Permaculture Management of Arable Soil Increases Soil Microbial Abundance, Nutrients, and Carbon Stocks Compared to Conventional Agriculture
Abstract
1. Introduction
- Greater microbial abundance, with increase in fungal biomass relative to bacterial.
- Higher soil organic matter and total carbon content from organic amendments and microbial activity.
- Greater nutrient retention.
2. Materials and Methods
2.1. Study Sites and Sampling Strategy
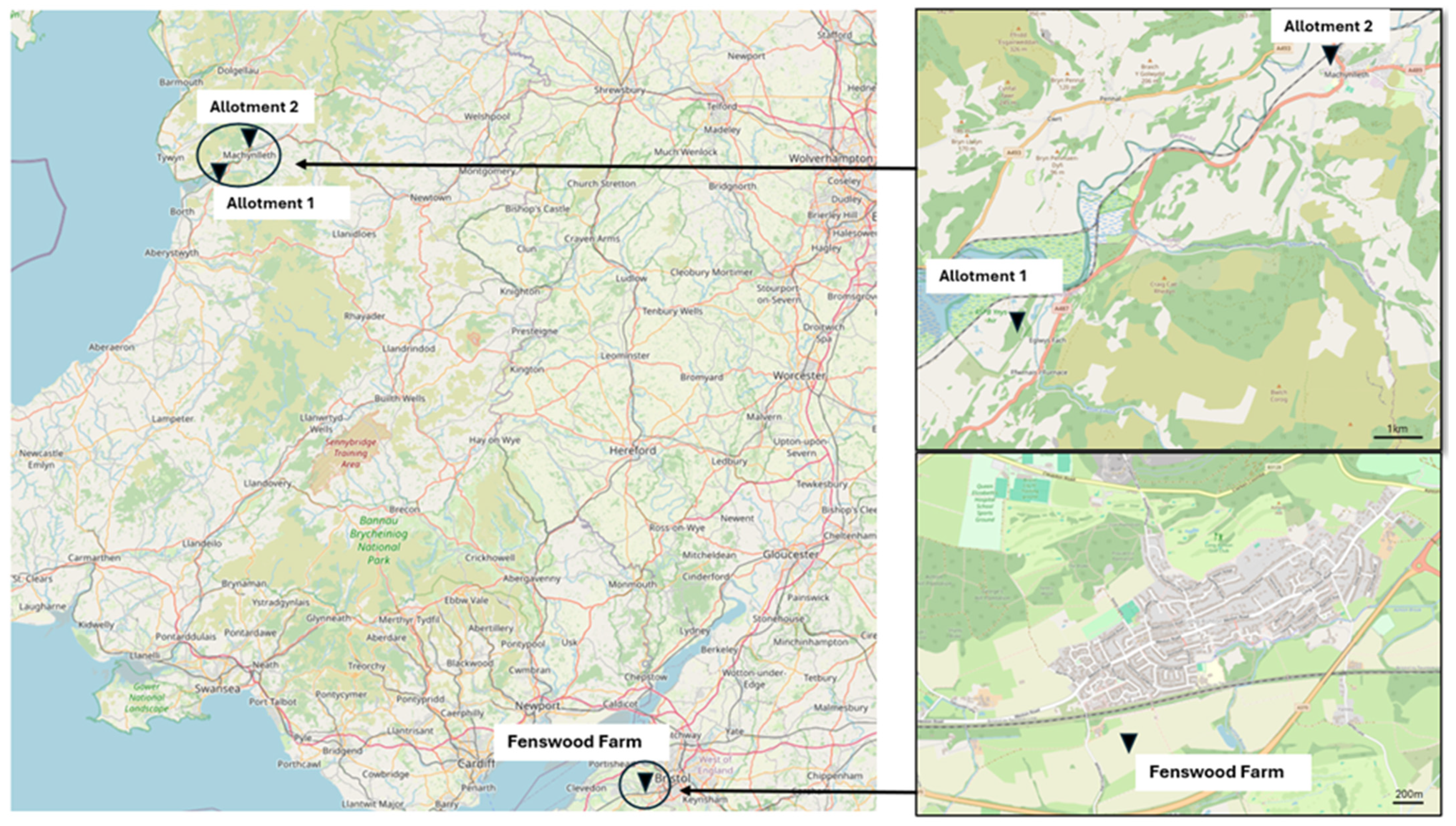
2.2. Soil Geochemical Properties
2.3. Phospholipid Fatty Acid (PLFA) Analysis
2.4. Greenhouse Gas Incubation
2.5. Statistical Analyses
3. Results
3.1. Soil Geochemical Properties
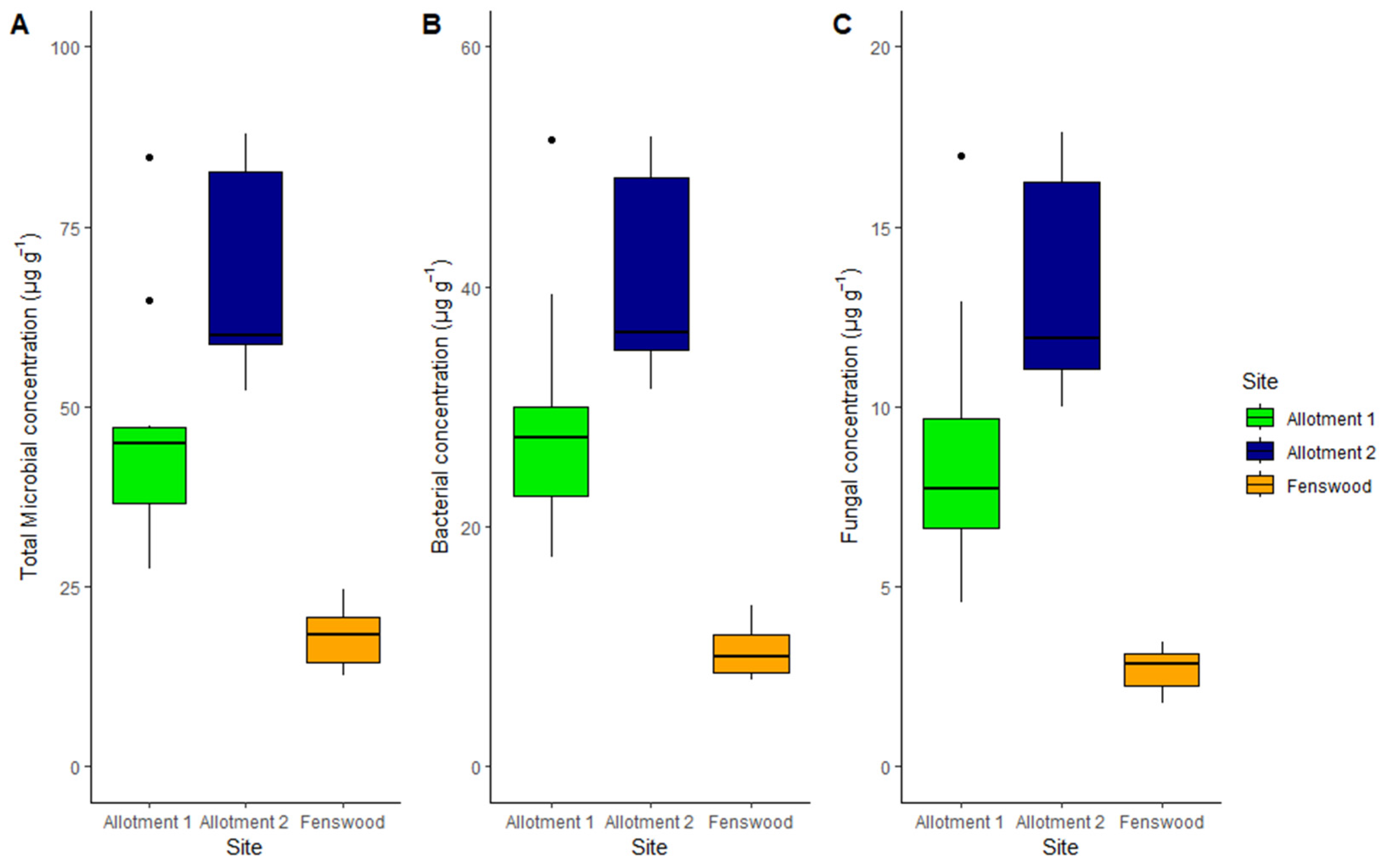
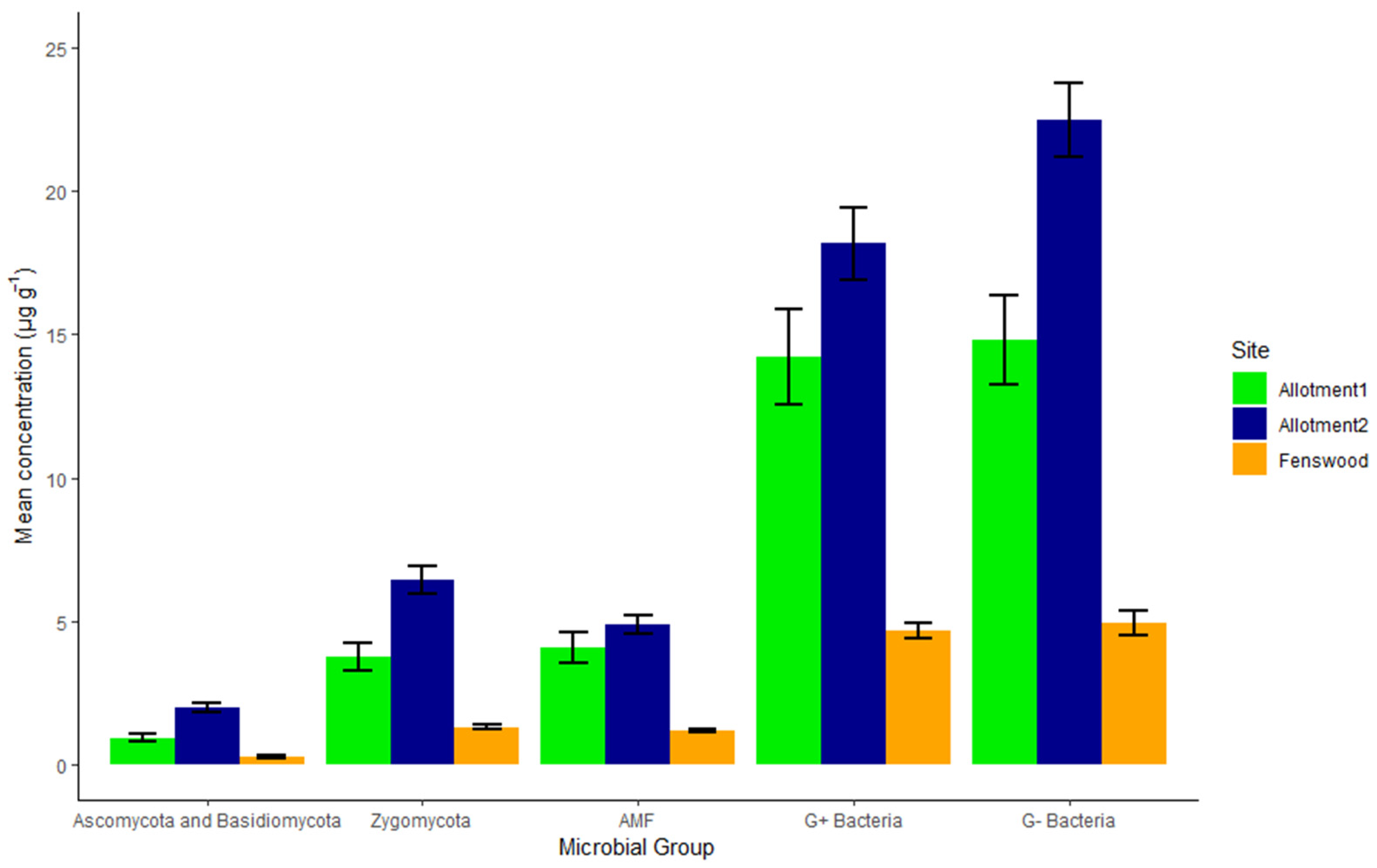
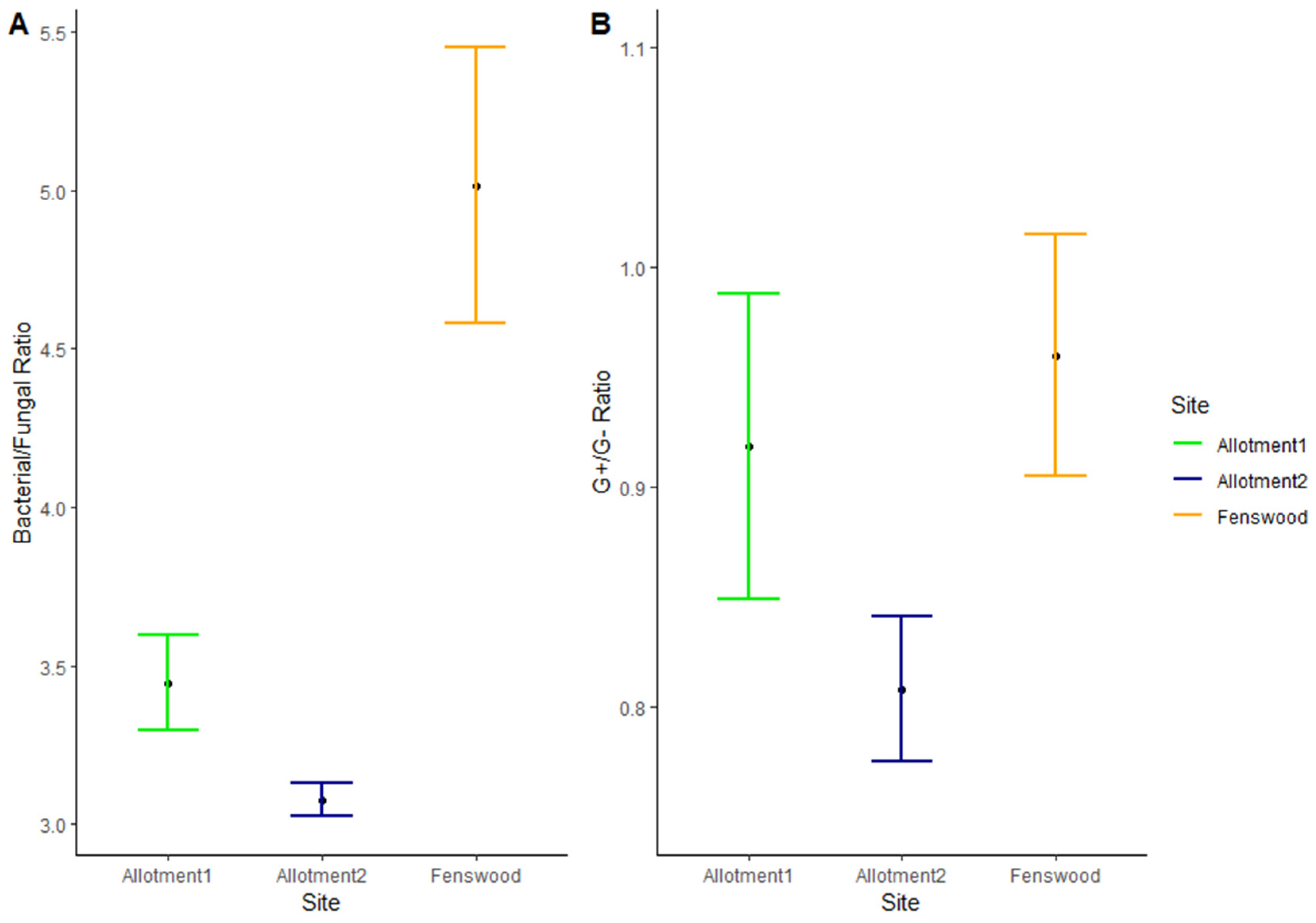
3.2. Microbial Abundance
3.3. Greenhouse Gas Fluxes
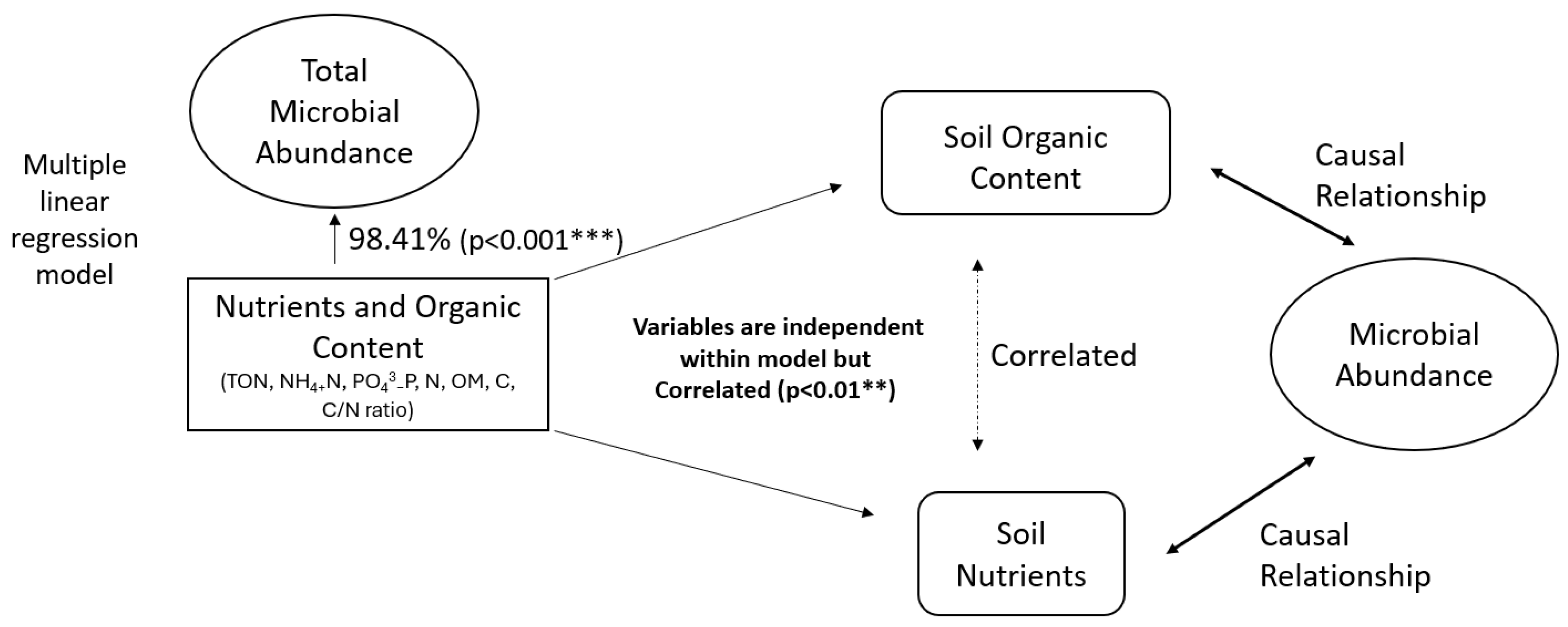
3.4. Controlling Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faltin, J. Failing Food Supply: Permaculture’s Potential. Bachelor’s Thesis, University of Nebraska-Lincoln, Lincoln, Nebraska, 2017. Available online: https://digitalcommons.unl.edu/envstudtheses/213/ (accessed on 29 January 2024).
- Hathaway, M.D. Agroecology and permaculture: Addressing key ecological problems by rethinking and redesigning agricultural systems. J. Environ. Stud. Sci. 2016, 6, 239–250. [Google Scholar] [CrossRef]
- Reganold, J.P. Comparison of soil properties as influenced by organic and conventional farming systems. Am. J. Altern. Agric. 1988, 3, 144–155. [Google Scholar] [CrossRef]
- Vanwalleghem. Soil erosion and conservation. In International Encyclopedia of Geography: People, the Earth, Environment and Technology: People, the Earth, Environment and Technology; 2017; pp. 1–10. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118786352.wbieg0381 (accessed on 29 January 2024). [CrossRef]
- United Nations, 2022. FAO Warns 90 per Cent of Earth’s Topsoil at Risk by 2050. UN News, Global Perspective Human Stories. United Nations. [Online]. Available online: https://news.un.org/en/story/2022/07/1123462 (accessed on 29 January 2024).
- Montgomery. Soil erosion and agricultural sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 13268–13272. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Reeve, J.R.; Hoagland, L.A.; Villalba, J.J.; Carr, P.M.; Atucha, A.; Cambardella, C.; Davis, D.R.; Delate, K. Organic farming, soil health, and food quality: Considering possible links. Adv. Agron. 2016, 137, 319–367. [Google Scholar]
- Tully, K.L.; McAskill, C. Promoting soil health in organically managed systems: A review. Org. Agric. 2020, 10, 339–358. [Google Scholar] [CrossRef]
- Hemenway, T. GAIA’S GARDEN a Guide to Home Scale Permaculture, 2nd ed.; Chelsea Green Publishing: White River Junction, VT, USA, 2009. [Google Scholar]
- Mollison, B.C.; Slay, R.M.; Girard, J.L.; Girard, J.L. Introduction to Permaculture. Yankee Permaculture. Publisher and Distributor of Permaculture Publications POB 52, Sparr FL 32192-0052 USA. 1991. Available online: https://www.academia.edu/download/62514467/Bill_Mollison_-_Permaculture_Design_Course_20200328-35259-mdyiv7.pdf (accessed on 15 October 2022).
- Leni-Konig. Beyond School Gardens: Permaculture Food Forests Enhance Ecosystem Services While Achieving Education for Sustainable Development Goals. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 2020.
- Minckler, S.J. Permaculture: The Need for Increased Science. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2019. [Google Scholar]
- Fiebrig, I.; Zikeli, S.; Bach, S.; Gruber, S. Perspectives on permaculture for commercial farming: Aspirations and realities. Org. Agric. 2020, 10, 379–394. [Google Scholar] [CrossRef]
- Morel, K.; Léger, F.; Ferguson, R.S. Permaculture. In Encyclopedia of Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 559–567. [Google Scholar] [CrossRef]
- De Tombeur, F.; Sohy, V.; Chenu, C.; Colinet, G.; Cornelis, J.T. Effects of permaculture practices on soil physicochemical properties and organic matter distribution in aggregates: A case study of the Bec-Hellouin Farm (France). Front. Environ. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- French, E.; Kaplan, I.; Iyer-Pascuzzi, A.; Nakatsu, C.H.; Enders, L. Emerging strategies for precision microbiome management in diverse agroecosystems. Nat. Plants 2021, 7, 256–267. [Google Scholar] [CrossRef]
- Bak, G.R.; Lee, G.J.; Lee, J.T.; Jee, S.N. Crop rotation affects biological properties of rhizosphere soil and productivity of Kimchi cabbage (Brassica rapa ssp. pekinensis) compared to monoculture. Hortic. Environ. Biotechnol. 2022, 63, 613–625. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Feeding and healing the world: Through regenerative agriculture and permaculture. Sci. Prog. 2012, 95, 345–446. [Google Scholar] [CrossRef] [PubMed]
- Sneha, S.; Anitha, B.; Sahair, R.A.; Raghu, N.; Gopenath, T.S.; Chandrashekrappa, G.K.; Basalingappa, M.K. Biofertilizer for crop production and soil fertility. Acad. J. Agric. Res. 2018, 6, 299–306. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Mäder, P.; Edenhofer, S.; Boller, T.; Wiemken, A.; Niggli, U. Arbuscular mycorrhizae in a long-term field trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Biol. Fertil. Soils 2000, 31, 150–156. [Google Scholar] [CrossRef]
- Sainju, U.M.; Stevens, W.B.; Caesar-TonThat, T.; Liebig, M.A. Soil greenhouse gas emissions affected by irrigation, tillage, crop rotation, and nitrogen fertilization. J. Environ. Qual. 2012, 41, 1774–1786. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, W.; Wang, L.; Hui, D.; Grove, J.H.; Yang, X.; Tao, B.; Goff, B. Greenhouse gas emissions and crop yield in no-tillage systems: A meta-analysis. Agric. Ecosyst. Environ. 2018, 268, 144–153. [Google Scholar] [CrossRef]
- Gao, Y.; Cabrera Serrenho, A. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Lassaletta, L.; Aguilera, E.; del Prado, A.; Garnier, J.; Billen, G.; Iglesias, A.; Sanchez, B.; Guardia, G.; Abalos, D.; et al. Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: A review. Agric. Ecosyst. Environ. 2017, 238, 5–24. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Wu, Y.; Liu, S.; Li, L.; Chen, W.; Wu, S.; Meng, Q.; Feng, H.; Siddique, K.H. Mitigating greenhouse gas emissions by replacing inorganic fertilizer with organic fertilizer in wheat–maize rotation systems in China. J. Environ. Manag. 2023, 344, 118494. [Google Scholar] [CrossRef]
- IPCC. IPCC, 2023: Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H.H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Joergensen. Phospholipid fatty acids in soil—Drawbacks and future prospects. Biol. Fertil. Soils 2022, 58, 1–6. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; JRAlves, B.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Digimap, 2023. Digimap. [Online]. Available online: https://digimap.edina.ac.uk (accessed on 12 January 2023).
- Rhodes, C.J. The imperative for regenerative agriculture. Sci. Prog. 2017, 100, 80–129. [Google Scholar] [CrossRef]
- OpenStreetMap.org 2023. OpenStreetMap. [Online]. Available online: www.openstreetmap.org/copyright/ (accessed on 23 February 2023).
- Pennock, D.; Yates, T.; Braidek, J. Soil Sampling Designs. In Soil Sampling and Methods of Analysis: Chapter 1; CRC Press: Boca Raton, FL, USA, 2008; pp. 863–869. [Google Scholar]
- Malvern Instruments Ltd. Mastersizer 3000 User Manual; Transport; Malvern Analytical Ltd.: Worcestershire, UK, 2017. [Google Scholar]
- Natural Resources Conservation Service USDA, nd. Soil Textural Calculator. Natural Resources Conservation Service. U.S. Department of Agriculutre. [Online]. Available online: https://www.nrcs.usda.gov/resources/education-and-teaching-materials/soil-texture-calculator (accessed on 19 December 2022).
- Buyer, J.S.; Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012, 61, 127–130. [Google Scholar] [CrossRef]
- Kroppenstedt, R.M. The genus Nocardiopsis. In The Prokaryotes 2; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.H., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1992; pp. 1139–1156. [Google Scholar]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Jiang, S.; An, X.; Shao, Y.; Kang, Y.; Chen, T.; Mei, X.; Dong, C.; Xu, Y.; Shen, Q. Responses of arbuscular mycorrhizal fungi occurrence to organic fertilizer: A meta-analysis of field studies. Plant Soil 2021, 469, 89–105. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Mäder, P.; Dubois, D.; Ineichen, K.; Boller, T.; Wiemken, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 2004, 138, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Edlinger, A.; Garland, G.; Hartman, K.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Hallin, S.; Valzano-Held, A.; Herzog, C.; Jansa, J.; et al. Agricultural management and pesticide use reduce the functioning of beneficial plant symbionts. Nat. Ecol. Evol. 2022, 6, 1145–1154. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Brussaard, L.; De Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef] [PubMed]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Peng, J.; Han, X.; Li, N.; Chen, K.; Yang, J.; Zhan, X.; Luo, P.; Liu, N. Combined application of biochar with fertilizer promotes nitrogen uptake in maize by increasing nitrogen retention in soil. Biochar 2021, 3, 367–379. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.X.; Wu, W.X.; Shi, D.Z.; Yang, M.; Zhong, Z.K. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Romaniuk, R.; Giuffré, L.; Costantini, A.; Nannipieri, P. Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol. Indic. 2011, 11, 1345–1353. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Krauss, M.; Wiesmeier, M.; Don, A.; Cuperus, F.; Gattinger, A.; Gruber, S.; Haagsma, W.K.; Peigné, J.; Palazzoli, M.C.; Schulz, F.; et al. Reduced tillage in organic farming affects soil organic carbon stocks in temperate Europe. Soil Tillage Res. 2022, 216, 105262. [Google Scholar] [CrossRef]
- Chitravadivu, C.; Balakrishnan, V.; Manikandan, J.; Elavazhagan, T.; Jayakumar, S. Application of food waste compost on soil microbial population in groundnut cultivated soil, India. Middle-East J. Sci. Res. 2009, 4, 90–93. [Google Scholar]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Bio/Technol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Sgouridis, F.; Ullah, S. Soil greenhouse gas fluxes, environmental controls, and the partitioning of N2O sources in UK natural and seminatural land use types. J. Geophys. Res. Biogeosci. 2017, 122, 2617–2633. [Google Scholar] [CrossRef]
- Akiyama, H.; Tsuruta, H.; Watanabe, T. N2O and NO emissions from soils after the application of different chemical fertilizers. Chemosphere-Glob. Chang. Sci. 2000, 2, 313–320. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Otieno, D.O.; K’Otuto, G.O.; Maina, J.N.; Kuzyakov, Y.; Onyango, J.C. Responses of ecosystem carbon dioxide fluxes to soil moisture fluctuations in a moist Kenyan savanna. J. Trop. Ecol. 2010, 26, 605–618. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Cantero-Martínez, C.; Bareche, J.; Arrúe, J.L.; Álvaro-Fuentes, J. Soil carbon dioxide and methane fluxes as affected by tillage and N fertilization in dryland conditions. Plant Soil 2014, 381, 111–130. [Google Scholar] [CrossRef]
- UK Climate Averages (nd). UK Climate Averages. MetOffice.gov.uk. [Online]. Available online: https://www.metoffice.gov.uk/research/climate/maps-and-data/uk-climate-averages (accessed on 4 May 2024).
- Biala, J.; Wilkinson, K.; Henry, B.; Singh, S.; Bennett-Jones, J.; De Rosa, D. The potential for enhancing soil carbon levels through the use of organic soil amendments in Queensland, Australia. Reg. Environ. Chang. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; Zelfde, M.V.T.; McCallum, I.; Luke McCormack, M.; Fisher, J.B.; Brundrett, M.C.; de Sá, N.C.; Tedersoo, L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019, 10, 5077. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Peng, C.; Wang, C.; Chen, H.; Liu, W.; Liu, Z.; Wang, H.; Li, H.; Chen, D.; Li, Y.; et al. Responses of soil CH4 fluxes to nitrogen addition in two tropical montane rainforests in southern China. For. Ecosyst. 2022, 9, 100031. [Google Scholar] [CrossRef]
- Chang, R.; Liu, X.; Wang, T.; Li, N.; Bing, H. Stimulated or inhibited response of methane flux to nitrogen addition depends on nitrogen levels. J. Geophys. Res. Biogeosciences 2021, 126, e2021JG006600. [Google Scholar] [CrossRef]
- Bodelier, L.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, C.; Zhang, J.; Li, Y.; Song, X. Nitrogen addition decreases methane uptake caused by methanotroph and methanogen imbalances in a Moso bamboo forest. Sci. Rep. 2021, 11, 5578. [Google Scholar] [CrossRef]
- Bell, G. The Permaculture Way: Practical Steps to Create a Self-Sustaining World; Chelsea Green Publishing: White River Junction, VT, USA, 2005. [Google Scholar]
- Suh, J. Towards sustainable agricultural stewardship: Evolution and future directions of the permaculture concept. Environ. Values 2014, 23, 75–98. [Google Scholar] [CrossRef]
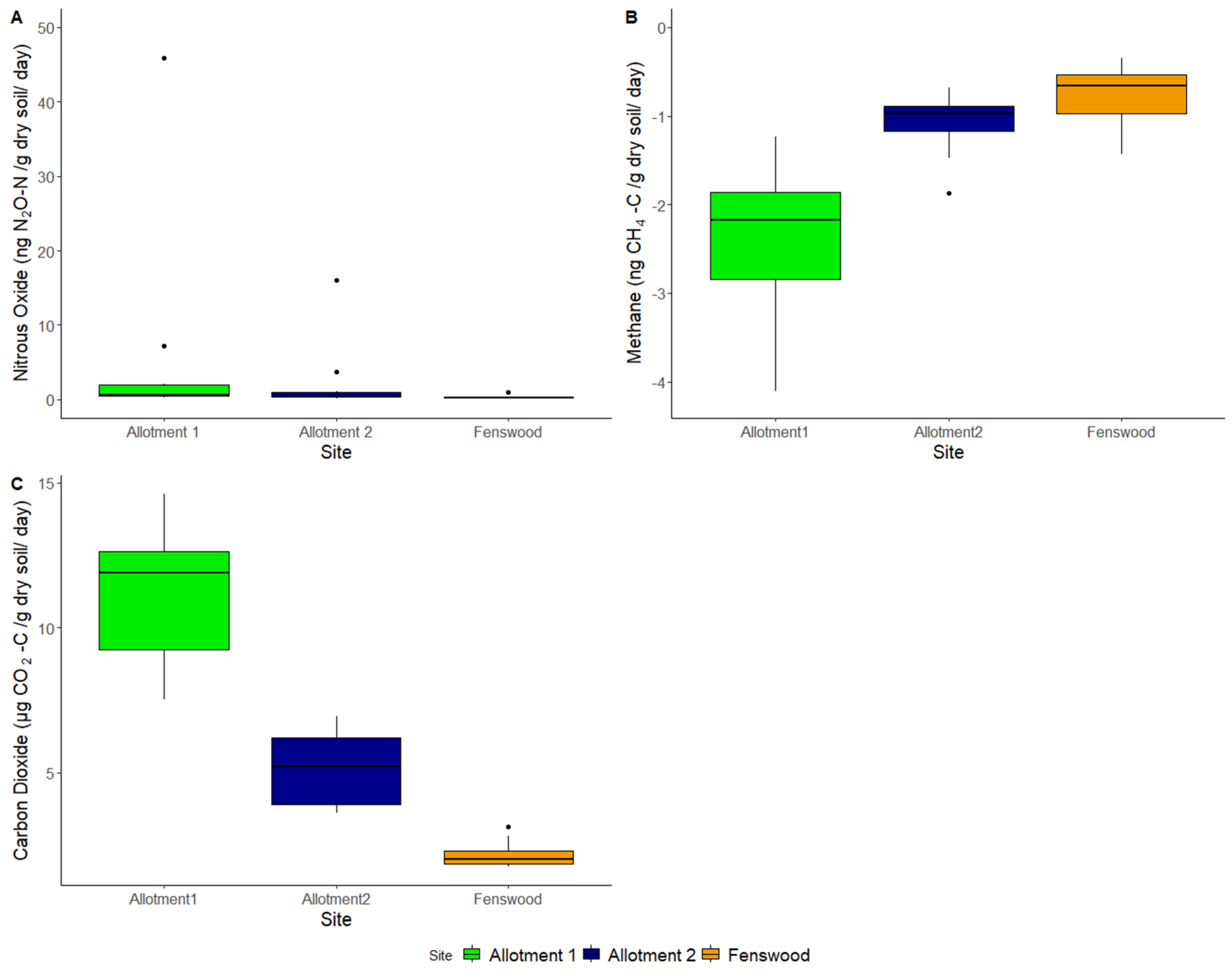
| Soil Geochemical Properties | Allotment 1: Permaculture (n = 10) | Allotment 2: Permaculture (n = 10) | Fenswood: Conventional Arable (n = 10) |
|---|---|---|---|
| Soil Type Classification | Silt | Silt loam | Silt loam |
| Soil Moisture (%) | 29.30 (0.86) A | 21.28 (1.13) B | 10.31 (0.47) C |
| Soil Temperature (°C) | 8.21 (0.09) A | 6.11 (0.15) B | 6.72 (0.02) C |
| pH | 6.93 (0.07) A | 7.39 (0.05) B | 7.57 (0.02) B |
| Total Microbial Abundance (µg g−1) | 46.67 (5.4) A | 68.23 (4.39) A | 17.99 (1.25) B |
| Bacterial Abundance (µg g−1) | 28.99 (3.27) A | 40.66 (2.55) A | 9.61 (0.65) B |
| Fungal Abundance (µg g−1) | 8.73 (1.21) A | 13.3 (0.94) B | 2.73 (0.17) C |
| Nitrogen (%) | 0.60 (0.03) A | 0.53(0.02) A | 0.16(0.001) B |
| Total Oxidised Nitrogen (Nitrate and Nitrite) (µg g−1) | 5.15(0.28) A | 3.61(0.21) B | 1.81(0.17) C |
| Ammonium (µg g−1) | 0.48(0.03) A | 0.28(0.02) A | 0.17(0.03) C |
| Phosphate (µg g−1) | 0.93(0.15) A | 1.06(0.15) B | 0.27(0.12) C |
| Organic Matter (%) | 16.48(0.88) A | 16.58(0.5) A | 5.51(0.26) B |
| Total Carbon (%) | 7.44(0.4) A | 9.33(0.31) B | 1.81(0.03) C |
| Multiple Regression Model Independent variables | Nitrous Oxide Flux | Methane Flux | Carbon Dioxide Flux |
|---|---|---|---|
| Total Carbon, TON, OM, NH4-N, Total Nitrogen, Bacterial Biomass, Fungal Biomass | 72.44% (p < 0.001 ***) | 48.9% (p < 0.01 **) | 56.92% (p < 0.01 **) |
| Durbin–Watson test (testing for autocorrelation) | 2.03 (p > 0.05 *) | 2.23 (p > 0.05 *) | 2.03 (p > 0.05 *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williamson, R.F.; Reay, M.; Sgouridis, F. Permaculture Management of Arable Soil Increases Soil Microbial Abundance, Nutrients, and Carbon Stocks Compared to Conventional Agriculture. Agronomy 2024, 14, 1446. https://doi.org/10.3390/agronomy14071446
Williamson RF, Reay M, Sgouridis F. Permaculture Management of Arable Soil Increases Soil Microbial Abundance, Nutrients, and Carbon Stocks Compared to Conventional Agriculture. Agronomy. 2024; 14(7):1446. https://doi.org/10.3390/agronomy14071446
Chicago/Turabian StyleWilliamson, Rose Frances, Michaela Reay, and Fotis Sgouridis. 2024. "Permaculture Management of Arable Soil Increases Soil Microbial Abundance, Nutrients, and Carbon Stocks Compared to Conventional Agriculture" Agronomy 14, no. 7: 1446. https://doi.org/10.3390/agronomy14071446
APA StyleWilliamson, R. F., Reay, M., & Sgouridis, F. (2024). Permaculture Management of Arable Soil Increases Soil Microbial Abundance, Nutrients, and Carbon Stocks Compared to Conventional Agriculture. Agronomy, 14(7), 1446. https://doi.org/10.3390/agronomy14071446






