Biomass Ash as a Substitute for Lime and Its Impact on Grassland Soil, Forage, and Soil Microbiota
Abstract
1. Introduction
2. Material and Methods
2.1. Field Trial and Sampling Strategy
2.2. Plant Cover and Yield
2.3. Forage Analysis
2.4. Soil/Biomass Ash/CS Physicochemical and Microbiological Measurements
2.5. Microbiome Profiling
2.6. Statistical Analyses
3. Results
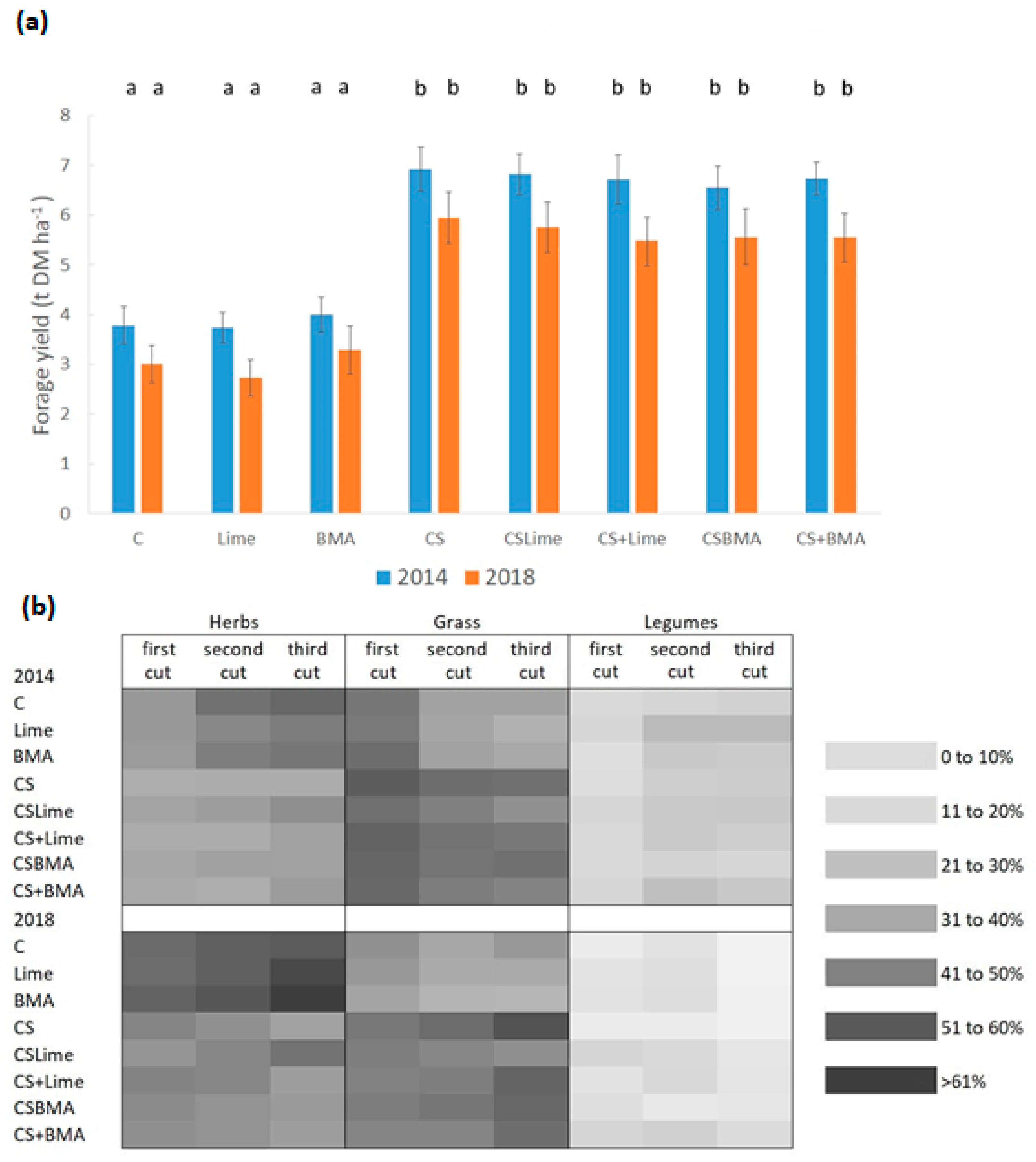
3.1. Yield, Plant Cover, and Forage Quality
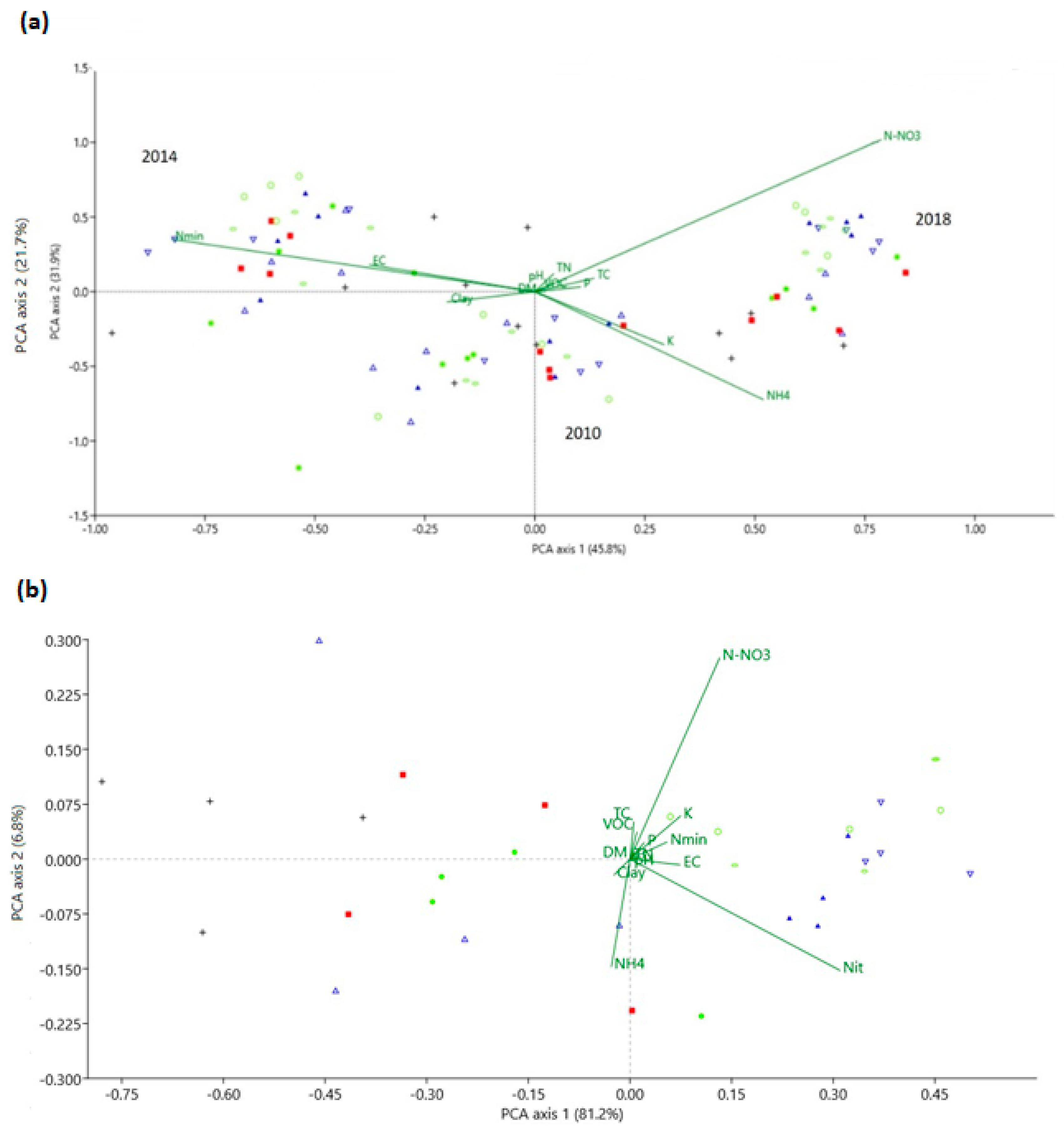
3.2. Soil Physicochemical Properties
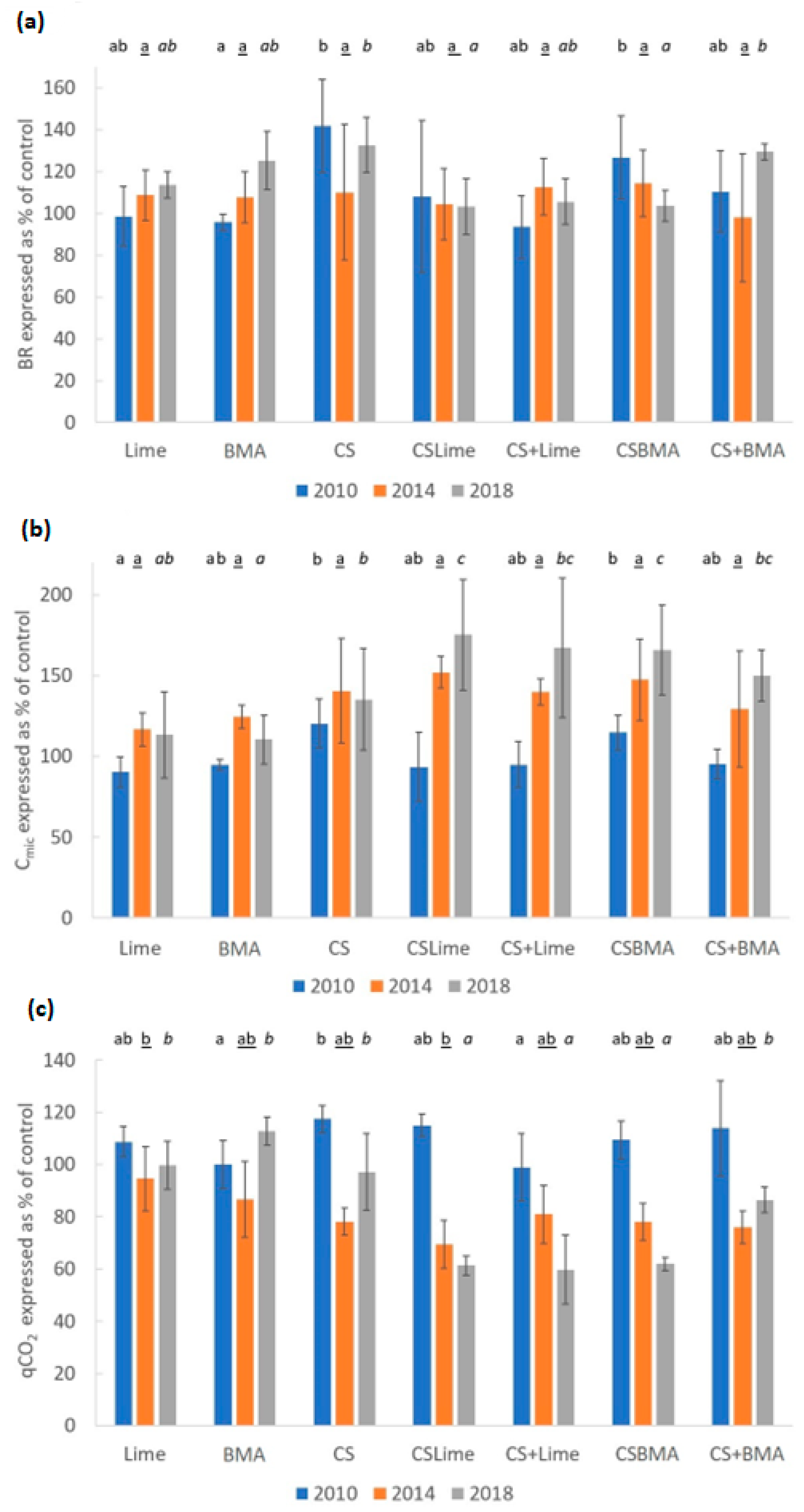
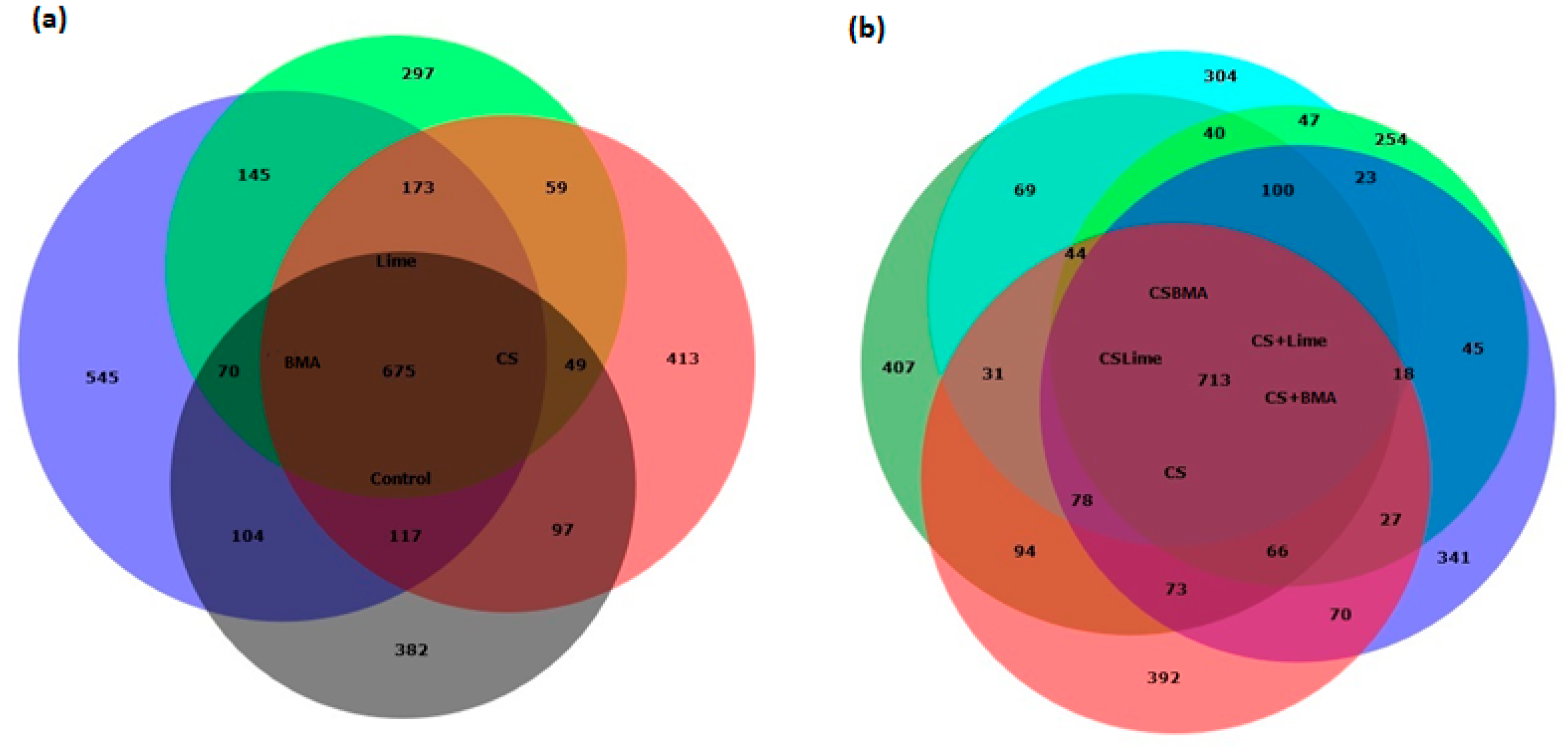
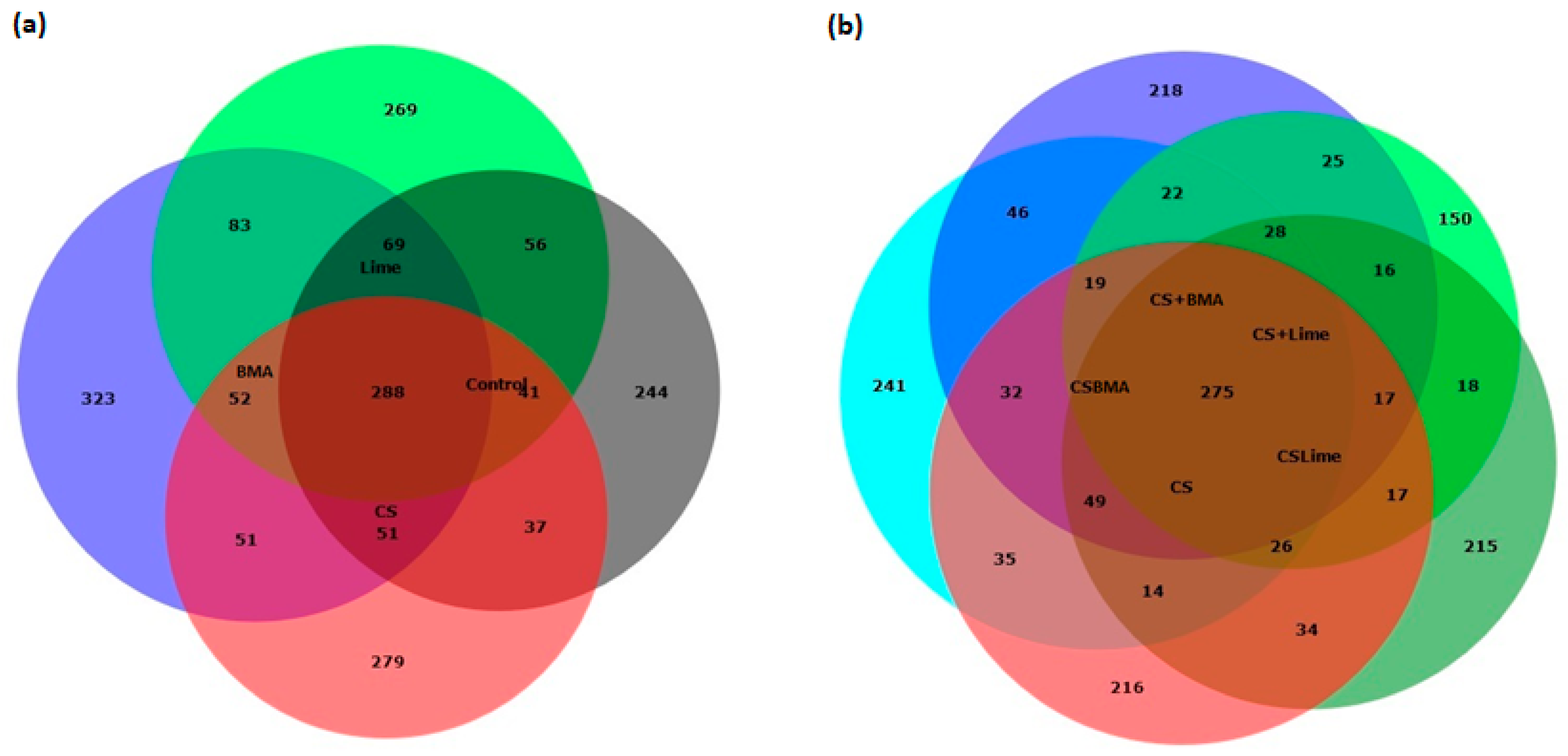
3.3. Microbial Properties and Community Composition
4. Discussion
4.1. Yield, Plant Cover, and Forage Quality
4.2. Soil Physicochemical Properties
4.3. Microbial Properties and Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banja, M.; Sikkema, R.; Jégard, M.; Motola, V.; Dallemand, J.F. Biomass for energy in the EU—The support framework. Energy Policy 2019, 131, 215–228. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/de/home (accessed on 17 November 2023).
- European Commission. A Policy Framework for Climate and Energy in the Period from 2020 to 2030. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Hassan, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef]
- Alexander, P.; Arneth, A.; Henry, R.; Maire, J.; Rabin, S.; Rounsevell, M.D.A. High energy and fertilizer prices are more damaging than food export curtailment from Ukraine and Russia for food prices, health and the environment. Nat. Food 2023, 4, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Riding, M.J.; Herbert, B.M.J.; Ricketts, L.; Dodd, I.; Ostle, N.; Semple, K.T. Harmonising conflicts between science, regulation, perception and environmental impact: The case of soil conditioners from bioenergy. Environ. Int. 2015, 75, 52–67. [Google Scholar] [CrossRef] [PubMed]
- BMKBundesministerium für Klimaschutz, Umwelt, Energie, Mobilität, Innovation und Technologie. Available online: https://www.bmk.gv.at/dam/jcr:04ca87f4-fd7f-4f16-81ec-57fca79354a0/BAWP_Statusbericht2021.pdf (accessed on 10 October 2013).
- Knapp, B.A.; Insam, H. Recycling of Biomass Ashes: Current Technologies and Future Research Needs. In Recycling of Biomass Ashes, 1st ed.; Insam, H., Knapp, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–16. [Google Scholar] [CrossRef]
- Fernández-Delgado Juárez, M.; Gómez-Brandón, M.; Knapp, A.; Stöhr, D.; Insam, H. Chemical and microbiological properties of alpine forest soils: Effects of pelletized ashes in a short-term trial. For. Ecol. Manag. 2015, 357, 42–49. [Google Scholar] [CrossRef]
- Kurzemann, F.R.; Fernández-Delgado Juárez, M.; Probst, M.; Gómez-Brandón, M.; Partl, C.; Insam, H. Effect of biomass fly ashes from fast pyrolysis bio-oil production on soil properties and plant yield. J. Environ. Manag. 2021, 298, 113479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado Juárez, M.; Prähauser, B.; Walter, A.; Insam, H.; Franke-Whittle, I.H. Co-composting of biowaste and wood ash, influence on a microbially driven-process. Waste Manag. 2015, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Gorazda, K.; Witek-Krowiak, A.; Moustakas, K. Recovery of fertilizer nutrients from materials—Contradictions, mistakes and future trends. Renew. Sustain. Energy Rev. 2019, 110, 485–498. [Google Scholar] [CrossRef]
- Mieldažys, R.; Jotautiene, E.; Jasinskas, A. The Opportunities of Sustainable Biomass Ashes and Poultry Manure Recycling for Granulated Fertilizers. Sustainability 2019, 11, 4466. [Google Scholar] [CrossRef]
- Maresca, A.; Hyks, J.; Astrup, T.F. Recirculation of biomass ashes onto forest soils: Ash composition, mineralogy and leaching properties. Waste Manag. 2017, 70, 127–138. [Google Scholar] [CrossRef]
- Fernández-Delgado Juárez, M.; Fabiani, G.; Mazzier, T.; Schönegger, D.; Pietramellara, G.; Gómez-Brandón, M.; Insam, H. Reclamation of Acid Soils with Biomass Ashes from Pyrolytic Wood Liquefaction. Waste Biomass Valorization 2020, 11, 5067–5078. [Google Scholar] [CrossRef]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilisers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Da Costa, T.P.; Quinteiro, P.; Tarelho, L.A.C.; Arroja, L.; Dias, A.C. Life cycle assessment of woody biomass ash for soil amelioration. Waste Manag. 2020, 101, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Park, N.D.; Michael Rutherford, P.; Thring, R.W.; Helle, S.S. Wood pellet fly ash and bottom ash as an effective liming agent and nutrient source for rye grass (Lolium perenne L.) and oats (Avena sativa). Chemosphere 2012, 86, 427–432. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Fernández-Delgado Juárez, M.; Waldhuber, S.; Knapp, A.; Partl, C.; Gómez-Brandón, M.; Insam, H. Wood ash effects on chemical and microbiological properties of digestate- and manure-amended soils. Biol. Fertil. Soils 2013, 49, 575–585. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Effect of management and climate on biochemical properties of grassland soils from Galicia (NW Spain). Eur. J. Soil Biol. 2010, 46, 136–143. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Anwar, M.Z.; Lanzén, A.; Kjøller, R.; Rønn, R.; Ekelund, F.; Jacobsen, C.S. Total RNA sequencing reveals multilevel microbial community changes and functional responses to wood ash application in agricultural and forest soil. FEMS Microbiol. Ecol. 2020, 96, fiaa016. [Google Scholar] [CrossRef]
- Schönegger, D.; Gómez-Brandón, M.; Mazzier, T.; Insam, H.; Hermanns, R.; Leijenhorst, E.; Bardelli, T.; Fernández-Delgado Juárez, M. Phosphorus fertilising potential of fly ash and effects on soil microbiota and crop. Resour. Conser. Recycl. 2018, 134, 262–270. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.B.; Jacobsen, C.S. Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Zimmermann, S.; Frey, B. Soil respiration and microbial properties in an acid forest soil: Effects of wood ash. Soil Biol. Biochem. 2002, 34, 1727–1737. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Siles, J.A.; Cajthaml, T.; García-Romera, I.; Tlustoš, P.; Száková, J. Effect of digestate and fly ash applications on soil functional properties and microbial communities. Eur. J. Soil Biol. 2015, 71, 1–12. [Google Scholar] [CrossRef]
- Brod, E.; Haraldsen, T.K.; Breland, T.A. Fertilization effects of organic waste resources and bottom wood ash: Results from a pot experiment. Agric. Food Sci. 2012, 21, 332–347. [Google Scholar] [CrossRef][Green Version]
- Johansen, J.L.; Nielsen, M.L.; Vestergård, M.; Mortensen, L.H.; Cruz-Paredes, C.; Rønn, R.; Kjøller, R.; Hovmand, M.; Christensen, S.; Ekelund, F. The complexity of wood ash fertilization disentangled: Effects on soil pH, nutrient status, plant growth and cadmium accumulation. Environ. Exp. Bot. 2021, 185, 104424. [Google Scholar] [CrossRef]
- Hale, S.E.; Nurida, N.L.; Jubaedah Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ. 2020, 719, 137455. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Mapsi; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- BMLRT Bundesministerium für Land- und Forstwirtschaft, Regionen und Wasserwirtschaft. Available online: https://info.bml.gv.at/dam/jcr:c27d33a3-d162-4632-9a63-c17491467447/Richtlinie%20f%C3%BCr%20den%20sachgerechten%20Einsatz%20von%20Pflanzenaschen%20zur%20Verwertung%20auf%20Land-%20und%20Forstwirtschaftlich%20genutzten%20Fl%C3%A4chen.pdf (accessed on 17 November 2013).
- BMLRT Bundesministerium für Klimaschutz, Umwelt, Energie, Mobilität, Innovation und Technologie. Available online: https://www.ages.at/download/sdl-eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJpYXQiOjE2MDk0NTkyMDAsImV4cCI6NDA3MDkwODgwMCwidXNlciI6MCwiZ3JvdXBzIjpbMCwtMV0sImZpbGUiOiJmaWxlYWRtaW4vQUdFU18yMDIyLzRfUEZMQU5aRS9EXHUwMGZjbmdlbWl0dGVsL0RcdTAwZmNuZ2VtaXR0ZWxfVW50ZXJzdWNodW5nZW4vUmljaHRsaW5pZW5fZnVlcl9kaWVfc2FjaGdlcmVjaHRlX0R1ZW5ndW5nX2ltX0Fja2VyYmF1X3VuZF9HcnVlbmxhbmRfN19BdWZsYWdlXzFfLnBkZiIsInBhZ2UiOjg0OX0.NJdQ-wVKIMEbxD25xFqUhRc55QIpxnbVeFYWnPaaw0s/Richtlinien_fuer_die_sachgerechte_Duengung_im_Ackerbau_und_Gruenland_7_Auflage_1_.pdf (accessed on 11 July 2024).
- Peratoner, G.; Pötsch, E.M. Methods to describe the botanical composition of vegetation in grassland research. Die Bodenkultur. J. Land Manag. Food Environ. 2019, 70, 1–18. [Google Scholar] [CrossRef]
- VDLUFA. Bestimmung Rohfett, WEENDER-Verfahren; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- VDLUFA. Bestimmung Rohfaser, WEENDER-Verfahren; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- VDLUFA. Bestimmung Rohprotein, DUMAS-Verbrennungsmethode; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- VDLUFA. Bestimmung Rohasche; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- VDLUFA. Bestimmung der Mengenelemente; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- Öhlinger, R. Maximum water-holding capacity. In Methods in Soil Biology, 1st ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margasin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 385–386. [Google Scholar]
- Kandeler, E. Nitrate. In Methods in Soil Biology, 1st ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margasin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 408–410. [Google Scholar]
- Kandeler, E. Ammonium. In Methods in Soil Biology, 1st ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margasin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 406–408. [Google Scholar]
- Kandeler, E. N-Mineralization under waterlogged conditions. In Methods in Soil Biology, 1st ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margasin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 141–144. [Google Scholar]
- Kandeler, E. Potential nitrification. In Methods in Soil Biology, 1st ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margasin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 146–149. [Google Scholar]
- Heinemeyer, O.; Insam, H.; Kaiser, E.A.; Walenzik, G. Soil microbial biomass and respiration measurements: An automated technique based on infra-red gas analysis. Plant Soil 1989, 116, 191–195. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- ÖNORM L 1086-1 Austrian Standards. Available online: https://shop.austrian-standards.at/action/de/public/details/518044/OENORM_L_1086-1_2014_03_15;jsessionid=BEF9832C9871F0717DAD63E77B17ABD5 (accessed on 6 May 2023).
- ÖNORM L 1085 Austrian Standards. Available online: https://shop.austrian-standards.at/action/de/public/details/510509/OENORM_L_1085_2013_11_15;jsessionid=CC2B6824556C5FA5651E964058B00685 (accessed on 6 May 2023).
- DIN CEN/TS 15290. Available online: https://www.beuth.de/en/pre-standard/din-cen-ts-15290/82690986 (accessed on 6 May 2023).
- DIN EN 15297. Available online: https://www.beuth.de/de/norm/din-en-15297/138427266 (accessed on 6 May 2023).
- DIN CEN/TS 14774. Available online: https://www.beuth.de/de/vornorm/din-cen-ts-14774-3/66876061 (accessed on 6 May 2023).
- ÖNORM EN Austrian Standards. Available online: https://shop.austrian-standards.at/action/de/public/details/311963/SN_EN_13137_2001_2001_08_01 (accessed on 6 May 2023).
- DIN 38414-4. Available online: https://www.beuth.de/de/norm/din-38414-4/1148060 (accessed on 6 May 2023).
- DIN EN 13370. Available online: https://www.beuth.de/de/norm/din-en-13370/60388488 (accessed on 6 May 2023).
- Caporaso, J.G.; Lauber, C.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York City, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria.
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Meth. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 178. Available online: http://palaeo-electronica.orghttp://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 17 November 2023).
- R: The R Project for Statistical Computing. The R Foundation. Available online: https://www.r-project.org/ (accessed on 4 November 2023).
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genet. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118445112 (accessed on 4 November 2023).
- Oksanen, J.S.G.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; Wagner, H.; et al. Vegan: Community Ecology Package. R Package Version. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 4 November 2023).
- Pugnaire, F.I.; Serrano, I.; Pardos, J. Constraints by water stress on plant growth. In Handbook of Plant and Crop Stress, 2nd ed.; Pessarakli, Marcel Dekker: New York City, NY, USA, 1999; pp. 271–283. [Google Scholar]
- ZAMG. Available online: https://www.zamg.ac.at/cms/de/produkte/klima/daten-und-statistiken (accessed on 17 November 2023).
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology, 1st ed.; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kuba, T.; Tschöll, A.; Partl, C.; Meyer, K.; Insam, H. Wood Ash Admixture to Organic Wastes Improves Compost and Its Performance. Agric. Ecosystem. Environm. 2008, 127, 43–49. [Google Scholar] [CrossRef]
- Asquer, C.; Cappai, G.; Carucci, A.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass ash characterisation for reuse as additive in composting process. Biomass Bioenergy 2019, 123, 186–194. [Google Scholar] [CrossRef]
- Rolka, E.; Żołnowski, A.C.; Wyszkowski, M.; Zych, W.; Skorwider-Namiotko, A. Wood Biomass Ash (WBA) from the Heat Production Process as a Mineral Amendment for Improving Selected Soil Properties. Energies 2023, 16, 5110. [Google Scholar] [CrossRef]
- Schellberg, J.; Möseler, B.M.; Kühbauch, W.; Rademacher, I.F. Long-term effects of fertilizer on soil nutrient concentration, yield, forage quality and floristic composition of a hay meadow in the Eifel mountains, Germany. Grass Forage Sci. 1999, 54, 195–207. [Google Scholar] [CrossRef]
- Herndl, M.; Kandolf, M.; Bohner, A.; Krautzer, B.; Graiss, W.; Schink, M. Wurzelparameter von Gräsern, Kräutern und Leguminosen als Grundlage zur Bewertung von Trockenheitstoleranz im Grünland. In Proceedings of the 1. Tagung der Österreichischen Gesellschaft für Wurzelforschung Pflanzenwurzel im System Boden-Pflanze-Atmosphäre, Raumberg-Gumpenstein, Austria, 13–14 September 2011; Österreichische Gesellschaft für Wurzelforschung. pp. 45–54. [Google Scholar]
- Pirhofer-Walzl, K.; Søegaard, K.; Høgh-Jensen, H.; Eriksen, J.; Sanderson, M.A.; Rasmussen, J.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef]
- Whitehead, D.C. Nutrient Elements in Grasslands: Soil Plant-Animal Relationships, 1st ed.; Whitehead, D.C., Ed.; CABI: Wallingford, UK, 2000; pp. 1–69. [Google Scholar]
- Aboltins, A.; Palabinskis, J.; Karps, O.; Jotautiene, E. Biomass Ash and Cattle Slurry Mixture Solid Fraction Extracting and Its Use in Agriculture. Engineering for Rural Development 2020. In Proceedings of the 21st International Scientific Conference Engineering for Rural Development, Jelgava, Lativa, 25–27 May 2020; Malinovska, L., Osadcuks, V., Eds.; Latvia University of Life Sciences and Technologies: Jelgava, Lativa, 2020; pp. 1967–1973. [Google Scholar]
- Hu, N.; Li, H.; Tang, Z.; Li, Z.; Li, G.; Jiang, Y.; Hu, X.; Lou, Y. Community size, activity and C:N stoichiometry of soil microorganisms following reforestation in a Karst region. Eur. J. Soil Biol. 2016, 73, 77–83. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of Organic Fertilizers on the Soil Microorganisms Responsible for N2O Emissions: A Review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, M.M.; Minarsch, E.-M.L.; Durai Raj, A.C.; Rineau, F.; Schröder, P. Changes of soil-rhizosphere microbiota after organic amendment application in a Hordeum vulgare L. short-term greenhouse experiment. Plant Soil 2020, 455, 489–506. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Manoeli, L.; Boldo, J.T.; Pereira, M.G.; Roesch, L.F.W. Shifts in soil bacterial community after eight years of land-use change. Syst. Appl. Microbiol. 2013, 36, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Korvigo, I.O.; Pershina, E.; Ivanova, E.A.; Matyuk, N.S.; Savos’kina, O.A.; Chirak, E.L.; Provorov, N.A.; Andronov, E.E. Effect of long-term application of agrotechnical techniques and crops on soil microbial communities. Microbiology 2016, 85, 231–242. [Google Scholar] [CrossRef]
- Armbruster, M.; Goodall, T.; Hirsch, P.R.; Ostle, N.; Puissant, J.; Fagan, K.C.; Pywell, R.F.; Griffiths, R.I. Bacterial and archaeal taxa are reliable indicators of soil restoration across distributed calcareous grasslands. Eur. J. Soil Sci. 2020, 72, 2430–2444. [Google Scholar] [CrossRef]
- Vázquez, E.; Teutscherova, N.; Pastorelli, R.; Lagomarsino, A.; Giagnoni, L.; Renella, G. Liming reduces N2O emissions from Mediterranean soil after-rewetting and affects the size, structure and transcription of microbial communities. Soil Biol. Biochem. 2020, 147, 107839. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Bossolani, J.W.; Crusciol, C.A.C.; Leite, M.F.A.; Merloti, L.F.; Moretti, L.G.; Pascoaloto, I.M.; Kuramae, E.E. Modulation of the soil microbiome by long-term Ca-based soil amendments boosts soil organic carbon and physicochemical quality in a tropical no-till crop rotation system. Soil Biol. Biochem. 2021, 156, 108188. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Yao, Q.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Short-term lime application impacts microbial community composition and potential function in an acid black soil. Plant Soil 2022, 470, 35–50. [Google Scholar] [CrossRef]
- Neilson, R.; Caul, S.; Fraser, F.C.; King, D.; Mitchell, S.M.; Roberts, D.M.; Giles, M.E. Microbial community size is a potential predictor of nematode functional group in limed grasslands. Appl. Soil Ecol. 2020, 156, 103702. [Google Scholar] [CrossRef]
- Yin, C.; Schlatter, D.C.; Kroese, D.R.; Paulitz, T.C.; Hagerty, C.H. Impacts of lime application on soil bacterial microbiome in dryland wheat soil in the Pacific Northwest. Appl. Soil Ecol. 2021, 168, 104113. [Google Scholar] [CrossRef]
- Franklin, R.B.; Mills, A.L. Multi-scale variation in spatial heterogeneity for microbial community structure in an eastern Virginia agricultural field. FEMS Microbiol. Ecol. 2003, 44, 335–346. [Google Scholar] [CrossRef]
| Grass Yield (t DM ha−1 Year−1) | Legume Yield (t DM ha−1 Year−1) | Herb Yield (t DM ha−1 Year−1) | ||||
|---|---|---|---|---|---|---|
| year | 2014 | 2018 | 2014 | 2018 | 2014 | 2018 |
| Treatment | ||||||
| C | 1.49 ± 0.20 aA | 1.12 ± 0.24 aA | 0.52 ± 0.05 aA | 0.20 ± 0.09 aB | 1.78 ± 0.23 aA | 1.70 ± 0.18 aA |
| Lime | 1.40 ± 0.17 aA | 0.89 ± 0.17 aA | 0.78 ± 0.15 abA | 0.24 ± 0.05 aB | 1.57 ± 0.31 aA | 1.60 ± 0.56 aA |
| BMA | 1.64 ± 0.20 aA | 0.99 ± 0.14 aB | 0.63 ± 0.19 aA | 0.32 ± 0.07 abB | 1.73 ± 0.24 aA | 1.99 ± 0.16 aA |
| CS | 3.84 ± 0.35 bA | 3.23 ± 0.69 bA | 1.03 ± 0.23 abA | 0.34 ± 0.16 abB | 2.07 ± 0.28 aA | 2.38 ± 0.40 aA |
| CS + BMA | 3.39 ± 0.10 bA | 2.64 ± 0.42 bB | 1.27 ± 0.28 bA | 0.90 ± 0.49 bB | 2.17 ± 0.27 aA | 2.21 ± 0.38 aA |
| CS + Lime | 3.56 ± 0.49 bA | 2.75 ± 1.05 bB | 1.07 ± 0.32 abA | 0.54 ± 0.34 abB | 2.09 ± 0.36 aA | 2.18 ± 0.33 aA |
| CSLime | 3.09 ± 0.27 bA | 2.52 ± 0.45 bA | 1.11 ± 0.18 abA | 0.67 ± 0.12 abB | 2.34 ± 0.37 aA | 2.38 ± 0.31 aA |
| CSBMA | 3.57 ± 0.36 bA | 2.77 ± 0.17 bB | 0.91 ± 0.09 abA | 0.57 ± 0.21 abB | 2.25 ± 0.36 aA | 2.21 ± 0.28 aA |
| Year | CoVar% | C | Lime | BMA | CS | CSLime | CS + Lime | CSBMA | CS + BMA | |
|---|---|---|---|---|---|---|---|---|---|---|
| DM (%) | 2010 | 1.5 | 78.2 | 78.3 | 77.8 | 77.0 | 77.3 | 77.4 | 76.8 | 76.7 |
| VOC (%) | 2010 | 8.3 | 6.8 | 6.3 | 6.5 | 7.4 | 6.2 | 6.2 | 6.6 | 6.8 |
| pH (CaCl2) | 2010 | 2.1 | 5.2 | 5.3 | 5.2 | 5.2 | 5.4 | 5.3 | 5.2 | 5.3 |
| EC (µS cm−1) | 2010 | 13.3 | 28.5 | 35.5 | 34.5 | 37.5 | 38.3 | 37.5 | 37.0 | 44.0 |
| NH4 (µg N gDM−1) | 2010 | 37.8 | 9.5 | 15.0 | 10.4 | 17.5 | 16.4 | 13.9 | 12.1 | 14.4 |
| N-NO3 (µg N g−1 DM) | 2010 | 19.2 | 9.3 | 11.6 | 10.1 | 9.0 | 8.7 | 8.9 | 8.0 | 9.2 |
| Nit. (ng N g−1 DM 5 h−1) | 2010 | 45.2 | 102.6 | 56.8 | 64.2 | 119.9 | 111.9 | 82.5 | 75.0 | 98.3 |
| TC (%) | 2010 | 8.4 | 2.3 | 2.1 | 2.1 | 2.3 | 2.1 | 2.2 | 2.3 | 2.9 |
| TN (%) | 2010 | 13.4 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 |
| P (mg kg−1) | 2010 | 13.7 | 32.8 | 34.0 | 33.8 | 36.5 | 40.5 | 34.5 | 38.0 | 39.8 |
| K (mg kg−1) | 2010 | 23.9 | 60.5 | 81.5 | 76.3 | 89.8 | 96.0 | 105.0 | 87.0 | 109.3 |
| Clay (%) | 2010 | 5.5 | 17.0 | 17.5 | 18.0 | 17.0 | 17.5 | 17.0 | 18.5 | 17.0 |
| Fe (mg kg−1) | 2010 | 8.9 | n.d. | n.d. | n.d. | 255 | 259 | 240 | 228 | 253 |
| Mn (mg kg−1) | 2010 | 9.2 | n.d. | n.d. | n.d. | 240 | 242 | 236 | 222 | 238 |
| B (mg kg−1) | 2010 | 41.7 | n.d. | n.d. | n.d. | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 |
| As (mg kg−1) | 2010 | 3.3 | 21.4 | 21.9 | 21.8 | 21.5 | 21.3 | 21.6 | 21.5 | 21.3 |
| Pb (mg kg−1) | 2010 | 6.9 | 27.8 | 26.5 | 27.6 | 28.0 | 25.9 | 26.7 | 27.4 | 28.4 |
| Cd (mg kg−1) | 2010 | 21.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Co (mg kg−1) | 2010 | 3.5 | 15.2 | 15.5 | 15.7 | 15.2 | 15.5 | 15.6 | 15.1 | 15.1 |
| Cr (mg kg−1) | 2010 | 5.7 | 54.6 | 56.8 | 56.7 | 54.7 | 54.7 | 55.9 | 54.4 | 55.1 |
| Cu (mg kg−1) | 2010 | 9.2 | 38.7 | 40.7 | 40.7 | 39.1 | 40.6 | 40.5 | 39.2 | 40.0 |
| Ni (mg kg−1) | 2010 | 6.2 | 40.3 | 42.6 | 42.6 | 40.6 | 42.0 | 42.6 | 40.9 | 40.6 |
| Mo (mg kg−1) | 2010 | 9.0 | 0.9 | 0.8 | 0.8 | 0.9 | 0.8 | 0.8 | 0.9 | 0.9 |
| Zn (mg kg−1) | 2010 | 1.8 | 97.4 | 97.8 | 99.9 | 99.3 | 97.0 | 97.9 | 97.8 | 98.9 |
| V (mg kg−1) | 2010 | 2.8 | 53.0 | 53.0 | 53.8 | 53.0 | 51.3 | 51.5 | 50.9 | 52.5 |
| Hg (mg kg−1) | 2010 | 9.8 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| DM (%) | 2014 | 1.8 | 74.2 | 73.1 | 73.2 | 72.8 | 72.9 | 72.8 | 73.0 | 72.4 |
| VOC (%) | 2014 | 23.6 | 5.6 | 6.6 | 6.5 | 7.2 | 7.1 | 7.2 | 7.4 | 6.4 |
| pH (CaCl2) | 2014 | 1.9 | 4.9 | 5.1 | 5.2 | 5.1 | 5.2 | 5.4 | 5.3 | 5.3 |
| EC (µS cm−1) | 2014 | 20.6 | 33.0 | 39.1 | 42.5 | 60.0 | 78.7 | 76.8 | 73.5 | 70.2 |
| WHC (%) | 2014 | 3.6 | 56.4 | 56.4 | 56.3 | 56.9 | 57.0 | 56.9 | 55.7 | 55.9 |
| NH4 (µg N gDM−1) | 2014 | 32.3 | 2.8 | 2.1 | 2.6 | 2.6 | 2.9 | 1.7 | 3.7 | 5.3 |
| N-mineralization (µg N g−1 DM d−1) | 2014 | 14.8 | 10.2 | 10.5 | 14.6 | 14.6 | 13.3 | 14.3 | 15.5 | 12.5 |
| N-NO3 (µg N g−1 DM) | 2014 | 25.4 | 14.4 | 13.7 | 15.9 | 21.7 | 30.5 | 28.1 | 27.1 | 39.9 |
| Nit. (ng N g−1 DM 5 h−1) | 2014 | 56.1 | 187 | 120 | 133 | 118 | 159 | 247 | 177 | 205 |
| TC (%) | 2014 | 28.5 | 2.3 | 2.4 | 2.3 | 2.5 | 2.5 | 2.3 | 2.5 | 2.8 |
| TN (%) | 2014 | 13.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 |
| P (mg kg−1) | 2014 | 8.2 | 32.5 | 31.0 | 31.3 | 37.8 | 32.3 | 32.8 | 35.5 | 35.8 |
| K (mg kg−1) | 2014 | 19.1 | 23.5 | 24.8 | 28.8 | 34.5 | 30.3 | 32.8 | 36.0 | 34.5 |
| Clay (%) | 2014 | 7.9 | 23.3 | 24.8 | 28.8 | 16.5 | 16.0 | 16.0 | 16.0 | 16.0 |
| Fe (mg kg−1) | 2014 | 6.3 | 290 | 259 | 273 | 307 | 284 | 274 | 269 | 269 |
| Mn (mg kg−1) | 2014 | 10.2 | 290 | 258 | 273 | 227 | 223 | 214 | 201 | 200 |
| B (mg kg−1) | 2014 | 16.0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| As (mg kg−1) | 2014 | 2.7 | 20.7 | 21.0 | 20.5 | 20.5 | 20.9 | 20.4 | 21.0 | 20.6 |
| Pb (mg kg−1) | 2014 | 11.5 | 24.3 | 22.7 | 25.0 | 24.7 | 21.7 | 23.8 | 23.4 | 23.1 |
| Cd (mg kg−1) | 2014 | 4.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Co (mg kg−1) | 2014 | 3.7 | 16.2 | 16.7 | 16.5 | 16.0 | 16.6 | 16.4 | 16.2 | 16.1 |
| Cr (mg kg−1) | 2014 | 5.0 | 59.2 | 60.8 | 59.7 | 57.8 | 60.5 | 59.6 | 57.9 | 58.1 |
| Cu (mg kg−1) | 2014 | 9.7 | 37.7 | 39.9 | 38.8 | 37.9 | 39.9 | 39.5 | 38.0 | 38.7 |
| Ni (mg kg−1) | 2014 | 5.5 | 47.7 | 50.2 | 49.6 | 47.6 | 50.4 | 49.6 | 47.8 | 47.6 |
| Mo (mg kg−1) | 2014 | 6.4 | 1.1 | 1.0 | 1.0 | 1.1 | 1.0 | 0.9 | 1.0 | 1.0 |
| V (mg kg−1) | 2014 | 2.7 | 57.7 | 57.2 | 57.3 | 57.3 | 57.9 | 57.2 | 56.1 | 56.6 |
| Hg (mg kg−1) | 2014 | 9.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| DM (%) | 2018 | 1.0 | 75.7 | 75.8 | 74.9 | 75.9 | 75.8 | 75.9 | 75.9 | 75.3 |
| VOC (%) | 2018 | 7.2 | 7.8 | 7.7 | 8.0 | 8.3 | 8.5 | 8.6 | 8.6 | 8.2 |
| pH (CaCl2) | 2018 | 1.0 | 5.0 | 5.3 | 5.4 | 5.2 | 5.4 | 5.5 | 5.4 | 5.6 |
| EC (µS cm−1) | 2018 | 8.9 | 15.5 | 22.3 | 24.0 | 23.5 | 30.8 | 27.0 | 26.8 | 30.0 |
| WHC (%) | 2018 | 4.4 | 35.6 | 34.6 | 34.5 | 31.5 | 35.3 | 36.1 | 35.6 | 34.8 |
| NH4 (µg N gDM−1) | 2018 | 13.4 | 9.6 | 10.6 | 10.8 | 10.9 | 9.5 | 7.8 | 8.5 | 8.7 |
| N-mineralization (µg N g DM−1 d−1) | 2018 | 11.2 | 16.3 | 23.1 | 20.0 | 25.6 | 26.8 | 25.8 | 26.1 | 26.5 |
| N-NO3 (µg N g−1 DM) | 2018 | 29.0 | 2.2 | 3.0 | 3.2 | 2.8 | 4.5 | 6.4 | 5.6 | 6.0 |
| Nit. (ng N g−1 DM 5 h−1) | 2018 | 35.3 | 155 | 429 | 306 | 336 | 872 | 954 | 786 | 1059 |
| TC (%) | 2018 | 10.9 | 3.4 | 3.0 | 2.7 | 3.2 | 3.6 | 3.0 | 3.4 | 3.4 |
| TN (%) | 2018 | 14.7 | 0.3 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 |
| P (mg kg−1) | 2018 | 10.8 | 40.5 | 37.5 | 35.5 | 43.0 | 39.0 | 42.0 | 44.3 | 49.0 |
| K (mg kg−1) | 2018 | 9.8 | 48.3 | 51.0 | 50.0 | 67.8 | 75.5 | 81.0 | 79.5 | 81.0 |
| Clay (%) | 2018 | 5.8 | 14.0 | 12.5 | 12.5 | 12.5 | 12.0 | 11.5 | 12.0 | 12.0 |
| Fe (mg kg−1) | 2018 | 8.3 | 314 | 282 | 282 | 338 | 315 | 298 | 291 | 300 |
| Mn (mg kg−1) | 2018 | 10.7 | 217 | 207 | 210 | 246 | 240 | 230 | 220 | 219 |
| B (mg kg−1) | 2018 | 14.8 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 |
| As (mg kg−1) | 2018 | 2.3 | 23.4 | 23.4 | 22.7 | 23.0 | 23.2 | 23.1 | 23.0 | 23.0 |
| Pb (mg kg−1) | 2018 | 8.3 | 28.5 | 26.2 | 25.4 | 27.7 | 25.0 | 24.6 | 26.3 | 27.6 |
| Cd (mg kg−1) | 2018 | 5.0 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Co (mg kg−1) | 2018 | 3.8 | 19.0 | 19.3 | 19.2 | 18.9 | 19.3 | 19.3 | 18.6 | 18.9 |
| Cr (mg kg−1) | 2018 | 5.8 | 65.3 | 65.7 | 65.8 | 62.9 | 64.8 | 63.8 | 61.7 | 63.0 |
| Cu (mg kg−1) | 2018 | 8.4 | 45.0 | 46.5 | 46.1 | 44.6 | 51.1 | 46.1 | 44.2 | 45.8 |
| Ni (mg kg−1) | 2018 | 6.6 | 49.4 | 51.5 | 51.2 | 48.3 | 50.9 | 50.6 | 48.2 | 48.3 |
| Mo (mg kg−1) | 2018 | 11.5 | 1.3 | 1.2 | 1.2 | 1.3 | 1.3 | 1.3 | 1.5 | 1.3 |
| Zn (mg kg−1) | 2018 | 1.6 | 126 | 121 | 122 | 125 | 126 | 123 | 122 | 125 |
| V (mg kg−1) | 2018 | 1.7 | 69.4 | 68.5 | 68.2 | 68.5 | 68.0 | 66.2 | 65.1 | 67.3 |
| Hg (mg kg−1) | 2018 | 17.0 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzemann, F.R.; Juárez, M.F.-D.; Probst, M.; Gómez-Brandón, M.; Spiegel, H.; Resch, R.; Insam, H.; Pötsch, E.M. Biomass Ash as a Substitute for Lime and Its Impact on Grassland Soil, Forage, and Soil Microbiota. Agronomy 2024, 14, 1568. https://doi.org/10.3390/agronomy14071568
Kurzemann FR, Juárez MF-D, Probst M, Gómez-Brandón M, Spiegel H, Resch R, Insam H, Pötsch EM. Biomass Ash as a Substitute for Lime and Its Impact on Grassland Soil, Forage, and Soil Microbiota. Agronomy. 2024; 14(7):1568. https://doi.org/10.3390/agronomy14071568
Chicago/Turabian StyleKurzemann, Felix R., Marina Fernández-Delgado Juárez, Maraike Probst, María Gómez-Brandón, Heide Spiegel, Reinhard Resch, Heribert Insam, and Erich M. Pötsch. 2024. "Biomass Ash as a Substitute for Lime and Its Impact on Grassland Soil, Forage, and Soil Microbiota" Agronomy 14, no. 7: 1568. https://doi.org/10.3390/agronomy14071568
APA StyleKurzemann, F. R., Juárez, M. F.-D., Probst, M., Gómez-Brandón, M., Spiegel, H., Resch, R., Insam, H., & Pötsch, E. M. (2024). Biomass Ash as a Substitute for Lime and Its Impact on Grassland Soil, Forage, and Soil Microbiota. Agronomy, 14(7), 1568. https://doi.org/10.3390/agronomy14071568









