 144, 34–42. [Google Scholar] [CrossRef]
- Kato, Y.; Okami, M. Root morphology, hydraulic conductivity and plant water relations of high-yielding rice grown under aerobic conditions. Ann. Bot. 2011, 108, 575–583. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, S.; Park, C.; Na, C.-I.; Kim, Y. Treatment with silicon fertilizer induces changes in root morphological traits in soybean (Glycine max L.) during early growth. J. Crop Sci. Biotech. 2020, 23, 445–451. [Google Scholar] [CrossRef]
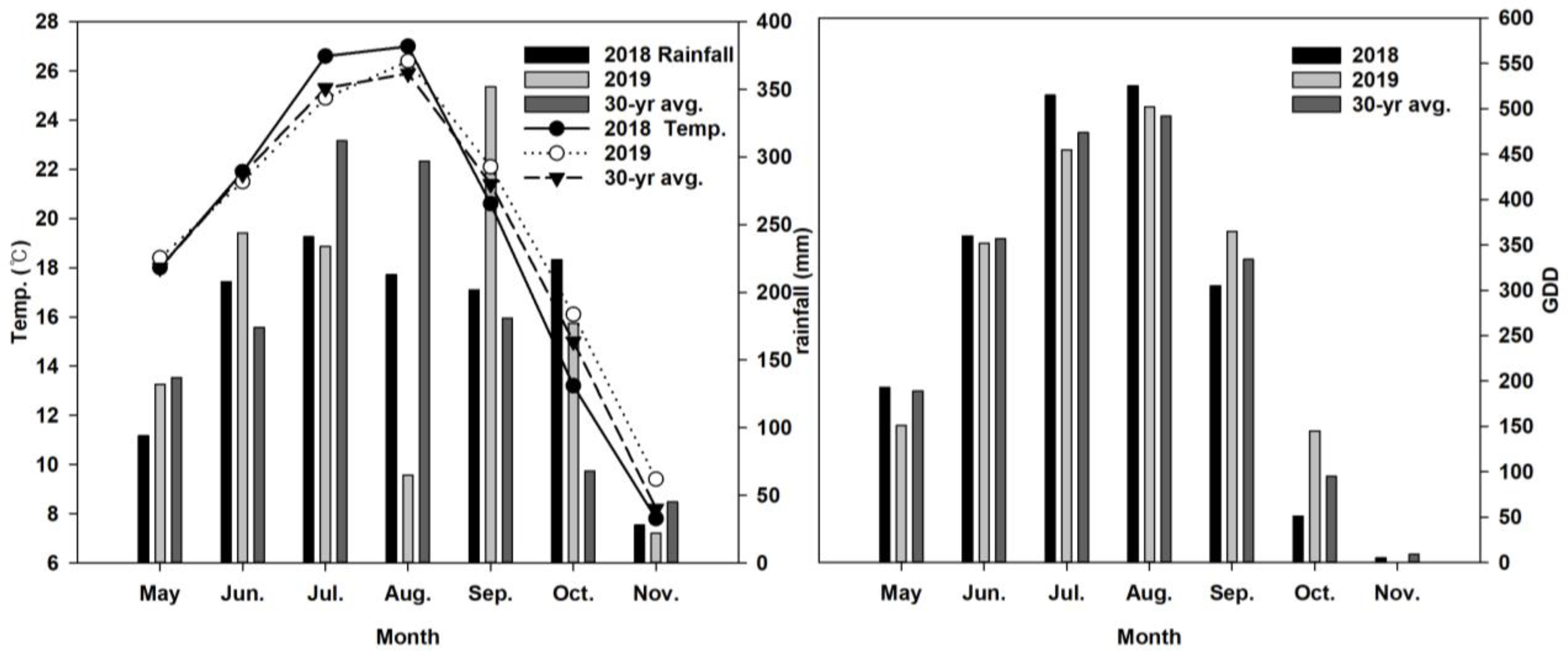

| Year | pH | EC | OM | Total N | Av. P2O5 | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|
| (1:5) | (dS m−1) | (g kg−1) | (g kg−1) | (mg kg−1) | (cmol+ kg−1) | |||
| 2018 | 6.3 | 0.20 | 7.0 | 0.37 | 104 | 0.22 | 2.84 | 0.49 |
| 2019 | 6.3 | 0.22 | 10.9 | 0.34 | 37 | 0.21 | 3.49 | 0.40 |
| Operations and Developmental Stages | 2018 | 2019 | |
|---|---|---|---|
| Plowing, leveling | 9 May | 7 May | |
| Preplant fertilizer application | 9 May | 7 May | |
| Sowing | 11 May | 8 May | |
| Summer harvest a | 2 August | 22 July | |
| Additional N fertilizer application | 10 August | 26 July | |
| Root sampling | Early | - | 19 August (DAS b 28) |
| Middle | 17 October (DAS 76) | 25 September (DAS 65) | |
| Late | 23 November (DAS 113) | 30 October (DAS 100) | |
| Boot stage | |||
| (Greenstar/Honeychew) | N.D. c/4 October | 21 October/20 September | |
| Full flowering stage | |||
| (Greenstar/Honeychew) | N.D./17 October | 28 October/4 October | |
| Source of Variation | Chlorophyll Content | LAI | ||
|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |
| N level (N) | <0.001 a | <0.001 | <0.001 | <0.001 |
| Ecotype (E) | 0.091 | 0.334 | 0.085 | 0.173 |
| Days after summer harvest (DAS) | <0.001 | <0.001 | <0.001 | <0.001 |
| N × E | 0.321 | 0.054 | 0.012 | 0.397 |
| N × DAS | 0.001 | <0.001 | <0.001 | <0.001 |
| E × DAS | 0.968 | <0.001 | 0.929 | <0.001 |
| N × E × DAS | 0.460 | 0.807 | 0.865 | 0.075 |
| Year | Treatments | Ratooning Period | ||||||
|---|---|---|---|---|---|---|---|---|
| DAS a 15 | DAS 30 | DAS 45 | DAS 60 | DAS 75 | DAS 90 | |||
| 2018 | N level | 0 N | - | 438 a b B c | 419 aB | 395 bB | 423 aC | - |
| 50 N | - | 527 aA | 449 bcA | 439 cA | 466 bB | - | ||
| 100 N | - | 543 aA | 450 cA | 432 cA | 492 bAB | - | ||
| 150 N | - | 537 aA | 462 cA | 450 cA | 503 bA | - | ||
| Polynomial contrast d | L ***Q *** | L ** | L * | L *** | ||||
| 2019 | N level | 0 N | 418 cB | 418 cC | 468 aB | 462 aB | 440 bB | 425 bcB |
| 50 N | 495 aA | 456 bcB | 467 bB | 475 abB | 474 bA | 445 cAB | ||
| 100 N | 507 aA | 497 abA | 480 bAB | 479 bB | 485 bA | 459 cA | ||
| 150 N | 500 aA | 497 aA | 495 aA | 507 aA | 489 aA | 460 bA | ||
| Polynomial contrast | L ***Q *** | L ***Q * | L * | L ** | L ** | NS | ||
| Source of Variation | Total Root Length | Total Root Surface Area | Total Root Volume | Tips | Root DM | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2019 | |
| N level (N) | <0.001 a | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ecotype (E) | 0.381 | 0.003 | 0.773 | 0.005 | 0.893 | 0.006 | 0.627 | 0.002 | 0.005 |
| Days after summer harvest (DAS) | <0.001 | <0.001 | 0.001 | <0.001 | 0.019 | <0.001 | 0.002 | 0.003 | <0.001 |
| N × E | 0.427 | 0.002 | 0.168 | 0.005 | 0.092 | 0.032 | 0.414 | <0.001 | 0.077 |
| N × DAS | 0.401 | 0.770 | 0.443 | 0.837 | 0.348 | 0.560 | 0.450 | 0.585 | 0.038 |
| E × DAS | 0.213 | <0.001 | 0.119 | <0.001 | 0.066 | <0.001 | 0.201 | 0.005 | <0.001 |
| N × E × DAS | 0.011 | 0.267 | 0.009 | 0.504 | 0.019 | 0.790 | 0.043 | 0.193 | 0.872 |
| Year | Treatments | Total Root Length | Total Root Surface Area | Total Root Volume | Tips | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| cm plant−1 | cm2 plant−1 | cm3 plant−1 | plant−1 | |||||||
| Greenstar | Honeychew | Greenstar | Honeychew | Greenstar | Honeychew | Greenstar | Honeychew | |||
| 2018 | N level | 0 N | 377 a b B c | 526 aB | 76 aB | 120 aB | 1.73 aB | 2.25 aC | 1780 aB | 2470 aB |
| 50 N | 1063 aA | 737 aAB | 234 aA | 165 aAB | 4.22 aA | 2.99 bBC | 4630 aA | 3380 aAB | ||
| 100 N | 1079 aA | 996 aA | 232 aA | 223 aA | 4.05 aA | 4.07 aAB | 4490 aA | 4490 aA | ||
| 150 N | 1123 aA | 982 aA | 215 aA | 226 aA | 3.35 aA | 4.21 aA | 4690 aA | 4380 aA | ||
| Polynomial contrast d | L **Q * | L ** | L **Q ** | L ** | L *Q *** | L ** | L **Q * | L * | ||
| Ratooning period | DAS a 76 | 992 aA | 964 aA | 201 aA | 216 aA | 3.41 aA | 3.93 aA | 4180 aA | 4340 aA | |
| DAS 113 | 829 aB | 657 aB | 177 aA | 151 aB | 3.27 aA | 2.83 aB | 3620 aA | 3020 aB | ||
| 2019 | N level | 0 N | 318 aC | 226 bB | 98 aC | 65 bB | 2.52 aC | 1.52 bB | 1240 aC | 800 bB |
| 50 N | 530 aB | 246 bB | 179 aB | 80 bB | 4.93 aB | 2.12 bB | 2110 aAB | 880 bB | ||
| 100 N | 512 aB | 399 bA | 186 aAB | 136 bA | 5.51 aAB | 3.80 bA | 1920 aB | 1460 bA | ||
| 150 N | 606 aA | 422 bA | 209 aA | 141 bA | 5.84 aA | 3.80 bA | 2370 aA | 1570 bA | ||
| Polynomial contrast | L ** | L *** | L ** | L *** | L ** | L *** | L ** | L *** | ||
| Ratooning period | DAS 28 | 319 aC | 198 bB | 106 aC | 67 bC | 2.91 aC | 1.86 bC | 1270 aC | 740 bB | |
| DAS 65 | 478 aB | 377 bA | 157 aB | 113 bB | 4.23 aB | 2.70 bB | 2020 aB | 1510 bA | ||
| DAS 100 | 677 aA | 394 bA | 241 aA | 137 bA | 6.95 aA | 3.86 bA | 2440 aA | 1280 bA | ||
| Treatments | Ecotype | |||
|---|---|---|---|---|
| g plant−1 | ||||
| Greenstar | Honeychew | Avg. | ||
| N level | 0 N | 1.22 | 0.72 | 0.97 C b |
| 50 N | 2.45 | 1.03 | 1.74 B | |
| 100 N | 2.78 | 1.82 | 2.30 A | |
| 150 N | 2.99 | 1.95 | 2.47 A | |
| Avg. | 2.36 a c | 1.38 b | ||
| Polynomial contrast d | L * | L *** | ||
| Ratooning period | DAS a 28 | 0.92 aC | 0.62 aC | |
| DAS 65 | 1.98 aB | 1.35 bB | ||
| DAS 100 | 4.19 aA | 2.18 bA | ||
| Ratooning period | ||||
| DAS 28 | DAS 65 | DAS 100 | ||
| N level | 0 N | 0.29 bB | 0.74 bC | 1.89 aC |
| 50 N | 0.67 cAB | 1.55 bB | 3.01 aB | |
| 100 N | 1.02 cA | 2.22 bA | 3.66 aA | |
| 150 N | 1.11 cA | 2.14 bAB | 4.16 aA | |
| Treatments | Total Root Length | Total Root Surface Area | Total Root Volume | Tips | Root DM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm plant−1 | cm2 plant−1 | cm3 plant−1 | plant−1 | g plant−1 | ||||||||
| Greenstar | Honeychew | Greenstar | Honeychew | Greenstar | Honeychew | Avg. | Greenstar | Honeychew | Avg. | Greenstar | Honeychew | |
| DAP a 26 | 217 a b B c | 229 aB | 50 aB | 53 aB | 0.95 | 1.00 | 0.98 B | 680 | 740 | 710 B | 0.23 aC | 0.27 aB |
| DAP 55 | 1209 aA | 738 bA | 243 aA | 165 bA | 3.93 | 2.99 | 3.46 A | 4520 | 2580 | 3550 A | 1.14 aB | 1.08 aA |
| DAP 75 | 1326 aA | 701 bA | 302 aA | 148 bA | 4.74 | 2.51 | 3.63 A | 4520 | 2780 | 3650 A | 2.10 aA | 1.10 bA |
| Avg. | 3.21 a | 2.17 b | 3240 a | 2030 b | ||||||||
| Source of variation | ||||||||||||
| Ecotype (E) | <0.001 d | <0.001 | 0.039 | 0.002 | 0.031 | |||||||
| DAP | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| E × DAP | 0.020 | 0.036 | 0.137 | 0.064 | 0.041 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.; Choi, M.; Lee, S.; Jo, C.; Kim, G.; Jeong, Y.; Lee, J.; Na, C. Effects of Ecotypes and Reduced N Fertilization on Root Growth and Aboveground Development of Ratooning Sorghum × Sudangrass Hybrids. Agronomy 2024, 14, 2073. https://doi.org/10.3390/agronomy14092073
Choi N, Choi M, Lee S, Jo C, Kim G, Jeong Y, Lee J, Na C. Effects of Ecotypes and Reduced N Fertilization on Root Growth and Aboveground Development of Ratooning Sorghum × Sudangrass Hybrids. Agronomy. 2024; 14(9):2073. https://doi.org/10.3390/agronomy14092073
Chicago/Turabian StyleChoi, Nayoung, Miri Choi, Sora Lee, Chaelin Jo, Gamgon Kim, Yonghyun Jeong, Jihyeon Lee, and Chaein Na. 2024. "Effects of Ecotypes and Reduced N Fertilization on Root Growth and Aboveground Development of Ratooning Sorghum × Sudangrass Hybrids" Agronomy 14, no. 9: 2073. https://doi.org/10.3390/agronomy14092073
APA StyleChoi, N., Choi, M., Lee, S., Jo, C., Kim, G., Jeong, Y., Lee, J., & Na, C. (2024). Effects of Ecotypes and Reduced N Fertilization on Root Growth and Aboveground Development of Ratooning Sorghum × Sudangrass Hybrids. Agronomy, 14(9), 2073. https://doi.org/10.3390/agronomy14092073






