Abstract
An excess of selenium (Se) can cause toxicity to plants. Treatment with melatonin (MT) can alleviate the stress conditions in plants. Limited research exists on the impact of MT on Se stress and uptake in fruit trees. To address Se stress and enhance Se accumulation in Cyphomandra betacea Sendt. (Solanum betaceum Cav.), the effects of exogenous MT (50–200 µmol L−1) on C. betacea growth and Se accumulation under Se stress were studied. MT increased the biomass, photosynthetic pigments levels, and peroxidase activity of C. betacea under Se stress. Only at concentrations of 150 and 200 µmol L−1 did MT increase superoxide dismutase and catalase activities, as well as soluble protein content in C. betacea leaves. MT treatment also boosted the Se content and bioconcentration factor of C. betacea under Se stress while reducing the translocation factor. The dose of MT was directly related to the Se content, and the highest levels of Se in roots and shoots were observed at 150 µmol L−1, which was an increase of 114% and 60%, respectively, compared with the control. In addition, correlation, principal component, cluster, grey, and path analyses revealed a strong correlation between root Se content and shoot biomass with the shoot Se content. Therefore, MT treatment can mitigate Se stress and enhance Se uptake in C. betacea, and the most effective dose of MT is 150 µmol L−1.
1. Introduction
Selenium (Se) is an important component of selenoamino acids and selenoproteins, which are involved in many aspects of plant metabolism; in addition, it is an elemental nutrient required by humans to prevent diseases [1]. Se is found in plants mainly in the form of organic Se, and the organic Se is often used to supplement foods [2,3]. Owing to the similar chemical properties with sulfur (S), Se is accumulated by plants through the metabolism and pathways of S [4,5]; however, plants differ in how they take up S [6]. An excess of Se can cause toxicity by causing oxidative stress and the denaturation of proteins, which can cause toxicity, including stunted growth, leaf yellowing, impaired nutrient uptake, and even death [6,7,8]. To increase Se uptake in plants to convert to organic Se for human foods, approaches, such as grafting and the application of exogenous phytohormones, have been utilized [9,10].
Melatonin (MT) is widely present in nature, and its most widely recognized role is its participation in and regulation of the responses of plants to stress [11]. As an endogenous scavenger of free radicals [12], treatment with MT can improve the antioxidant capacity of plants and scavenge free radicals [13,14]. Under salt stress, treatment with MT increases the contents of betaine and soluble sugars to enhance tolerance [15], and it also improves the efficiency of photosynthesis and ionic homeostasis [16]. Under heavy metal stress, treatment with MT promotes growth and increases the contents of photosynthetic pigments [17]. Treatment with MT increased the uptake of nutrients by apples under drought stress by facilitating the absorption of ions [18], and it also increased the uptake of nutrients in pistachio nuts under salt stress [19]. Treatment with MT can reduce the nickel uptakes in plants under nickel stress [20]. For Se uptake, treatment with MT improved the contents of Se in grapes and teas [21] and promoted the uptake of Se in the medicinal plant Plantago asiatica L. [22]. Thus, treatment with MT may promote Se uptake in plants. However, the action mechanism of MT on Se uptake in fruit trees has not been reported.
Cyphomandra betacea Sendt. (Solanum betaceum Cav.) has ornamental and edible values, and its fruits are rich in vitamin C, minerals, and pectin [23]. However, C. betacea is less effective at accumulating Se [24]. Thus, increasing the content of Se in C. betacea can increase its commercial value. If exogenous MT is applied to C. betacea, its Se capacity could be improved. Therefore, the effects of exogenous MT on the Se uptake of C. betacea under Se stress (0.1 mg L−1) [25] were investigated, and the aim of this study was to determine the best dose of MT to promote C. betacea growth and its Se uptake. The results of this study may provide a reference to produce C. betacea that is enriched in Se.
2. Materials and Methods
2.1. Materials
In September 2023, the seeds were collected from a fruiting tree of C. betacea planted at the Chengdu Campus of Sichuan Agricultural University (30°42′ N, 103°51′ E). They were then planted in a tray filled with perlite and placed in a greenhouse with specific conditions. During the day, the greenhouse had 14 h of light at 10,000 Lux, a temperature of 25 °C, and a relative humidity of 70%, while at night, it had 10 h of darkness at 0 Lux, a temperature of 20 °C, and a relative humidity of 90% [26]. Hoagland solution was used to irrigate each tray every 3 days until the seedlings of C. betacea reached approximately 10 cm in height.
The MT was obtained from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China).
2.2. Experimental Design
C. betacea uniform seedlings were planted in nine-hole trays filled with perlite. Six seedlings were planted in each tray. The trays were placed in the greenhouse as described in Section 2.1. Hoagland nutrient solution containing 0.1 mg L−1 Se (in the form of sodium selenite (Na2SeO3)) was irrigated every 3 d [25]. Each tray was irrigated with 100 mL. Various doses of MT (0, 50, 100, 150, and 200 µmol L−1) [27] were sprayed on both sides of C. betacea leaves, and each tray was sprayed with 25 mL of MT. Each treatment was repeated three times (18 plants in total) with one tray as a replicate. The seedlings of C. betacea were sprayed with the same dose of MT again after 15 d. Foil was used to limit the spray contact of the growth medium during each spraying application.
2.3. Determination of the Parameters
After one month from the initial treatment with MT, the third mature leaf from the top was collected to measure the levels of chlorophyll a, chlorophyll b, and carotenoids, activities of POD, SOD, and CAT, and soluble protein content. The photosynthetic pigments were analyzed using the acetone–ethanol mixed extraction method [28]. The activities of POD, SOD, and CAT were assayed using the methods used in previous studies [28,29]. The content of soluble protein was determined by staining with Coomassie bright blue [28]. Then, the plants were harvested and washed, and the roots were immersed in 20 mol L−1 EDTA-Na2 solution for 15 min to exchange the selenite of the root surface. The samples were dried to a constant weight. The dry weights (biomass) of the roots and shoots were recorded. The samples were digested and reduced to determine the Se contents using a hydride generation-atomic fluorescence spectrometry (AFS-9700; Beijing Haiguang Instrument Co., Ltd., Beijing, China) [30]. The bioconcentration factor (BCF) of Se was calculated as the Se content in plants/Se concentration in media (Hoagland nutrient solution), and the translocation factor (TF) of Se was calculated as the shoot Se content/root Se content [31].
2.4. Statistical Analysis
The data of all treatments with three biological replicates were analyzed using SPSS 26.0 (IBM, Armonk, NY, USA). All the data were analyzed with a one-way analysis of variance (ANOVA) and Duncan’s Multiple Range Test. Regression analysis, Pearson’s correlation analysis, principal component analysis (PCA), cluster analysis, and grey relational analysis were conducted [32,33].
3. Results
3.1. Biomass
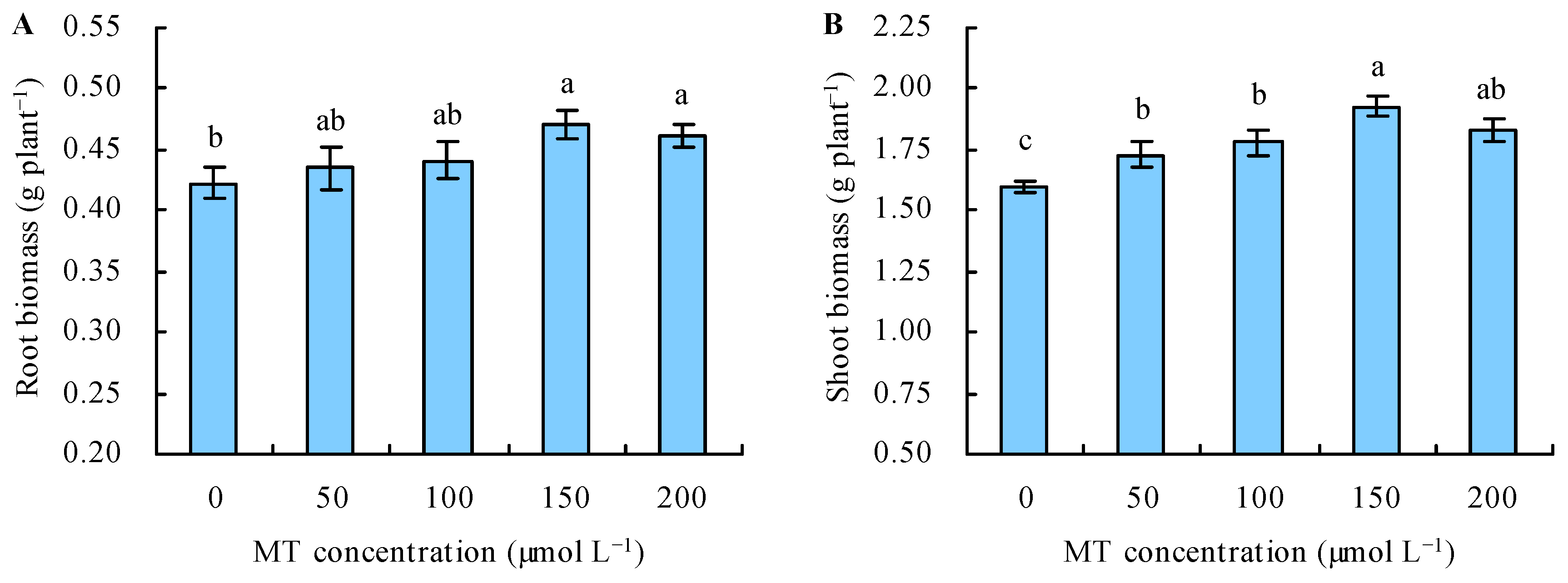
Under Se stress, with the increase in the dose of MT, the biomass increased when this dose ≤ 150 µmol L−1, and it decreased when the dose of MT > 150 µmol L−1 (Figure 1A,B). The application of MT at 50 and 100 µmol L−1 did not affect the root biomass, while treatments at 150 and 200 µmol L−1 increased it by 12% and 9%, respectively, compared with the control. All of the MT treatments increased the shoot biomass. Treatments with 50, 100, 150, and 200 µmol L−1 of MT increased the shoot biomass by 8%, 12%, 21%, and 15%, respectively, compared with the control.
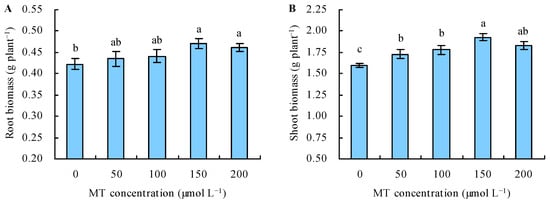
Figure 1.
Root (A) and shoot (B) biomass were treated with the different concentrations of melatonin (MT) under Se stress. Different lowercase letters indicate that the significant difference is at the 0.05 level.
3.2. Contents of the Photosynthetic Pigments
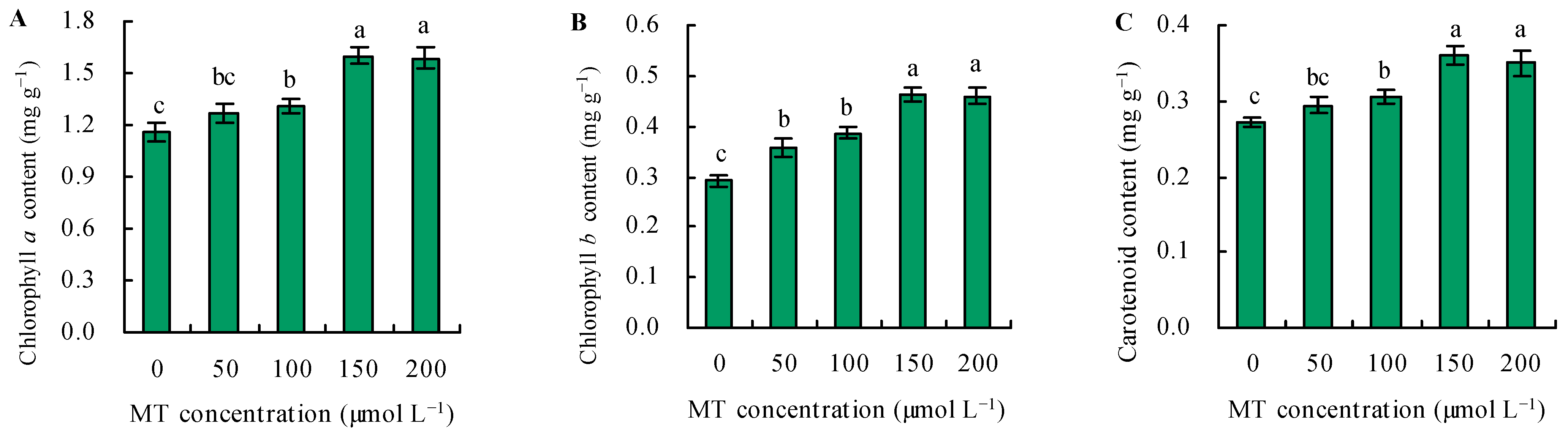
The contents of photosynthetic pigments increased in parallel with the dose of MT under Se stress (Figure 2A–C). Treatment with MT at 50 µmol L−1 did not affect the contents of chlorophyll a and carotenoids but increased the content of chlorophyll b. Treatments with 100, 150, and 200 µmol L−1 of MT increased the contents of photosynthetic pigments, and these parameters reached their maximum following treatment with 150 and 200 µmol L−1 of MT. Treatment with 150 µmol L−1 of MT increased the contents of photosynthetic pigments by 37%, 59%, and 32%, respectively, and increased these parameters by 36%, 57%, and 28%, respectively, at 200 µmol L−1, compared with the control.
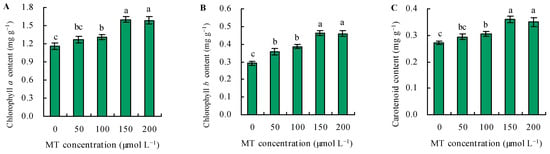
Figure 2.
The contents of chlorophyll a (A), chlorophyll b (B), and carotenoid (C) were treated with different concentrations of melatonin (MT) under Se stress. Different lowercase letters indicate that the significant difference is at the 0.05 level.
3.3. Activities of Antioxidant Enzymes and the Contents of Soluble Protein
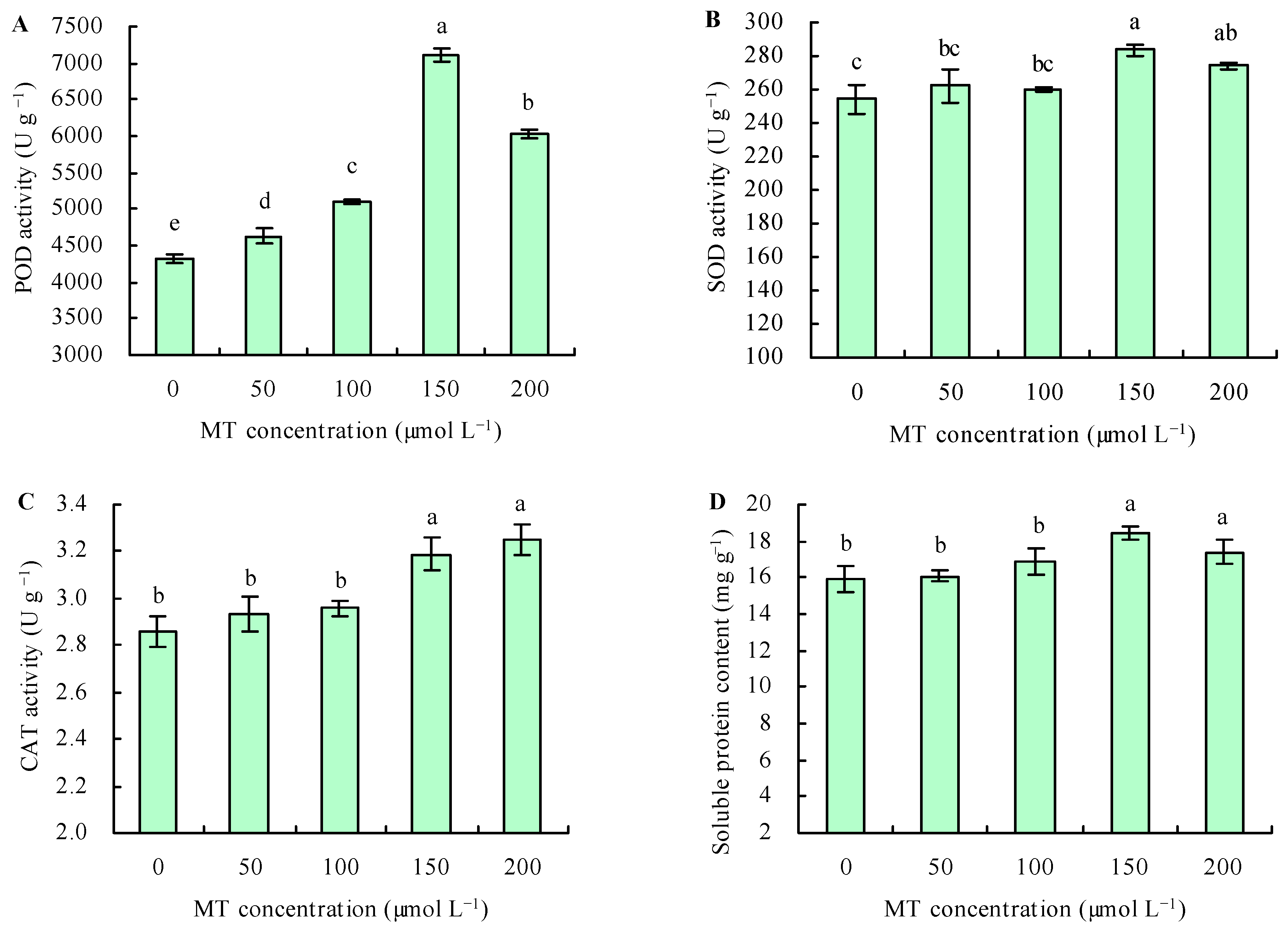
The different doses of MT increased the activity POD under Se stress (Figure 3A). Compared with the control, treatments with 50, 100, 150, and 200 µmol L−1 of MT increased the POD activity by 7%, 18%, 64%, and 39%, respectively. Under Se stress, treatments with 50 and 100 µmol L−1 of MT had no significant effects on the activities of SOD and CAT and the content of soluble protein in C. betacea, while treatment at 150 and 200 µmol L−1 increased these parameters (Figure 3B–D). Compared with the control, treatment with MT at 150 µmol L−1 increased the activities of SOD and CAT and the content of soluble protein by 11%, 11%, and 16%, respectively; moreover, treatment at 200 µmol L−1 increased these parameters by 8%, 14%, and 9%, respectively.
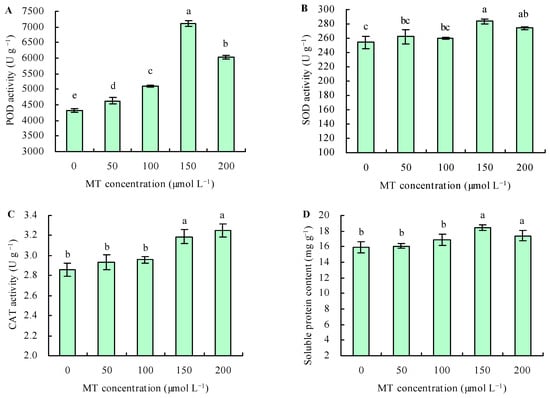
Figure 3.
The activities of POD (A), SOD (B), and CAT (C) and soluble protein content (D) were treated with different concentrations of melatonin (MT) under Se stress. Different lowercase letters indicate that the significant difference is at the 0.05 level.
3.4. Content and Transport of Se
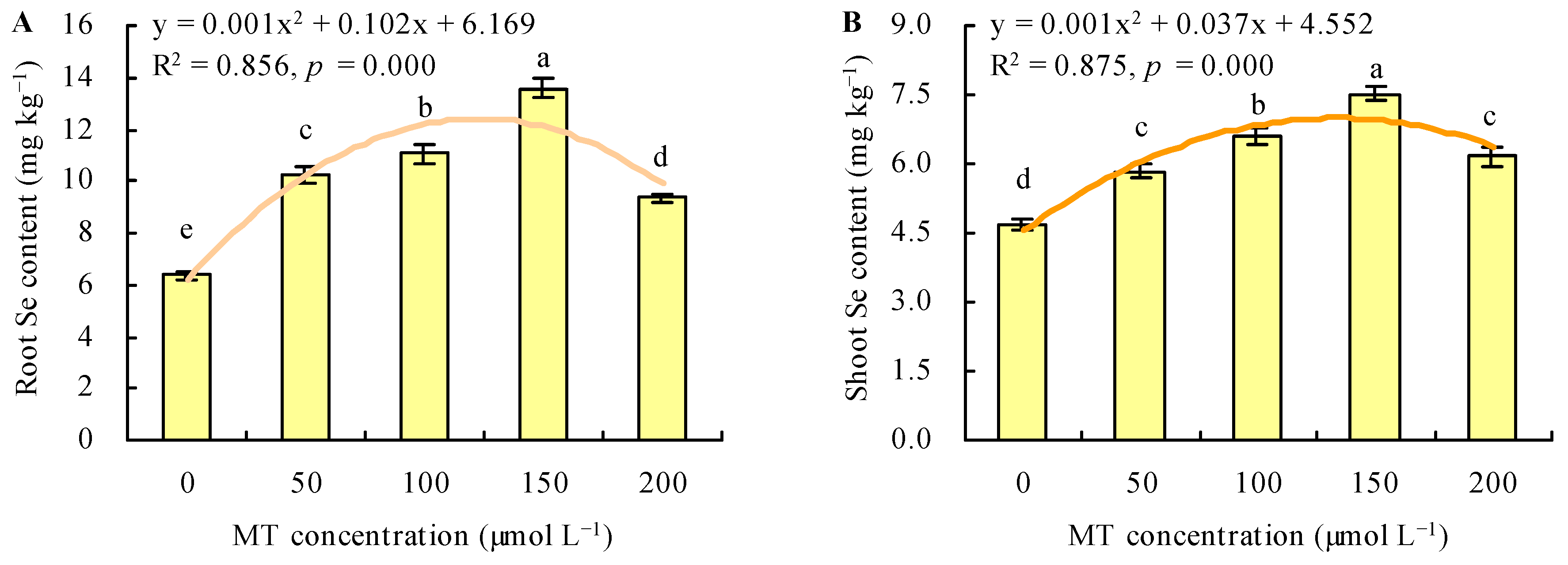
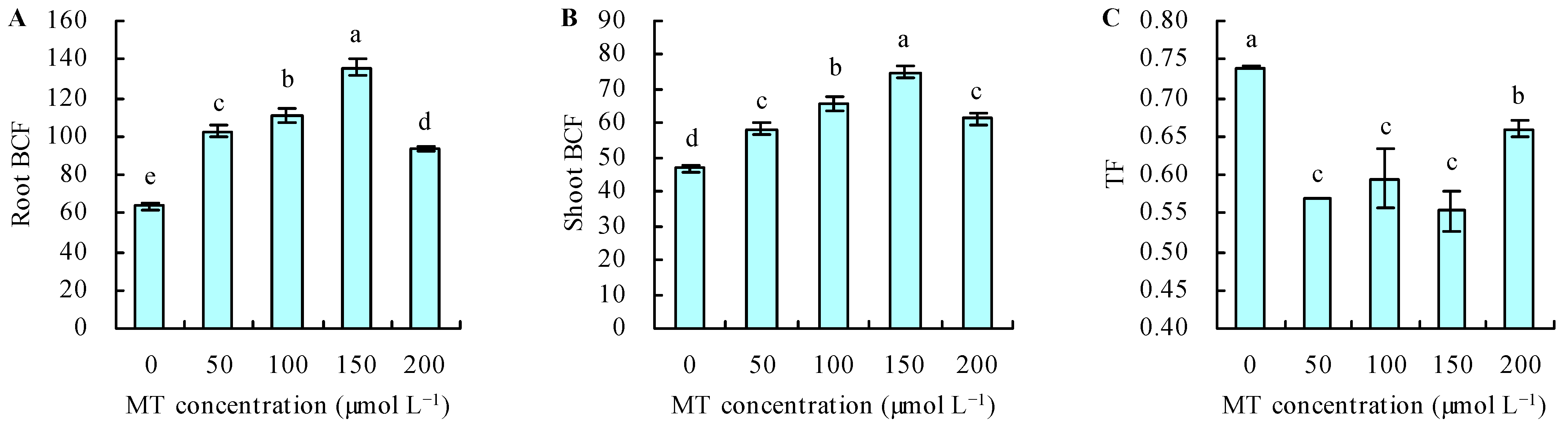
MT treatment increased the Se content under Se stress (Figure 4A,B). With the increase in MT dose, the Se content increased when the dose of MT ≤ 150 µmol L−1, and it decreased when the dose of MT > 150 µmol L−1. The dose of MT had a regression relationship with the Se content and Se had the maximal contents following treatment with 150 µmol L−1 of MT. Compared with the control, treatment with MT at 50, 100, 150, and 200 µmol L−1 increased the root Se content by 61%, 74%, 114%, and 47%, respectively, and increased the shoot Se content by 24%, 40%, 60%, and 31%, respectively. The different doses of MT also increased the root and shoot BCFs under Se stress (Figure 5A,B). The maximal root and shoot BCFs were obtained at 150 µmol L−1 of MT. However, MT treatment decreased the TF under Se stress (Figure 5C).
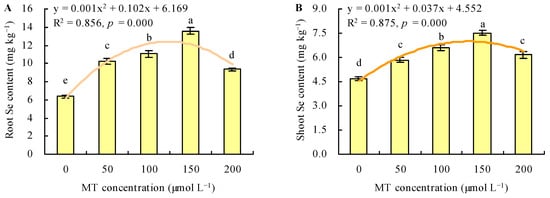
Figure 4.
The Se contents in roots (A) and shoots (B) were treated with different doses of melatonin (MT) under Se stress. Different lowercase letters indicate that the significant difference is at the 0.05 level. The orange curve is a quadratic polynomial regression fit of MT dose to root Se content or shoot Se content.
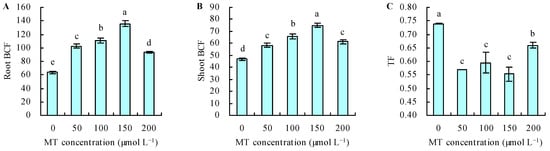
Figure 5.
Root bioconcentration factor (BCF) (A), shoot BCF (B), and translocation factor (TF) (C) were treated with different concentrations of melatonin (MT) under Se stress. Different lowercase letters indicate that the significant difference is at the 0.05 level. BCF = Se content in plants/Se concentration in media; TF = shoot Se content/root Se content.
3.5. Correlation Analysis
The biomass had a highly significant (p < 0.01) positive correlation with the contents of photosynthetic pigments, antioxidant enzyme activity, soluble protein content, and Se content (Table 1). The Se content was significantly (p < 0.01) and positively correlated with the contents of photosynthetic pigments and soluble protein and the activities of POD and SOD. The content of Se in the shoots was also significantly (p < 0.01) and positively correlated with the content of Se in the roots and significantly (0.05 < p ≤ 0.01) and positively correlated with the activity of CAT.

Table 1.
Correlations among the different parameters.
3.6. PCA and Cluster Analysis
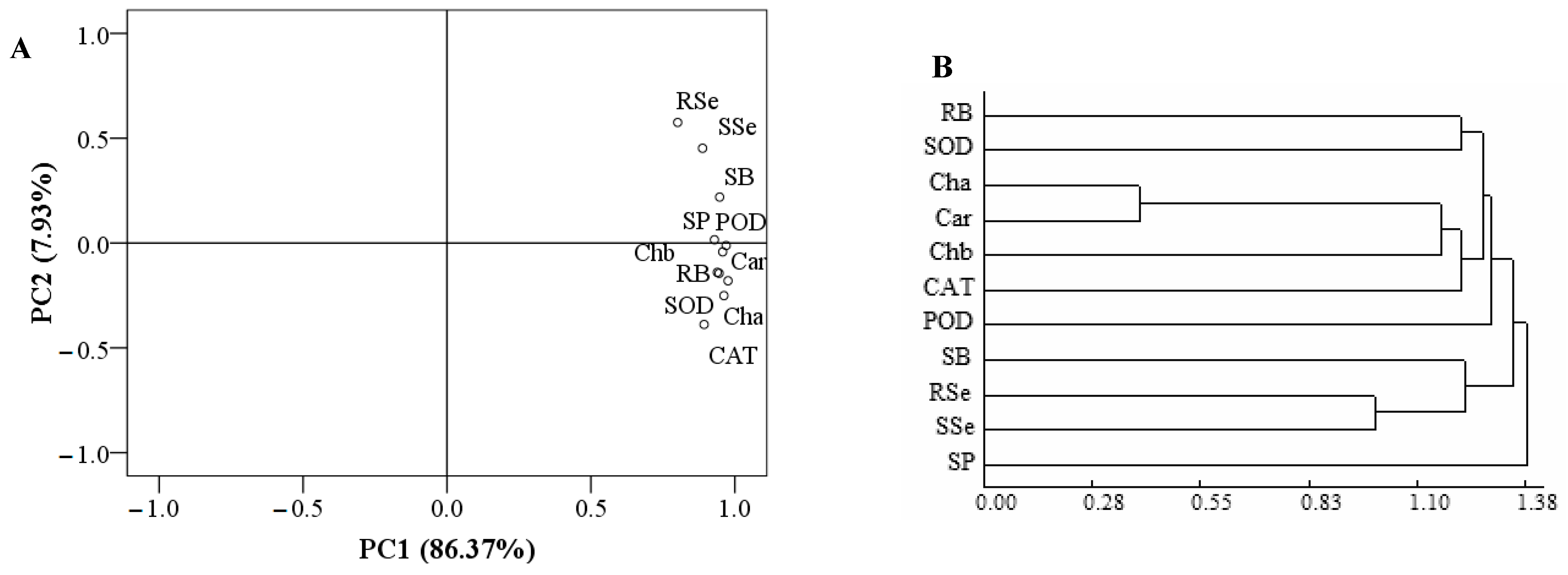
The total variance of the two components of the PCA was 94.30%; that of PC1 was 86.37%, while that of PC2 was 7.93% (Figure 6A). The contents of Se in roots and shoots and shoot biomass were closely related. Similarly, the other parameters were also closely related. As shown by a cluster analysis (Figure 6B), the parameters were divided into three categories when the decision distance < 1.3. The first category was composed of the root biomass; activities of SOD, CAT, and POD; and the contents of photosynthetic pigments. The second category was composed of the contents of Se in roots and shoots and shoot biomass. The third category was composed of the soluble protein content.
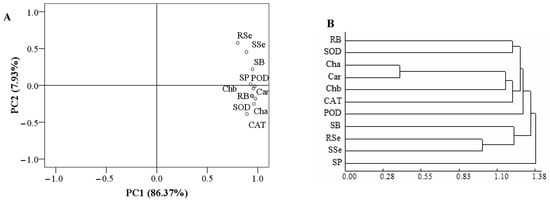
Figure 6.
PCA and cluster analysis: (A): PCA; (B): cluster analysis. RB = root biomass; SB = shoot biomass; Cha = chlorophyll a content; Chb = chlorophyll b content; Car = carotenoid content; POD = POD activity; SOD = SOD activity; CAT = CAT activity; SP = soluble protein content; RSe = root Se content; SSe = shoot Se content.
3.7. Grey and Path Analyses
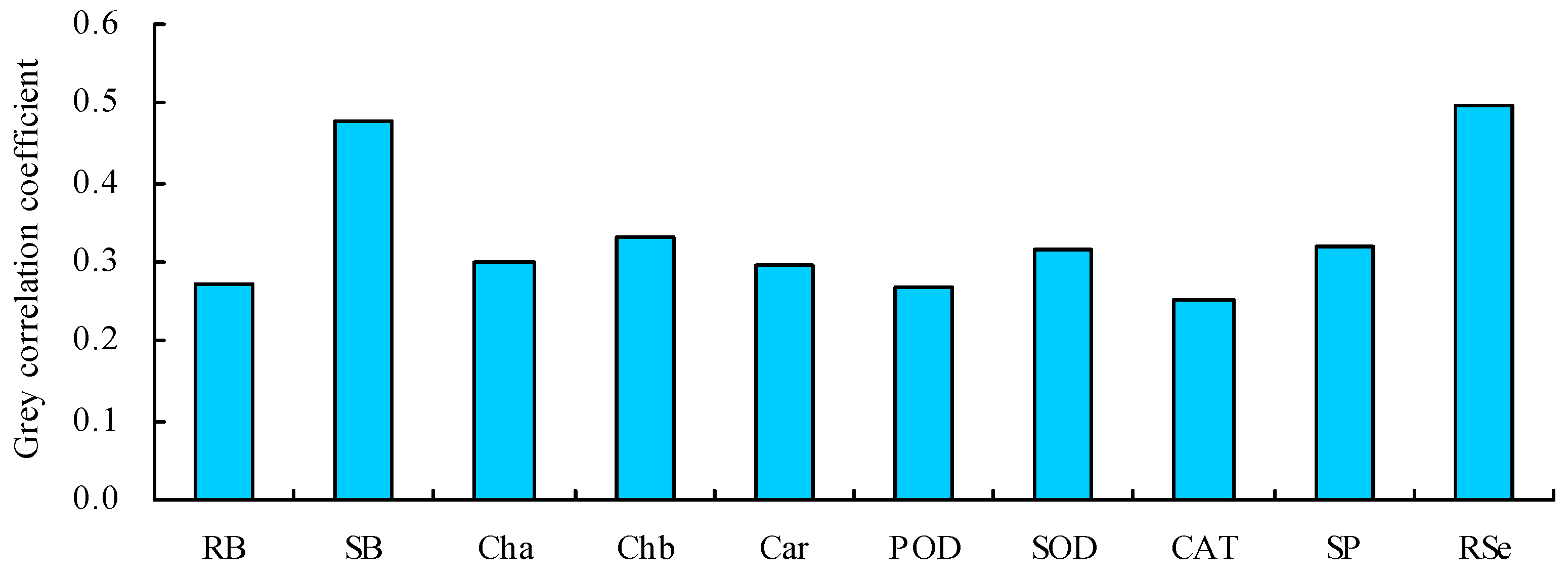
The grey correlation coefficients of the various parameters with the shoot Se content were 0.253–0.499 (Figure 7). The grey correlation coefficients of the root Se content and shoot biomass were much higher than those of the other parameters. Thus, the root Se content and shoot biomass were the closest relationships with the shoot Se content. A path analysis also showed that the root Se content and shoot biomass had the top two largest total path positive coefficients with the shoot Se content, which were much higher than those of the other parameters (Table 2).
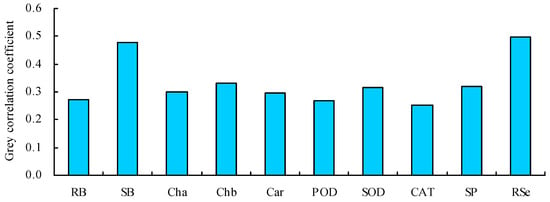
Figure 7.
Grey correlation coefficients of the different parameters with the shoot Se content. RB = root biomass; SB = shoot biomass; Cha = chlorophyll a content; Chb = chlorophyll b content; Car = carotenoid content; POD = POD activity; SOD = SOD activity; CAT = CAT activity; SP = soluble protein content; RSe = root Se content.

Table 2.
Path coefficients of the different parameters with the shoot Se content.
4. Discussion
MT can be used to protect against stress [34,35]. Treatment with MT increased the biomass of rice and sugar beet under salt stress and drought stress, respectively [36,37], and mitigated flooding stress in soybean [38]. Treatment with MT increased the biomass of grape and P. asiatica under Se stress [21,22]. In this study, treatment with MT increased the biomass of C. betacea to some extent under Se stress. It has been shown that MT can regulate the transport of indole-3-acetic acid (IAA), thus promoting plant growth [39], and MT and IAA have synergistic or cumulative effects or both [40,41]. A suitable amount of exogenous MT can act as a chemical signal to upregulate the IAA signal transducer genes’ expressions, which induces the plant to produce higher amounts of IAA and thus promotes its growth [42]. However, high doses of MT inhibit the biosynthesis of IAA, and, thus, inhibit plant growth [39]. The effective dose of MT for plant growth varies from plant to plant [43,44].
MT can regulate the biosynthesis and degradation of chlorophyll [45]. Under salt stress, treatment with MT increased the content of chlorophyll in cucumber, which suggests that treatment with MT alleviates the effect of salt stress on photosynthesis [46], and treatment with MT mitigates the inhibition of the photosynthetic rate induced by salt treatment by the inhibiting the closure of stomata [47]. Under nitrogen dioxide stress, treatment with MT reduced the degradation of chlorophyll in tobacco and increased the chlorophyll content by upregulating the chlorophyll biosynthesis-related genes’ expressions [48]. In this study, treatment with MT increased the photosynthetic pigment contents in C. betacea to some degree under Se stress. This result may be related to the MT's ability to reduce the expressions of chlorophyllase, and, thus, inhibit the degradation of chlorophyll [49]. The other reason could be that MT can protect the integrity of the chloroplasts and thus it does not impede the biosynthesis of chlorophyll [50].
Under stress conditions, MT is important for maintaining the enzymatic antioxidant system of plants [51,52]. The mechanism of its action is that MT can act as a signaling molecule to participate in the signal transduction pathway to implement physiological functions [35]. Under salt stress, treatment with MT increased the content of soluble protein in cotton [53]. Under chromium (Cr) stress, treatment with MT increased the activities of antioxidant enzymes to reduce the oxidative stress response of maize, and MT is thought to function as a multidirectional signaling molecule to promote the levels of gene expression and participate in transcription [54]. Under Se stress, treatment with MT increased the activities of antioxidant enzymes in grape and P. asiatica and also increased their content of soluble protein [21,22]. In this study, the different doses of MT increased the POD activity of C. betacea, and treatment with 150 and 200 µmol L−1 MT increased the activities of SOD and CAT and the content of soluble protein of C. betacea under Se stress. This effect could be due to the fact that MT acts as a strong antioxidant and can directly or indirectly scavenge reactive oxygen species (ROS) in plants during stress and thus improve the antioxidant enzyme activity [53,55]. MT and its metabolites also play a role in some signaling pathways and act as signaling molecules to scavenge ROS to enhance the antioxidant capacity of plants when they are under stress [56].
Treatment with MT can promote nutrient uptakes by plants [27,57]. Under iron stress, treatment with MT increased the uptake of iron and redistributed it in peaches and cucumbers [58,59]. Under Se stress, treatment with MT promotes the Se uptakes by grape and P. asiatica [21,22]. In this study, treatments with MT increased the Se contents in C. betacea under Se stress. The dose of MT had a regression relationship with the Se content, indicating that MT could promote Se accumulation in C. betacea. Because MT can increase the content of some amino acids in plant roots [60], these amino acids are secreted by plant roots and combined with inorganic Se in the soil to become organic Se in proteins through biotransformation [61]. The forms of Se uptake and transport in C. betacea affected by MT need further study. In addition, treatment with MT also increased the BCF of C. betacea, while it decreased the TF of C. betacea. Therefore, MT could promote the Se uptake using C. betacea but inhibit the transport of Se from the roots to shoots. Inorganic Se can be directly absorbed and utilized by plants in soil, which includes selenate (SeO42−) and selenite (SeO32−) [62]. The inorganic Se that is absorbed by the roots is converted into selenocysteine (SeCys) or selenomethionine (SeMet), which are easily immobilized in roots, and only a small portion is transported to the aboveground parts through the xylem [63]. Thus, the content of Se in the roots of C. betacea was higher than that in shoots, which induced the decrease in TF. Moreover, the shoot Se content was positively correlated with other parameters. A PCA and cluster analysis showed that the contents of Se in roots and shoots and shoot biomass were classified as a category. Grey and path analyses further showed that the content of Se in roots and shoot biomass had the top two largest contributions to the content of Se in shoots. These results indicate that the content of Se in roots and shoot biomass was closely associated with the content of Se in shoots, which highlighted their significant role in promoting Se uptake by C. betacea under Se stress.
5. Conclusions
The growth of C. betacea is promoted under Se stress through treatment with MT, leading to increased biomass, photosynthetic pigment contents, and antioxidant enzyme activities. Thus, MT helps to alleviate the stress caused by Se on C. betacea. MT treatment enhances the Se content and BCF of C. betacea while reducing the TF. The dose of MT used is inversely correlated with Se content, with the highest levels found at 150 µmol L−1. The relationship between root Se content and shoot biomass is crucial for Se uptake. Therefore, MT treatment can support the growth of C. betacea and its Se absorption under Se stress. Further research should explore the application of MT in horticultural crops to enhance the production of selenium-enriched products.
Author Contributions
Conceptualization, X.W. and L.L.; investigation, X.W., R.D., Y.X., Z.D. and D.Z.; writing—original draft preparation, X.W. and J.D.; writing—review and editing, L.L.; supervision, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. Plant samples were collected from the university research area. Study protocol must comply with relevant institutional, national, and international guidelines and legislation. Our experiment follows the relevant institutional, national, and international guidelines and legislation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Thiry, C.; Ruttens, A.D.; Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits EA, H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Wei, X.; Bie, Y.; Zhou, H.; Deng, L.; Lin, L.; Liao, M. Effects of mutual grafting Solanum photeinocarpum from two ecosystems on physiology and selenium absorption of their offspring under selenium stress. Acta Physiol. Plant. 2021, 43, 96. [Google Scholar] [CrossRef]
- Huan, Y.; Yang, L.; Liu, Q.; Lin, L.; Liao, M.; Wang, Z.; Liang, D.; Xia, H.; Tang, Y.; Lv, X.; et al. Effects of indole acetic acid on the growth and selenium absorption characteristics of Cyphomandra betacea seedlings. Acta Physiol. Plant. 2021, 43, 74. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in Plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar] [CrossRef]
- Zou, J.N. Effects of Exogenous Melatonin on Photosynthesis and Growth of Soybean under Drought Stress; Heilongjiang Bayi Agricultural University: Daqing, China, 2019. [Google Scholar]
- Wang, P.; Sun, X.; Wang, N.; Tan, D.X.; Ma, F. Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J. Pineal Res. 2015, 58, 479–489. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; You, X.Y.; Bao, R.F.; Wang, J.; Lv, X.L.; Liang, D.; Xia, H.; Liao, M.A.; Lin, L.J. Effects of melatonin on growth and selenium accumulation of grape seedlings. Chin. J. Soil Sci. 2022, 53, 1453–1460. [Google Scholar] [CrossRef]
- Liao, R.Y.; Huang, K.W.; Li, K.Q. Effect of different concentrations of melatonin on selenium accumulation in Plantago asiatica L. J. Chin. Med. Mater. 2018, 41, 1539–1542. [Google Scholar] [CrossRef]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective potential of tamarillo (Cyphomandra betacea) epicarp extracts obtained by sustainable extraction process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sun, J.; Cui, T.; Zhou, X.; Liao, M.; Huan, Y.; Yang, L.; Wu, C.; Xia, X.; Wang, Y.; et al. Selenium accumulation characteristics of Cyphomandra betacea (Solanum betaceum) seedlings. Physiol. Mol. Biol. Plants 2020, 26, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Deng, Q.; Wang, X.; Luo, X.; Liao, M.; et al. Gibberellic acid promotes selenium accumulation in Cyphomandra betacea under selenium stress. Front. Plant Sci. 2022, 13, 968768. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, J.; Deng, L.; Zhou, H.; Bie, Y.; Jing, Q.; Lin, L.; Wang, J.; Liao, M. Effects of diethyl aminoethyl hexanoate on the physiology and selenium absorption of grape seedlings. Acta Physiol. Plant. 2021, 43, 115. [Google Scholar] [CrossRef]
- Xia, H.; Yang, C.; Liang, Y.; He, Z.; Guo, Y.; Lang, Y.; Wei, J.; Tian, X.; Lin, L.; Deng, H.; et al. Melatonin and arbuscular mycorrhizal fungi synergistically improve drought toleration in kiwifruit seedlings by increasing mycorrhizal colonization and nutrient uptake. Front. Plant Sci. 2022, 13, 1073917. [Google Scholar] [CrossRef]
- Hao, Z.B.; Cang, J.; Xu, Z. Plant Physiology Experiment; Harbin Institute of Technology Press: Harbin, China, 2004. [Google Scholar]
- Lin, L.; Wu, C.; Jiang, W.; Liao, M.; Tang, Y.; Wang, J.; Lv, X.; Liang, D.; Xia, H.; Wang, X.; et al. Grafting increases cadmium accumulation in the post-grafting generations of the potential cadmium-hyperaccumulator Solanum photeinocarpum. Chem. Ecol. 2020, 36, 685–704. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soils, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Rastmanesh, F.; Moore, F.; Keshavarzi, B. Speciation and phytoavailability of heavy metals in contaminated soils in Sarcheshmeh area, Kerman Province, Iran. Bull. Environ. Contam. Toxicol. 2010, 85, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Z.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Tang, Y.; Wang, X.; Deng, Q.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Q.; Xu, X.; Liao, M.; Lin, L.; Hu, R.; Luo, X.; Wang, Z.; Wang, J.; Deng, Q.; et al. An amino acid fertilizer improves the emergent accumulator plant Nasturtium officinale R. Br. phytoremediation capability for cadmium-contaminated paddy soils. Front. Plant Sci. 2022, 13, 1003743. [Google Scholar] [CrossRef]
- Sun, C.L.; Liu, L.J.; Wang, L.X.; Li, B.H.; Jin, C.W.; Lin, X.Y. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Mei, S.Y.; Zhai, Y.N.; Geng, G.; Yu, L.H.; Wang, Y.G. Effects of melatonin on the growth of sugar beet (Beta vulgaris L.) seedlings under drought stress. J. Plant Growth Regul. 2023, 42, 5116–5130. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Chen, Z.Y.; Yang, B.X.; Komatsu, S.; Zhou, S.L. Proteomic analysis reveals the effects of melatonin on soybean root tips under flooding stress. J. Proteom. 2021, 232, 104064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; You, J.; Li, J.Z.; Wang, Y.P.; Chan, Z.L. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Growth activity, rooting capacity, and tropism, three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin promotes adventitious and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, D.; Wang, J.; Tian, B.; Li, Y.; Sun, G.; Zhang, H. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.R.; Chen, S.C. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Danilova, E.D.; Zlobin, I.E.; Kuznetsov, V.V.; Efimova, M. Exogenic melatonin reduces the toxic effect of polymetallic stress on barley plants. Dokl. Biochem. Biophys. 2021, 499, 228–232. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Yang, X.X.; Ren, J.H.; Lin, X.Y.; Yang, Z.P.; Deng, X.P.; Ke, Q.B. Melatonin alleviates chromium toxicity in maize by modulation of cell wall polysaccharides biosynthesis, glutathione metabolism, and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef]
- Ren, J.H.; Ye, J.; Yin, L.; Li, G.X.; Deng, X.P.; Wang, S.W. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin application improves berry coloration, sucrose synthesis, and nutrient absorption in ‘Summer Black’ grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. Amst. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Wu, C.; Xu, Y.; Wang, J.; Lv, X.; Xia, H.; Liang, D.; Huang, Z.; Tang, Y. Melatonin promotes iron reactivation and reutilization in peach plants under iron deficiency. Int. J. Mol. Sci. 2023, 24, 16133. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, B.; Qiu, D.; Xie, Z.; Dai, S.; Li, C.; Xu, S.; Zheng, Y.; Li, S.; Jiang, M. Melatonin enhances metallic oxide nanoparticle stress tolerance in rice via inducing tetrapyrrole biosynthesis and amino acid metabolism. Environ. Sci. Nano 2021, 8, 2310–2323. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.Q.; Wu, H. Selenium accumulation, speciation, and its effect on nutritive value of Flammulina velutipes (golden needle mushroom). Food Chem. 2021, 350, 128667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Pan, G.P.; Chen, J.; Hu, Q.H. Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 2003, 253, 437–443. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).