Effects of Drought at Anthesis on Flag Leaf Physiology and Gene Expression in Diverse Wheat (Triticum aestivum L.) Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Layout
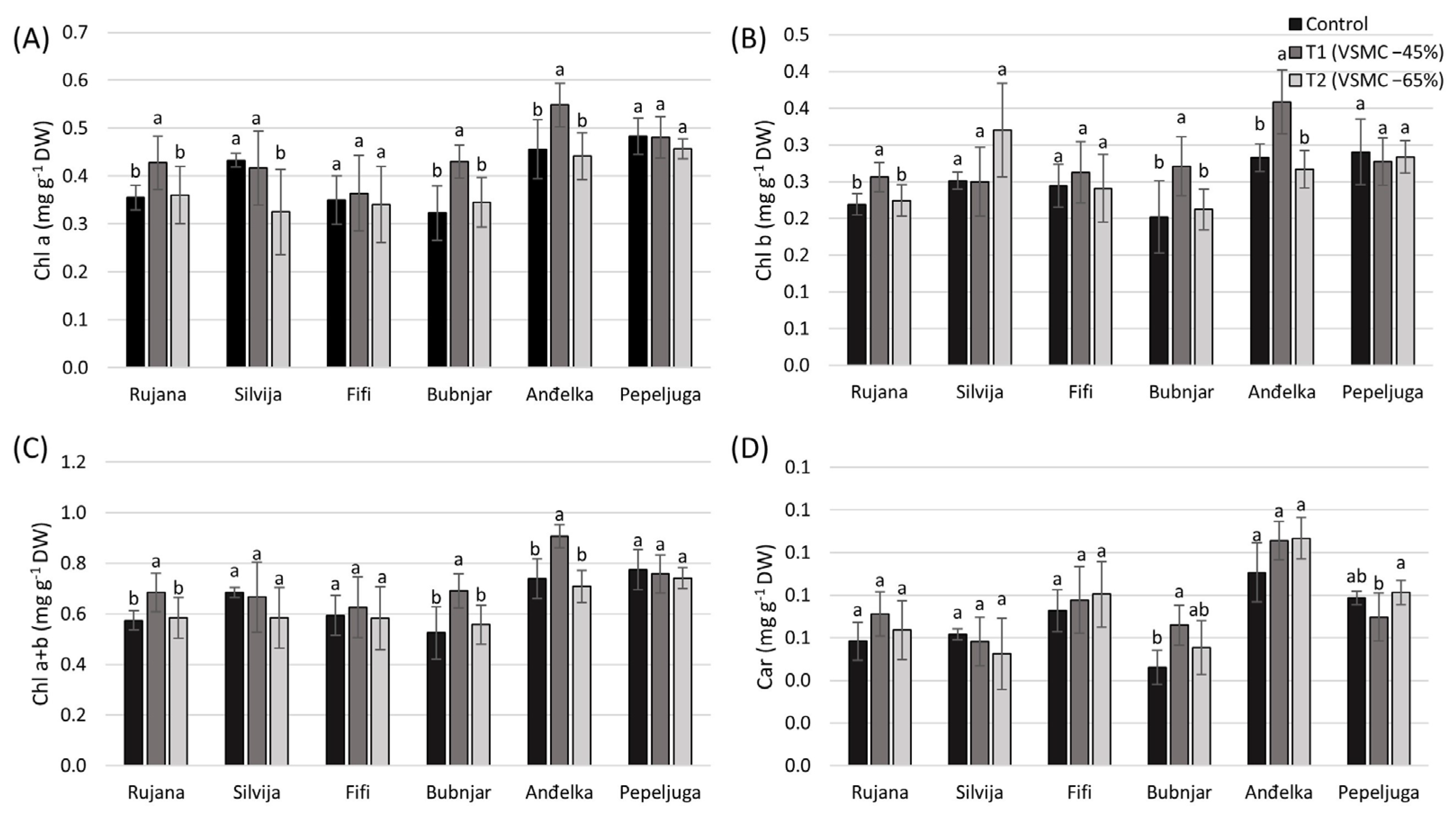
2.2. Determination of the Photosynthetic Pigments
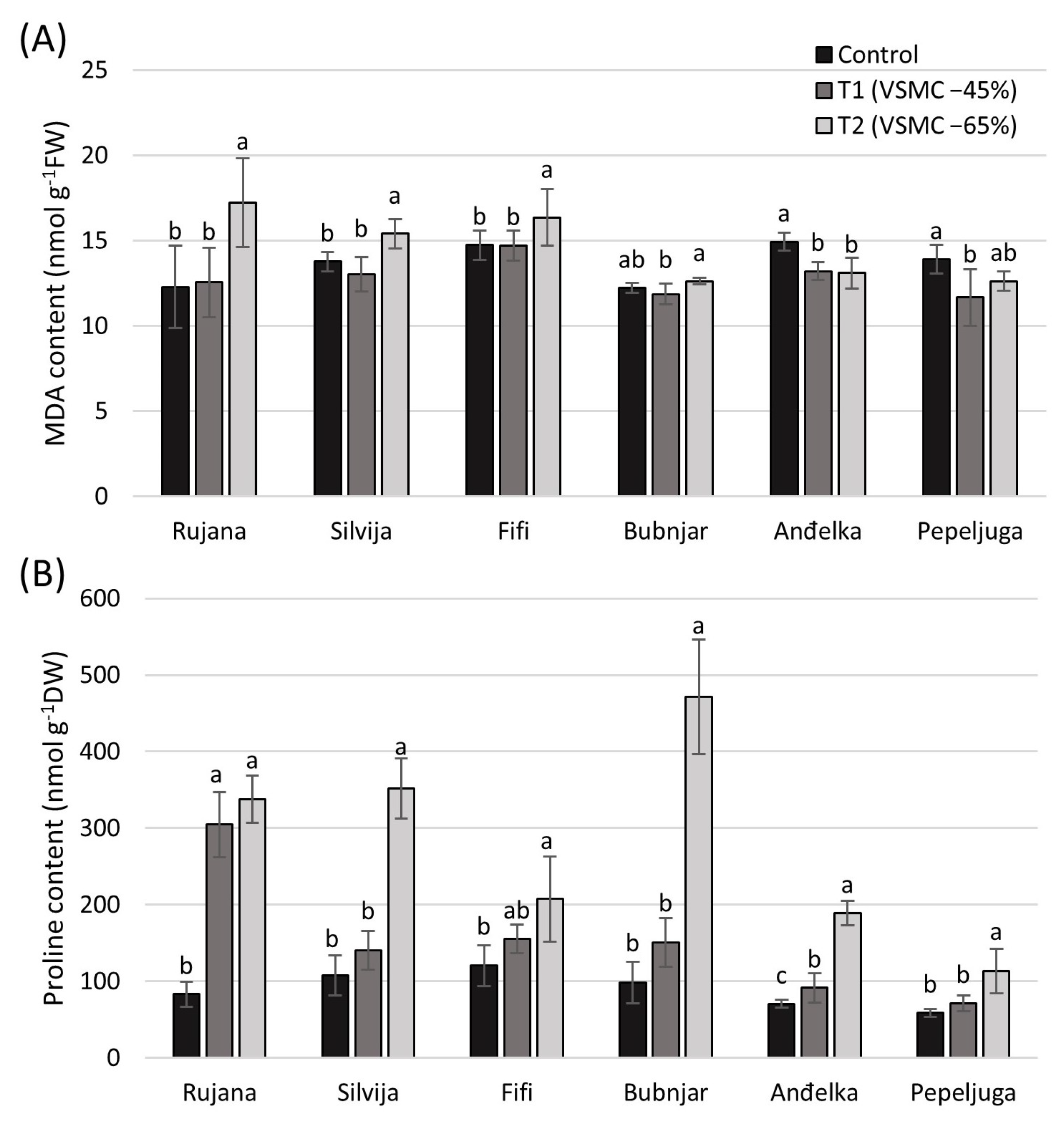
2.3. Determination of Malondialdehyde (MDA)
2.4. Determination of the Proline Content
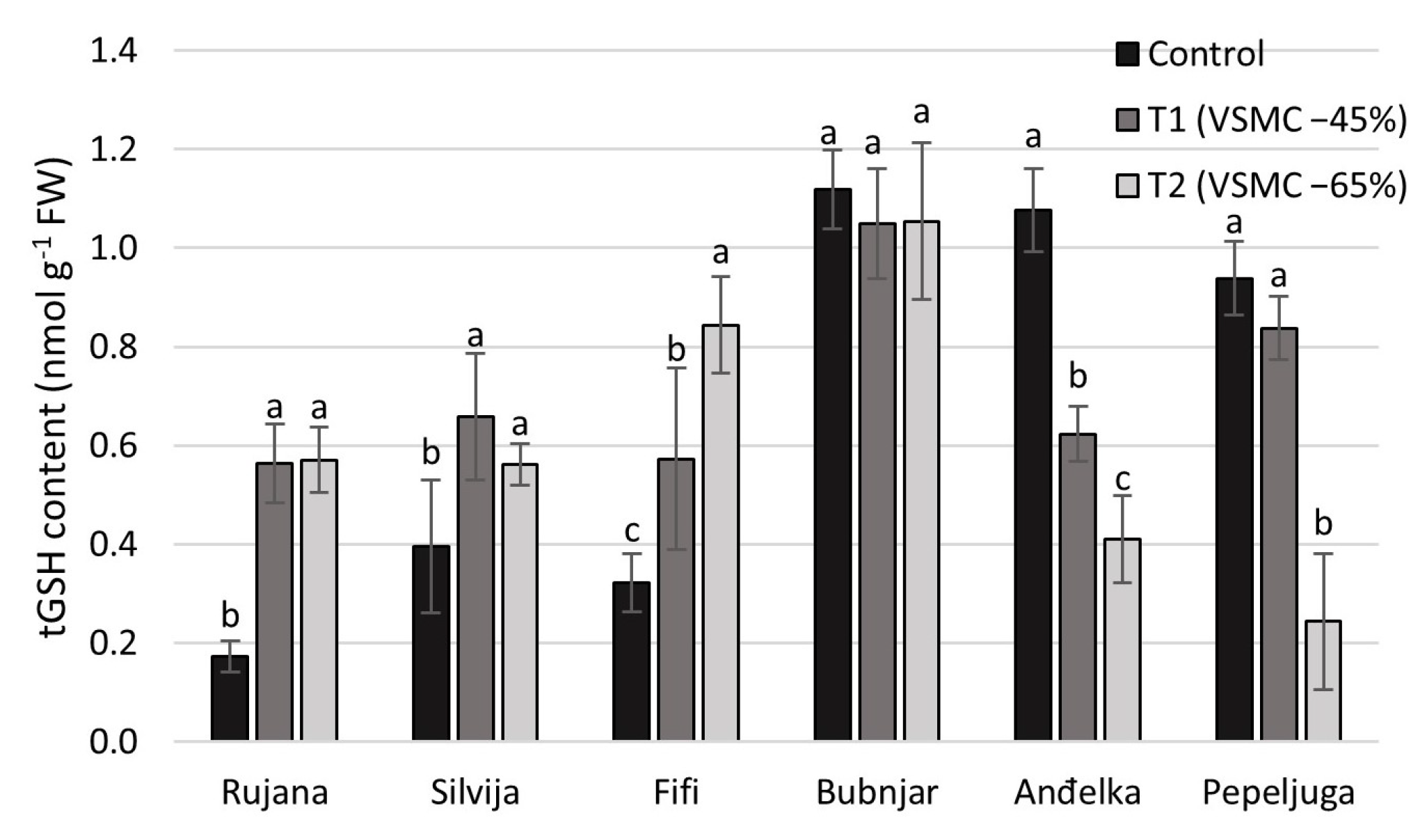
2.5. Determination of the Glutathione Content
2.6. Antioxidant Enzyme Activity Determination
2.7. Molecular Analysis
2.7.1. RNA Extraction and cDNA Synthesis
2.7.2. QPCR Analysis
2.8. Grain Morphology
2.9. Data Analysis
3. Results
3.1. Content of Chlorophylls and Carotenoids
3.2. Content of Malondialdehyde (MDA) and Proline
3.3. Content of Total Glutathione (tGSH)
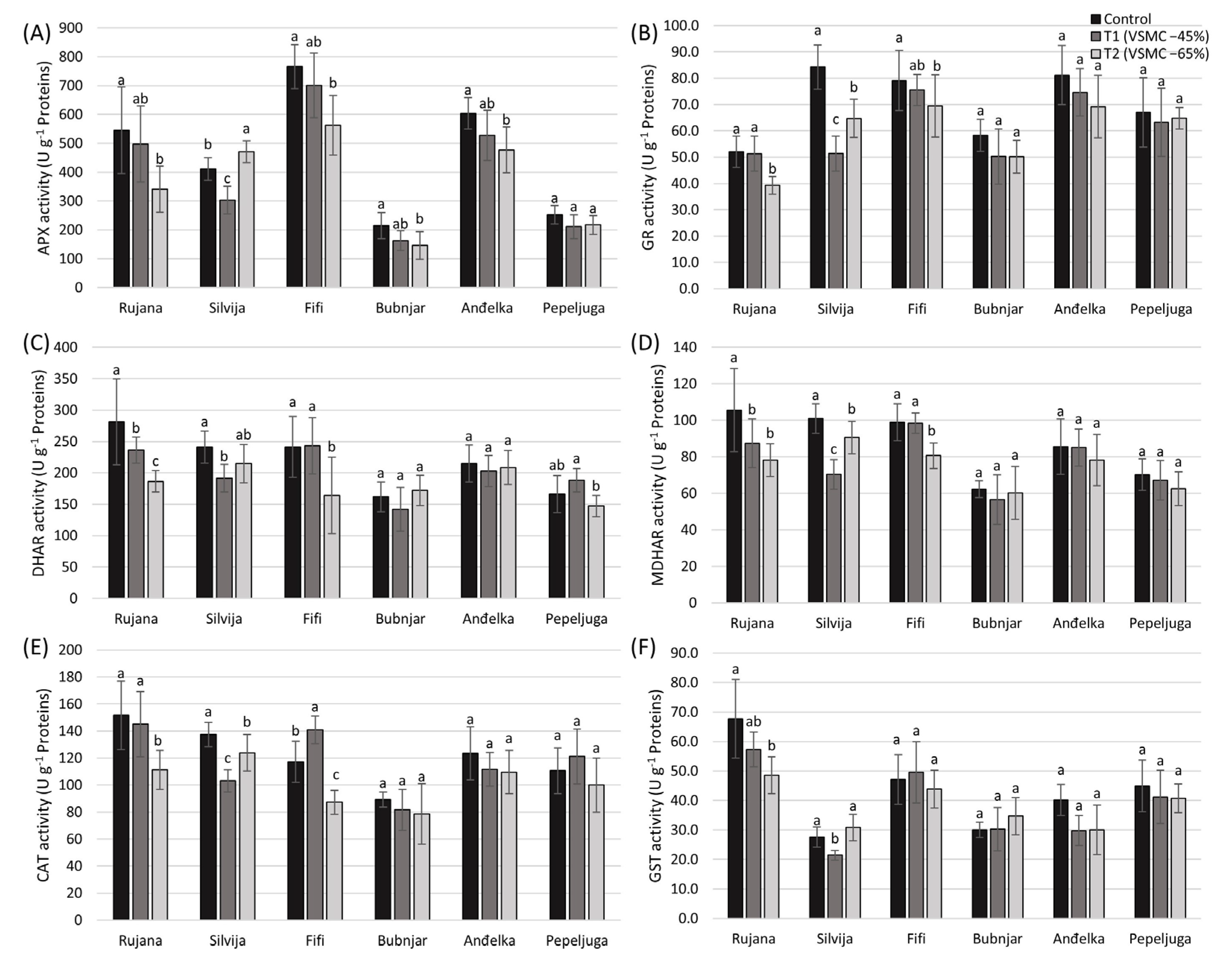
3.4. Enzymatic Activity
3.4.1. Activity of Ascorbate Peroxidase (APX)
3.4.2. Activity of Glutathione Reductase (GR)
3.4.3. Activity of Dehydroascorbate Reductase (DHAR) and Monodehydroascorbate Reductase (MDHAR)
3.4.4. Activity of Catalase (CAT)
3.4.5. Activity of Glutathione S-Transferase (GST)
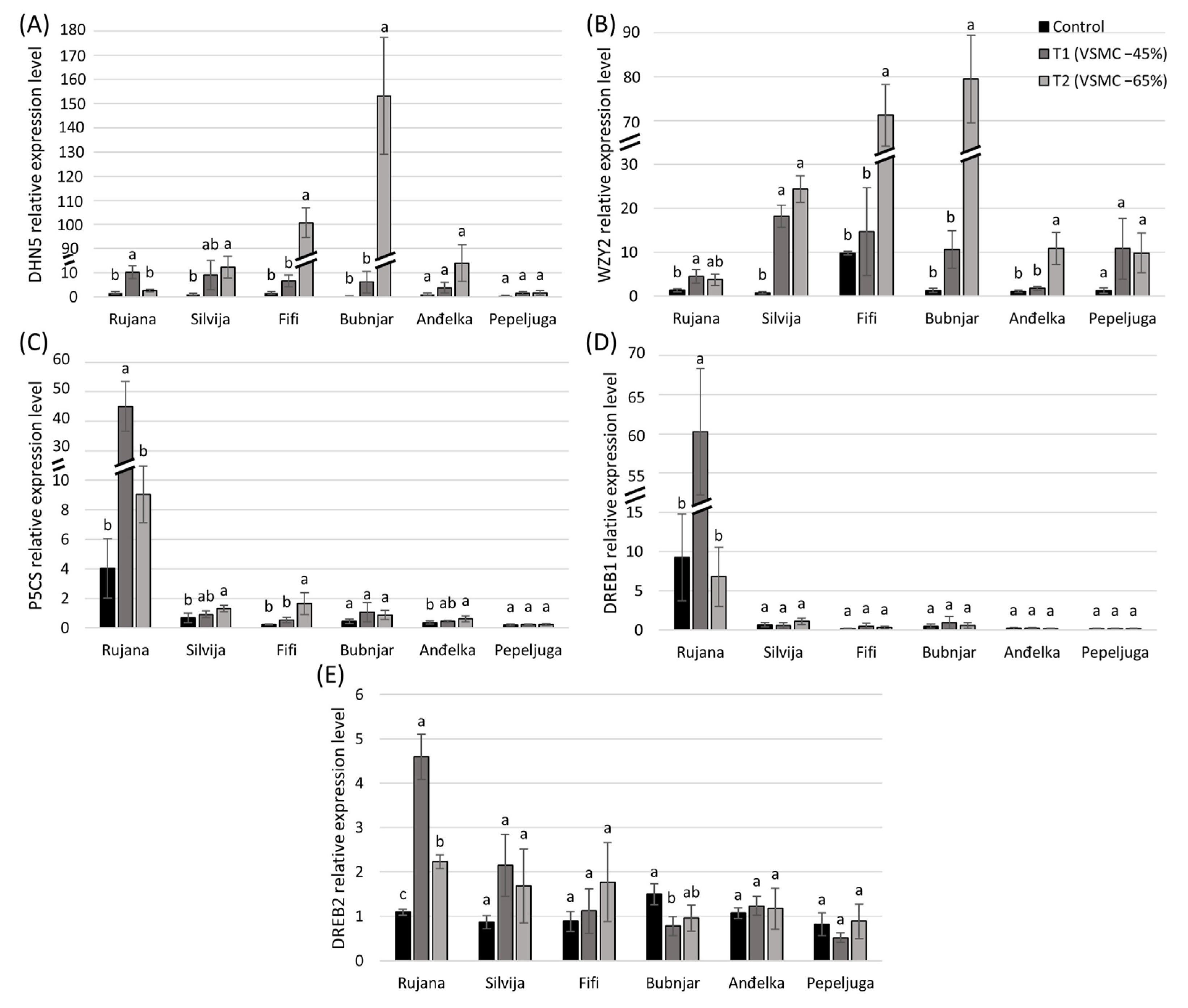
3.5. Relative Expression of Genes
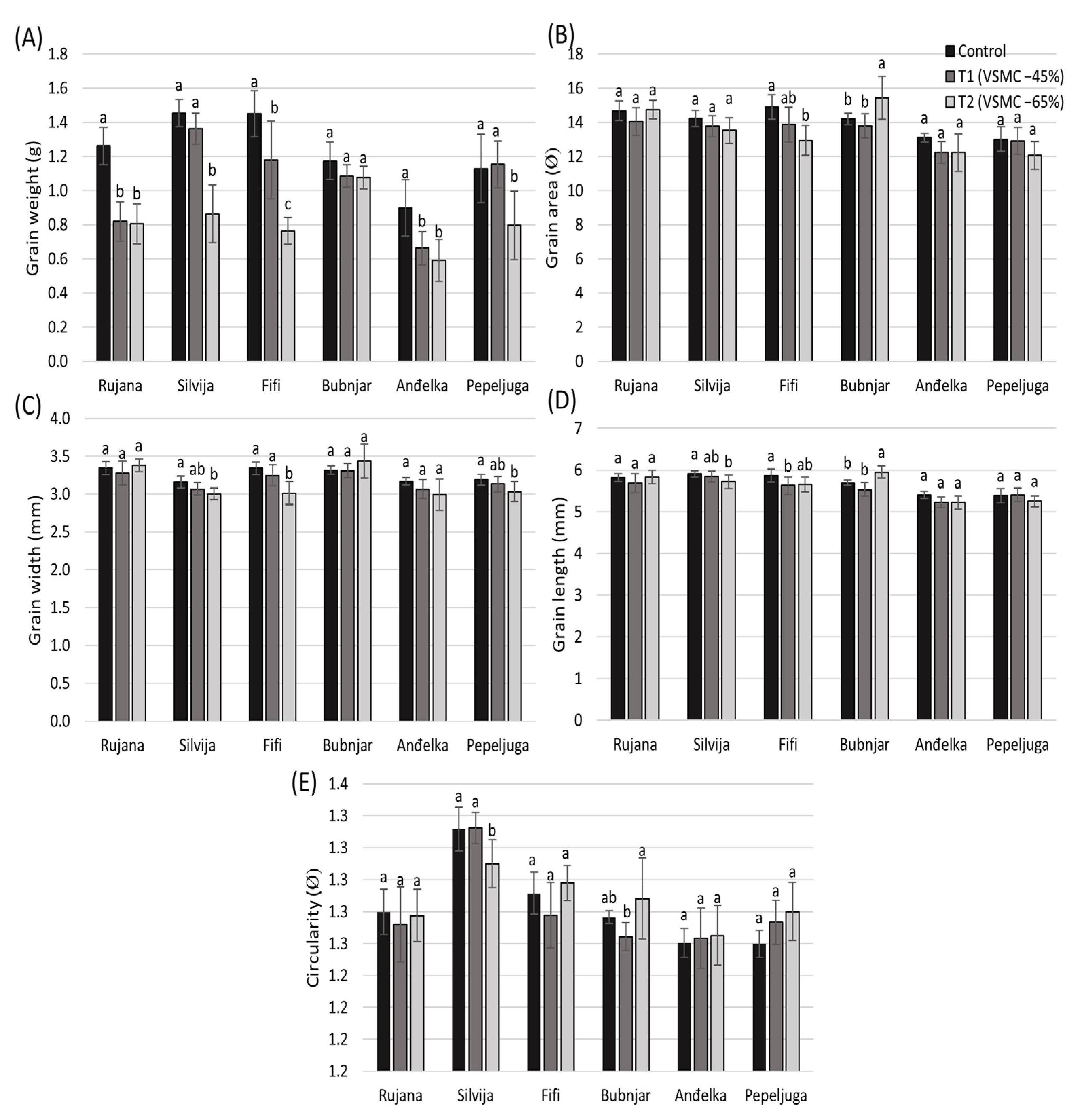
3.6. Grain Morphology
3.7. Correlation Analysis
4. Discussion
4.1. Pigments Involved in Photosynthesis during Drought
4.2. Content of Malondialdehyde (MDA) and Proline
4.3. ROS-Scavenging System
4.4. The Expression of Genes (P5CS, DHN5, WZY2, DREB1, and DREB2) under Drought
4.5. Postharvest Traits of Grain Morphology Influenced by Drought
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.; Momčilović, I.; Ristic, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Yusa, A.; Berry, P.J.; Cheng, J.; Ogden, N.; Bonsal, B.; Stewart, R.; Waldick, R. Climate change, drought and human health in Canada. Int. J. Environ. Res. Public Health 2015, 12, 8359–8412. [Google Scholar] [CrossRef] [PubMed]
- Begna, T. Effects of drought stress on crop production and productivity. Int. J. Res. Stud. Agric. Sci. 2020, 6, 34–43. [Google Scholar] [CrossRef]
- Wan, C.; Dang, P.; Gao, L.; Wang, J.; Tao, J.; Qin, X.; Feng, B.; Gao, J. How does the environment affect wheat yield and protein content response to drought? A meta-analysis. Front. Plant Sci. 2022, 13, 896985. [Google Scholar] [CrossRef] [PubMed]
- FAO. Crop Prospects and Food Situation. Available online: https://openknowledge.fao.org/handle/20.500.14283/cb7877en (accessed on 23 March 2024).
- Duvnjak, J.; Lončarić, A.; Brkljačić, L.; Šamec, D.; Šarčević, H.; Salopek-Sondi, B.; Španić, V. Morpho-physiological and hormonal response of winter wheat varieties to drought stress at stem elongation and anthesis stages. Plants 2023, 12, 3. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Zeng, Y.; Shah, A.N.; Yar, M.M.; Ullah, A.; Ali, M. Conferring of drought tolerance in wheat (Triticum aestivum L.) Genotypes using seedling indices. Front. Plant Sci. 2022, 13, 961049. [Google Scholar] [CrossRef]
- Ma, J.; Li, R.; Wang, H.; Li, D.; Wang, X.; Zhang, Y.; Zhen, W.; Duan, H.; Yan, G.; Li, Y. Transcriptomics analyses reveal wheat responses to drought stress during reproductive stages under field conditions. Front. Plant Sci. 2017, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic improvement of wheat for drought tolerance: Progress, challenges and opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Al Ayoubi, S.; Sher, A.; Bibi, Y.; Ahmad, S.; Shen, Z.; Jenks, M. Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 2021, 28, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Liu, S.; Rudd, J.C.; Xue, Q.; Payton, P.; Finlayson, S.A.; Mahan, J.; Akhunova, A.; Holalu, S.V.; Lu, N. Physiology and transcriptomics of water-deficit stress responses in wheat cultivars TAM 111 and TAM 112. J. Plant Physiol. 2014, 171, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Mazat, J.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish, S.R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Roitsch, T.; Liu, F. Activities of leaf and spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC Plant Biol. 2020, 20, 400. [Google Scholar] [CrossRef]
- Vuković, R.; Štolfa Čamagajevac, I.; Vuković, A.; Šunić, K.; Begović, L.; Mlinarić, S.; Sekulić, R.; Sabo, N.; Španić, V. Physiological, biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants 2022, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Durmaz, E.; Akpınar, B.A.; Budak, H. The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol. Biochem. 2011, 49, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF Family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Gene Regul. 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.G.; Zhang, W.K.; He, S.J.; Zhang, J.S.; Liu, Q.; Chen, S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Zhang, L. Identification of upstream transcription factors and an interacting PP2C protein of dehydrin WZY2 gene in wheat. Plant Signal Behav. 2019, 14, 1678370. [Google Scholar] [CrossRef]
- Zotova, L.; Kurishbayev, A.; Jatayev, S.; Khassanova, G.; Zhubatkanov, A.; Serikbay, D.; Sereda, S.; Sereda, T.; Shvidchenko, V.; Lopato, S. Genes encoding transcription factors TaDREB5 and TaNFYC-A7 are differentially expressed in leaves of bread wheat in response to drought, dehydration and ABA. Front. Plant Sci. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Abedini, R.; Ghanegolmohammadi, F.; Pishkamrad, R.; Pourabed, E.; Jafarnezhad, A.; Shobbar, Z.S.; Shahbazi, M. Plant dehydrins: Shedding light on structure and expression patterns of dehydrin gene family in barley. J. Plant Res. 2017, 130, 747–763. [Google Scholar] [CrossRef]
- Brini, F.; Yamamoto, A.; Jlaiel, L.; Takeda, S.; Hobo, T.; Dinh, H.Q.; Hattori, T.; Masmoudi, K.; Hanin, M. Pleiotropic effects of the wheat dehydrin DHN-5 on stress responses in Arabidopsis. Plant Cell Physiolog. 2011, 52, 676–688. [Google Scholar] [CrossRef]
- Saibi, W.; Feki, K.; Mahmoud, R.B.; Brini, F. Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 2015, 242, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 25 March 2024).
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiolog. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ma, F.W.; Cheng, L. Exposure of the shaded side of apple fruit to full sun leads to up-regulation of both the xanthophyll cycle and the ascorbate-glutathione cycle. Hortic. Sci. 2004, 39, 887A–887. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Racker, E. Glutathione reductase from bakers’ yeast and beef liver. J. Biol. Chem. 1955, 217, 855–865. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Arrieta, D.V.; Rodríguez, Y.Y.P.; Ramírez, A.M.J.; Bettin, A.M.V.; Avilez, M.A.Q.; Cárcamo, J.A.A.; Garcia-Castaño, S.G.; González, L.M.M.; Cordero, E.D.F.; et al. Can chlorophyll a fluorescence and photobleaching be a stress signal under abiotic stress in Vigna unguiculata L.? Sustainability 2022, 14, 15503. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, G.; Tang, Y.; Zhou, C.; Zhang, L.; Lv, J. The spike plays important roles in the drought tolerance as compared to the flag leaf through the phenylpropanoid pathway in Wheat. Plant Physiol. Biochem. 2020, 152, 100–111. [Google Scholar] [CrossRef]
- García-Valenzuela, X.; García-Moya, E.; Rascón-Cruz, Q.; Herrera-Estrella, L.; Aguado-Santacruz, G.A. Chlorophyll accumulation is enhanced by osmotic stress in graminaceous chlorophyllic cells. J. Plant Physiol. 2005, 162, 650–661. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, B. Drought stress-induced oxidative stress and antioxidative responses in four wheat (Triticum aestivum L.) varieties. Arch. Agron. Soil Sci. 2012, 58, 617–630. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant. 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, M.; Siddiqui, N.; Rohman, M.; Jagadish, S.V.K.; Ahmed, J.U.; Hassan, M.M.; Hossain, A.; Islam, T. Physiological and biochemical dissection reveals a trade-off between antioxidant capacity and heat tolerance in bread wheat (Triticum aestivum L.). Antioxidants 2021, 10, 351. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Seleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop. Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Sultan, M.A.R.F.; Hui, L.; Yang, L.J.; Xian, Z.H. Assessment of drought tolerance of some Triticum L. species through physiological indices. Czech J. Genet. Plant Breed. 2012, 48, 178–184. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Suravajhala, P.; Rathnagiri, P.; Sreenivasulu, N. Intriguing role of proline in redox potential conferring high temperature stress tolerance. Front. Plant Sci. 2022, 13, 867531. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Peršić, V.; Ament, A.; Antunović Dunić, J.; Drezner, G.; Cesar, V. PEG-induced physiological drought for screening winter wheat genotypes sensitivity–integrated biochemical and chlorophyll a fluorescence analysis. Front. Plant Sci. 2022, 13, 987702. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Li, Y.; Guo, R.; Liu, E.; Liu, X.; Gu, F.; Yang, Z.; Li, S.; Zhong, X.; et al. Wheat genotypes with higher yield sensitivity to drought overproduced proline and lost minor biomass under severer water stress. Front. Plant Sci. 2022, 13, 1035038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Keleş, Y.; Öncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kaur, N.; Gupta, A.N. Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.). Indian J. Biochem. Biophys. 2012, 49, 257–265. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant defences in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Funct. Plant Biol. 2005, 32, 45–53. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy-Lameta, A.; Ferrari-Iliou, R.; Contour-Ansel, D.; Pham-Thi, A.T.; Zuily-Fodil, Y. Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves. Ann. Bot. 2006, 97, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Herbinger, K.; Tausz, M.; Wonisch, A.; Soja, G.; Sorger, A.; Grill, D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol. Biochem. 2002, 40, 691–696. [Google Scholar] [CrossRef]
- Loggini, B.; Scartazza, A.; Brugnoli, E.; Navari-Izzo, F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought1. Plant Physiol. 1999, 119, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Khattab, H. Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline condition. Aust. J. Basic Appl. Sci. 2007, 1, 323–334. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the sntioxidant defence and methylglyoxal detoxification systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.V.C.; Reddy, A.R. Glutathione Reductase: A Putative Redox Regulatory System in Plant Cells; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–147. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Duan, R.; Fan, J.; Jiao, P.; Sun, H.; Guan, S.; Liu, S. Overexpression of maize Glutathione S-Transferase ZmGST26 decreases drought resistance of Arabidopsis. Agronomy 2022, 12, 2948. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, S.I.; Kim, J.J.; Shin, S.Y.; Kwak, S.S.; Lee, C.H.; Park, H.M.; Kim, Y.H.; Kim, I.S.; Yoon, H.S. Over-expression of dehydroascorbate reductase improves salt tolerance, environmental adaptability and productivity in Oryza Sativa. Antioxidants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Melandri, G.; AbdElgawad, H.; Riewe, D.; Hageman, J.A.; Asard, H.; Beemster, G.T.; Kadam, N.; Jagadish, K.; Altmann, T.; Ruyter-Spira, C.; et al. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020, 71, 669–683. [Google Scholar] [CrossRef]
- Chen, Z.; Gallie, D.R. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006, 142, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, R.; Qu, Y.; Miao, Z.; Zhang, Y.; Shen, X.; Wang, T.; Dong, J. Overexpression of MtCAS31 enhances drought tolerance in transgenic Arabidopsis by reducing stomatal density. New Phytol. 2012, 195, 124–135. [Google Scholar] [CrossRef]
- Liu, H.; Yu, C.; Li, H.; Ouyang, B.; Wang, T.; Zhang, J.; Wang, X.; Ye, Z. Overexpression of ShDHN, a dehydrin gene from solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci. 2015, 231, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Du, D.; An, Y.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Overexpression of prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, M.; Cui, Y.; Kong, L.; Wang, H. Genome-wide survey of the dehydrin genes in bread wheat (Triticum aestivum L.) and its relatives: Identification, evolution and expression profiling under various abiotic stresses. BMC Genom. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, L.; Wang, L.; Jiang, B.; Wen, J. Cloning and sequence analysis of a new dehydrin gene (wzy2) from wheat. J. Northwest A F Univ.-Nat. Sci. Ed. 2009, 37, 93–99. [Google Scholar]
- Zhu, W.; Zhang, L.; Zhang, N.; Xing, Y.; Jiang, B. The clone of wheat dehydrin-like gene Wzy2 and its functional analysis in Pichia pastoris. Afr. J. Biotechnol. 2012, 11, 9549–9558. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, E.; Gao, L.; Mao, X.; Zhou, R.; Jia, J. Isolation, expression analysis and chromosomal location of P5CR gene in common wheat (Triticum aestivum L.). S. Afr. J. Bot. 2008, 74, 705–712. [Google Scholar] [CrossRef]
- De Ronde, J.A.; Cress, W.A.; Krüger, G.H.J.; Strasser, R.J.; Staden, J.V. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J. Plant Physiol. 2004, 161, 1211–1224. [Google Scholar] [CrossRef]
- Nocek, B.; Chang, C.; Li, H.; Lezondra, L.; Holzle, D.; Collart, F.; Joachimiak, A. Crystal structures of Δ1-Pyrroline-5-Carboxylate reductase from human pathogens Neisseria meningitides and Streptococcus pyogenes. J. Mol. Biol. 2005, 354, 91–106. [Google Scholar] [CrossRef]
- Hien, D.; Jacobs, M.; Angenon, G.; Hermans, C.; Thanh, T.; Le Van Son, T.; Roosens, N. Proline accumulation and D 1-pyrroline-5-carboxylate synthetase gene properties in three rice cultivars differing in salinity and drought tolerance. Plant Sci. 2003, 165, 1059–1068. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Q.; Zhu, X.; Li, X.; Fan, X.; Hui, M.; Ye, Q.; Liu, G.; Liu, X. Transcription factor VvDREB2A from Vitis vinifera improves cold tolerance. Int. J. Mol. Sci. 2023, 24, 9381. [Google Scholar] [CrossRef]
- Arroyo Herrera, A.; Figueroa-Yáñez, L.; Castaño, E.; Santamaría, J.; Pereira-Santana, A.; Espadas, J.; Sánchez-Teyer, L.; Espadas-Gil, F.; Alcaraz, L.D.; López-Gómez, R. A novel Dreb2-type gene from carica papaya confers tolerance under abiotic stress. Plant Cell Tissue Organ. Cult. 2016, 125, 119–133. [Google Scholar] [CrossRef]
- Yousfi, S.; Márquez, A.J.; Betti, M.; Araus, J.L.; Serret, M.D. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J. Integr. Plant Biol. 2016, 58, 48–66. [Google Scholar] [CrossRef]
- Hwang, J.E.; Lim, C.J.; Chen, H.; Je, J.; Song, C.; Lim, C.O. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Mol. Cell 2012, 33, 135–140. [Google Scholar] [CrossRef]
- Kurahashi, Y.; Terashima, A.; Takumi, S. Variation in dehydration tolerance, ABA sensitivity and related gene expression patterns in D-genome progenitor and synthetic hexaploid wheat lines. Int. J. Mol. Sci. 2009, 10, 2733–2751. [Google Scholar] [CrossRef]
- Brinton, J.; Simmonds, J.; Minter, F.; Leverington-Waite, M.; Snape, J.; Uauy, C. Increased pericarp cell length underlies a major quantitative trait locus for grain weight in hexaploid wheat. New Phytol. 2017, 215, 3. [Google Scholar] [CrossRef]
- Evers, A.D.; Cox, R.I.; Shaheedullah, M.Z.; Withey, R.P. Predicting milling extraction rate by image analysis of wheat grains. Asp. Appl. Biol. 1990, 25, 417–426. [Google Scholar]
- Russo, M.; Ficco, D.; Laidò, G.; Marone, D.; Papa, R.; Blanco, A.; Gadaleta, A.; De Vita, P.; Mastrangelo, A. A dense durum wheat × T. dicoccum linkage map based on SNP markers for the study of seed morphology. Mol. Breed. 2014, 34, 1579–1597. [Google Scholar] [CrossRef]
- Wu, Q.H.; Chen, Y.X.; Zhou, S.H.; Fu, L.; Chen, J.J.; Xiao, Y.; Zhang, D.; Ouyang, S.H.; Cui, Y.; Zhang, D.Y.; et al. High-density genetic linkage map construction and QTL mapping of grain shape and size in the wheat population Yanda1817 × Beinong6. PLoS ONE 2015, 10, 0118144. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Baker, D.A. The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul. 2001, 35, 81–91. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Leverington-Waite, M.; Turner, A.S.; Brinton, J.; Korzun, V.; Snape, J.; Uauy, C. Identification and independent validation of a stable yield and thousand grain weight QTL on chromosome 6A of hexaploid wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Alghabari, F.; Lukac, M.; Jones, H.E.; Gooding, M.J. Effect of Rht alleles on the tolerance of wheat grain set to high temperature and drought stress during booting and anthesis. J. Agron. Crop. Sci. 2014, 200, 36–45. [Google Scholar] [CrossRef]
- Kino, R.I.; Pellny, T.K.; Mitchell, R.A.C.; Gonzalez-Uriarte, A.; Tosi, P. High post-anthesis temperature effects on bread wheat (Triticum aestivum L.) grain transcriptome during early grain-filling. BMC Plant Biol. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef]
- Dhakal, A. Effect of drought stress and management in wheat—A review. Food Agribus. Manag. 2021, 2, 62–66. [Google Scholar] [CrossRef]
| Wheat Cultivar | Origin | Registration Year | Drought Tolerance |
|---|---|---|---|
| Bubnjar | AIO, HR | 2016 | tolerant |
| Pepeljuga | AIO, HR | 2017 | medium tolerant |
| Anđelka | AIO, HR | 2008 | medium tolerant |
| Rujana | AIO, HR | 2017 | medium sensitive |
| Fifi | AIO, HR | 2016 | medium sensitive |
| Silvija | AIO, HR | 2010 | sensitive |
| Target Gene | GenBank Accession No. | Product Length (bp) | Forward Primer | Tm and %CG | Reverse Primer | Tm and %CG |
|---|---|---|---|---|---|---|
| P5CS | KT868850 | 85 | ccggtgaatggcagagtaat | 60 °C, 50% | ccccacggagaactttaaca | 60 °C, 50% |
| DHN5 | AY619566 | 99 | agaagaagggcatcatggac | 59.1 °C, 50% | ggcacctccactctcagaag | 60 °C, 60% |
| WZY2 | KF112871 | 142 | tcgttcgtcgtggtagtctg | 59.9 °C, 55% | atgaccttgctgtccgtagg | 60 °C, 55% |
| DREB1 | DQ195070 | 80 | gttggtacccaacccaagtg | 60.1 °C, 55% | aacagaacgaagcagggcta | 60 °C, 50% |
| DREB2 | AY781345.1 | 121 | ccacagctcgttcaaagtga | 60 °C, 50% | atgccattcaaaaaccaagc | 60 °C, 40% |
| actin | AK457930 | 215 | tgaccgtatgagcaaggag | 58 °C, 53% | ccagacaactcgcaacttag | 60 °C, 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duvnjak, J.; Sarcevic, H.; Vukovic, R.; Spanic, V. Effects of Drought at Anthesis on Flag Leaf Physiology and Gene Expression in Diverse Wheat (Triticum aestivum L.) Genotypes. Agronomy 2024, 14, 1522. https://doi.org/10.3390/agronomy14071522
Duvnjak J, Sarcevic H, Vukovic R, Spanic V. Effects of Drought at Anthesis on Flag Leaf Physiology and Gene Expression in Diverse Wheat (Triticum aestivum L.) Genotypes. Agronomy. 2024; 14(7):1522. https://doi.org/10.3390/agronomy14071522
Chicago/Turabian StyleDuvnjak, Jurica, Hrvoje Sarcevic, Rosemary Vukovic, and Valentina Spanic. 2024. "Effects of Drought at Anthesis on Flag Leaf Physiology and Gene Expression in Diverse Wheat (Triticum aestivum L.) Genotypes" Agronomy 14, no. 7: 1522. https://doi.org/10.3390/agronomy14071522
APA StyleDuvnjak, J., Sarcevic, H., Vukovic, R., & Spanic, V. (2024). Effects of Drought at Anthesis on Flag Leaf Physiology and Gene Expression in Diverse Wheat (Triticum aestivum L.) Genotypes. Agronomy, 14(7), 1522. https://doi.org/10.3390/agronomy14071522







