How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
3. Results
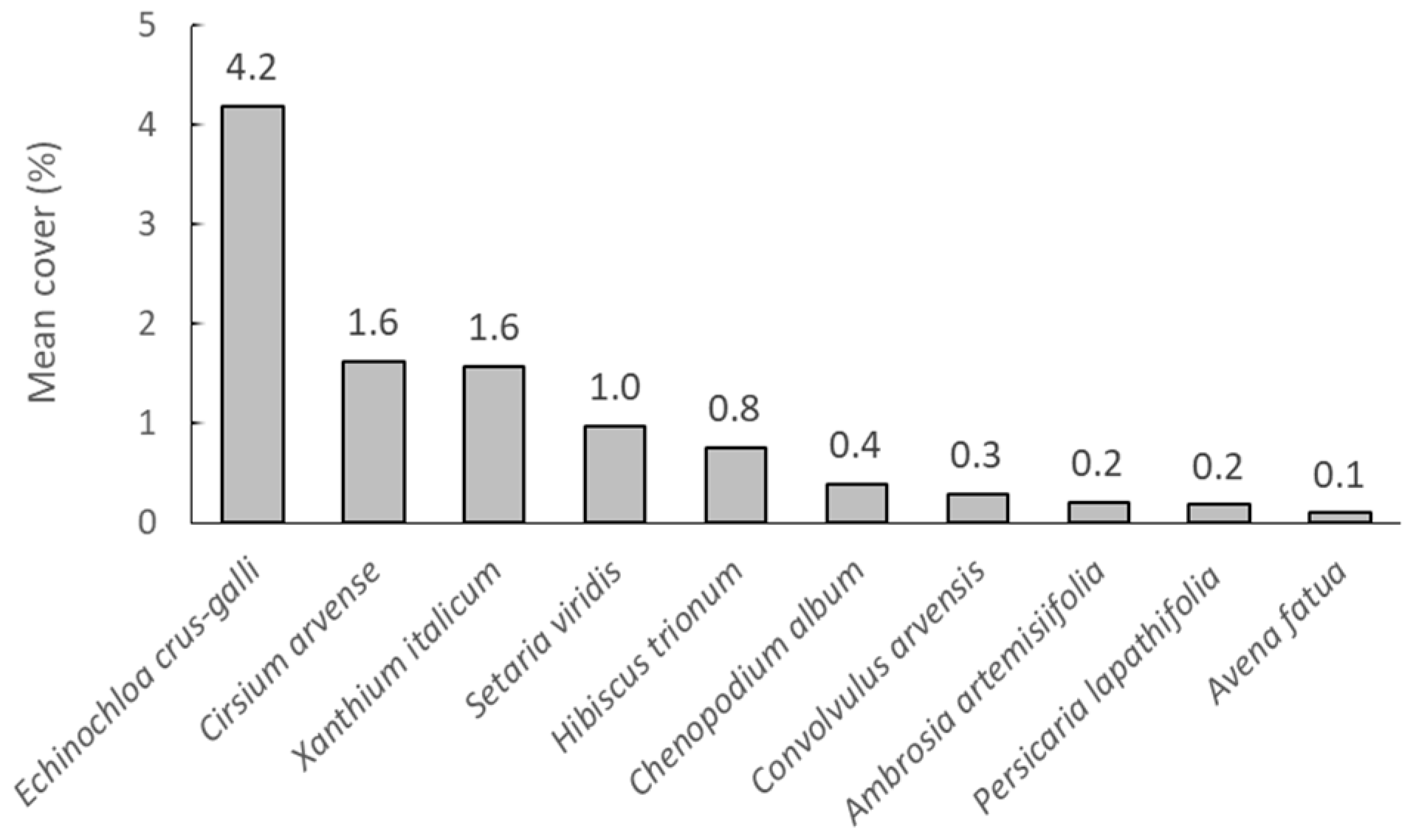
3.1. Weed Composition
3.2. Effect of Variables on Total Weed Coverage, Species Richness, Diversity, and Canary Grass Yield
3.3. Effect of Variables on Weed Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boye, J.I.; Achouri, A.; Raymond, N.; Cleroux, C.; Weber, D.; Koerner, T.B.; Hucl, P.; Patterson, C.A. Analysis of glabrous canary seeds by ELISA, mass spectrometry, and Western blotting for the absence of cross-reactivity with major plant food allergens. J. Agric. Food Chem. 2013, 61, 6102–6112. [Google Scholar] [CrossRef] [PubMed]
- Clayton, W.D.; Vorontsova, M.S.; Harman, K.T.; Williamson, H. GrassBase—The Online World Grass Flora. 2016. Available online: http://www.kew.org/data/grasses-db/index.htm (accessed on 24 March 2024).
- Abdel-Aal, E.S.M.; Hucl, P.J.; Sosulski, F.W. Structural and compositional characteristics of canaryseed (Phalaris canariensis L.). J. Agric. Food Chem. 1997, 45, 3049–3055. [Google Scholar] [CrossRef]
- Hedrick, U.P. (Ed.) Phalaris canariensis Linn. Gramineae. Canary grass. In Sturtevant’s Edible Plants of the World; Dover Publications: New York, NY, USA, 1972; p. 487. [Google Scholar]
- Baldini, R.M. The genus Phalaris L. (Gramineae) in Italy. Webbia 1993, 47, 1–53. [Google Scholar] [CrossRef]
- Cogliatti, M.; Bongiorno, F.; Dalla Valle, H.; Rogers, W.J. Canaryseed (Phalaris canariensis L.) accessions from nineteen countries show useful genetic variation for agronomic traits. Can. J. Plant Sci. 2011, 91, 37–48. [Google Scholar] [CrossRef]
- Pelikán, J. Evaluation of yields in canary grass (Phalaris canariensis L.) varieties. Rostl. Výroba 2000, 46, 471–475. [Google Scholar]
- Abdel-Aal, E.S.M.; Hucl, P.; Patterson, C.A.; Gray, D. Fractionation of hairless canary seed (Phalaris canariensis) into starch, protein, and oil. J. Agric. Food Chem. 2010, 58, 7046–7050. [Google Scholar] [CrossRef] [PubMed]
- Cogliatti, M. Canaryseed crop. Sci. Agropecu. 2012, 1, 75–88. [Google Scholar] [CrossRef]
- L’Hocine, L.; Achouri, A.; Mason, E.; Pitre, M.; Martineau-Côté, D.; Sirois, S.; Karboune, S. Assessment of protein nutritional quality of novel hairless canary seed in comparison to wheat and oat using in vitro static digestion models. Nutrients 2023, 15, 1347. [Google Scholar] [CrossRef] [PubMed]
- Rikal, L.I.; de Figueiredo, A.K.; Riccobene, I.C. Physicochemical and functional properties of canaryseed (Phalaris canariensis L.) with and without spicules flour. Cereal Cemistry 2023, 100, 904–913. [Google Scholar] [CrossRef]
- Estrada-Salas, P.A.; Montero-Morán, G.M.; Martínez-Cuevas, P.P.; González, C.; Barba de la Rosa, A.P. Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J. Agric. Food Chem. 2014, 62, 427–433. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.C.; Aguilar-Toalá, J.E.; Liceaga, A.M. Hairless canary seeds (Phalaris canariensis L.) as a potential source of antioxidant, antihypertensive, antidiabetic, and antiobesity biopeptides. Food Prod. Process. Nutr. 2021, 3, 6. [Google Scholar] [CrossRef]
- AusGrass2 (Grasses of Australia) 2015. Available online: https://ausgrass2.myspecies.info (accessed on 24 March 2024).
- POWO (Plants of the World Online) Royal Botanic Gardens: London, UK, 2020. Available online: https://powo.science.kew.org/ (accessed on 24 March 2024).
- USDA-ARS. Germplasm Resources Information Network (GRIN). National Germplasm Resources Laboratory: Beltsville, MD, USA, 2020. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysimple.aspx (accessed on 24 March 2024).
- BSBI (Botanical Society of Britain & Ireland). Online Atlas of the British and Irish Flora. 2020. Available online: https://www.brc.ac.uk/plantatlas/ (accessed on 24 March 2024).
- Verloove, F. Catalogue of neophytes in Belgium (1800–2005). Scr. Bot. Belg. 2006, 39, 1–89. [Google Scholar]
- Verloove, F. Poaceae. In Manual of the Alien Plants of Belgium; National Botanic Garden of Belgium: Meise, Belgium, 2020; Available online: http://alienplantsbelgium.be/ (accessed on 24 March 2024).
- Jóvér, J.; Czimbalmos, Á.; Fitosné Hornok, M. Canary Grass: Also Grown for Pasta and Birdseed, 2018. Available online: https://agroforum.hu/szakcikkek/novenytermesztes-szakcikkek/fenymag-vagyis-kanarikoles-tesztanak-es-madarelesegnek-termesztik/ (accessed on 24 March 2024). (In Hungarian).
- FAO (Food and Agriculture Organization). Online Database: Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 14 March 2024).
- Putnam, D.H.; Miller, P.R.; Hucl, P. Potential for production and utilization of annual canarygrass. Cereal Foods World 1996, 41, 75–83. [Google Scholar]
- PRIMAG. Fénymag (Canary grass). Online Seed Catalogue 2024. Available online: https://www.primag.hu/termekek/vetomagok/gabonafelek/fenymag (accessed on 14 March 2024). (In Hungarian).
- PFAF, Plants for a Future Database. Dawlish, UK, 2020. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Phalaris+canariensis (accessed on 24 March 2024).
- Cogliatti, M. Manejo del cultivo de alpiste (Phalaris canariensis L.). In El cultivo del Alpiste (Phalaris canariensis L.); Cogliatti, M., Ed.; Universidad Nacional del Centro de la Provincia de Buenos Aires: Azul, Argentina, 2014; pp. 38–54. (In Spanish) [Google Scholar]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Pinke, G. Ökológiai és agrotechnikai tényezők hatása a szántóföldi gyomtársulások faj- és jellegösszetételére. Bot. Közlemények 2016, 103, 249–262, (In Hungarian with English abstract). [Google Scholar] [CrossRef]
- Ford, J.F.; Norton, R.M.; Knights, S.E.; Flood, R.G. High sowing rates reduce seed weight in canary seed (Phalaris canariensis L.). In Proceedings of the 10th Australian Agronomy Conference; Rowe, B., Donaghy, D., Mendham, N., Eds.; Australian Society of Agronomy: Hobart, TAS, Australia, 2001; pp. 1–5. [Google Scholar]
- Cholango-Martinez, L.P.; Zhang, X.M.; Hucl, P.J.; Kutcher, H.R. First report of fusarium head blight, caused by Fusarium graminearum, on annual canarygrass (Phalaris canariensis) in Saskatchewan, Canada. Plant Dis. 2016, 100, 1780–1781. [Google Scholar] [CrossRef] [PubMed]
- Cholango-Martinez, L.P.; Halliday, J.R.; Hucl, P.J.; Kutcher, H.R. First report of ergot (Claviceps purpurea) on canary grass (Phalaris canariensis) in Saskatchewan, Canada. Plant Disease 2019, 103, 2682. [Google Scholar] [CrossRef]
- Cordo, H.A.; Logarzo, G.; Braun, K.; Di Iorio, O.R. Catálogo de Insectos Fitófagos de la Argentina y sus Plantas Asociadas (Catalog of Phytophagus Insects of Argentina and Their Assiciated Plants; Sociedad Entomológica Argentina: Buenos Aires, Argentina, 2004; 734p. [Google Scholar]
- NÉBIH (National Food Chain Safety Office). Növényvédő Szerek Adatbázisa (Online Database of Registered Pesticides in Hungary). Available online: https://novenyvedoszer.nebih.gov.hu/Engedelykereso/kereso (accessed on 14 March 2024).
- Holt, N.W.; Hunter, J.H. Annual canarygrass (Phalaris canariensis) tolerance and weed control following herbicide application. Weed Sci. 1987, 35, 673–677. [Google Scholar] [CrossRef]
- Nordmeyer, H.; Häusler, A. Einfluss von Bodeneigenschaften auf die Segetalflora von Ackerflächen (Impact of soil proper-ties on weed distribution within agricultural fields). J. Plant Nutr. Soil Sci. 2004, 167, 328–336. [Google Scholar] [CrossRef]
- Hashem, A. Weedsmart: Does Soil pH Affect Weed Management? 2017. Available online: https://www.graincentral.com/cropping/weedsmart-does-soil-ph-affect-weed-management (accessed on 14 March 2024).
- Repsiene, R.; Ozeraitiene, D. Manuring effect on the soil properties and crop rotation yield. Zemdirbyste 2006, 93, 199–209. [Google Scholar]
- Forcella, F. Real-time assessment of seed dormancy and seedling growth for weed management. Seed Sci. Res. 1998, 8, 201–210. [Google Scholar] [CrossRef]
- Hakansson, S. Weeds and Weed Management on Arable Land—An Ecological Approach; CABI: Cambridge, MA, USA, 2003; pp. 56–80. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Limitation of salt stress to plant growth. In Plant Toxicology; Hock, B., Elstner, C.F., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 91–224. [Google Scholar]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kaur, P.; Mahajan, G.; Randhawa, R.K.; Singh, H.; Kang, M.S. Global warming and its possible impact on agriculture in India. Adv. Agron. 2014, 123, 65–121. [Google Scholar] [CrossRef]
- Hungarian Meteorological Service: Csapadék Szélsőségek Változása (Changes in Precipitation Extremes). Available online: https://www.met.hu/eghajlat/eghajlatvaltozas/megfigyelt_hazai_valtozasok/homerseklet_es_csapadektrendek/csapadek_szelsosegek/ (accessed on 16 May 2024). (In Hungarian).
- Hungarian Meteorological Service: Hőségindexek (Heat Indices). Available online: https://www.met.hu/eghajlat/eghajlatvaltozas/megfigyelt_hazai_valtozasok/hosegindexek/ (accessed on 16 May 2024). (In Hungarian).
- Hungarian Meteorological Service: Éghajlati Körzetek Változása (Changes in Climate Zones). Available online: https://www.met.hu/eghajlat/eghajlatvaltozas/megfigyelt_hazai_valtozasok/eghajlati_korzetek_valtozasa/ (accessed on 16 May 2024). (In Hungarian).
- Patterson, D.T. Weeds in a changing climate. Weed Sci. 1995, 43, 685–700. [Google Scholar] [CrossRef]
- Patterson, D.T.; Westbrook, J.K.; Joyce, R.J.V.; Lingren, P.D.; Rogasik, J. Weeds, insects, and diseases. Clim. Chang. 1999, 43, 711–727. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Soussana, J.F.; Howden, S.M. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef]
- Weber, E.; Gut, D. A survey of weeds that are increasingly spreading in Europe. Agron. Sustain. Dev. 2005, 25, 109–121. [Google Scholar] [CrossRef]
- Clements, D.R.; DiTommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Hanzlik, K.; Gerowitt, B. Occurrence and distribution of important weed species in German winter oilseed rape fields. J. Plant Dis. Prot. 2012, 119, 107–120. [Google Scholar] [CrossRef]
- Giannini, A.; Biasutti, M.; Held, I.M.; Sobel, A.H. A global perspective on African climate. Clim. Chang. 2008, 90, 359–383. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, G.J. Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 2002, 50, 448–460. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Herms, C.P.; Cardina, J. Weed seedbank community composition in a 35-yr-old tillage and rotation experiment. Weed Sci. 2006, 54, 263–273. [Google Scholar] [CrossRef]
- Kenneth, T.J.; Norman, C.M. Weed and soil management: A balancing act. In Encyclopedia of Soils in the Environment, 2nd ed.; Goss, M.J., Oliver, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 439–449. [Google Scholar] [CrossRef]
- Auskalniene, O.; Kadziene, G.; Janusauskaite, D.; Suproniene, S. Changes in weed seed bank and flora as affected by soil tillage systems. Zemdirbyste 2018, 105, 221–226. [Google Scholar] [CrossRef]
- Búvár, G.; Hadászi, L.; Fodor, I. A forgatás nélküli talajművelés gyomszabályozási vonatkozásai (Weed control aspects of no-tillage systems). Gyak. Agrofórum 2000, 11, 90–92. (In Hungarian) [Google Scholar]
- Pekrun, C.; Claupein, W. The implication of stubble tillage for weed population dynamics in organic farming. Weed Res. 2006, 46, 414–423. [Google Scholar] [CrossRef]
- Kismányoky, A. Effect of Agrotechnical Factors to Crop Plants and Weeds. Ph.D. Thesis, University of Pannonia, Keszthely, Hungary, 2010. Available online: http://konyvtar.uni-pannon.hu/doktori/2010/Kismanyoky_Andras_theses_en.pdf (accessed on 24 March 2024).
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin, Germany, 1955; pp. 28–40. [Google Scholar]
- Hanf, M. Ackerunkräuter Europas mit ihren Keimlingen und Samen, 4th ed.; Eugen Ulmer: Stuttgart, Germany, 1999; pp. 301–321. (In German) [Google Scholar]
- Zimdahl, R. Fundamentals of Weed Science, 5th ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 260–281. [Google Scholar]
- Zalai, M.; Dorner, Z.; Kolozsvári, L.; Szalai, M. What does the precision of weed sampling of maize fields depend on? Növényvédelem 2012, 48, 451–456, (In Hungarian with English abstract). [Google Scholar]
- van der Maarel, E.; Franklin, J. Vegetation ecology: Historical notes and outline. In Weed Ecology, 2nd ed.; van ver Maarel, E., Franklin, J., Eds.; Wiley-Blackwell: Oxford, UK, 2013; pp. 1–27. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2016; pp. 342–358. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.; Heiberger, R.M. Analysis of variance, designed experiments. In Statistical Models in S, 1st ed.; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; pp. 145–194. [Google Scholar]
- Soper, H.E.; Young, A.W.; Cave, B.M.; Lee, A.; Pearson, K. On the distribution of the correlation coefficient in small samples. Appendix II to the papers of “Student” and R.A. Fisher. A co-operative study. Biometrika 1917, 11, 328–413. [Google Scholar] [CrossRef]
- Yandell, B.S. Practical Data Analysis for Designed Experiments, 1st ed.; Chapman & Hall; CRC: Boca Raton, FL, USA, 1997; pp. 86–104. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; pp. 34–50. [Google Scholar]
- Lososová, Z.; Chytry, M.; Cimalová, S.; Kropác, Z.; Otypková, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Nagy, M.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; et al. Change in the spread of common ragweed in Hungary in the light of the National Arable Weed Surveys (1947–2019). In Proceedings of the International Ragweed Society Conference, Budapest, Hungary, 8–9 September 2022. [Google Scholar] [CrossRef]
- Hakansson, S. Seasonal variation in the emergence of annual weeds—An introductory investigation in Sweden. Weed Res. 1983, 23, 313–324. [Google Scholar] [CrossRef]
- Harker, K.N.; O’Donovan, J.T. Recent weed control, weed management, and integrated weed management. Weed Technol. 2013, 27, 1–11. [Google Scholar] [CrossRef]
- Kovács, E.B.; Dorner, Z.; Csík, D.; Zalai, M. Effect of environmental, soil and management factors on weed flora of field pea in South-East Hungary. Agronomy 2023, 13, 1864. [Google Scholar] [CrossRef]
- Andersson, T.N.; Milberg, P. Weed flora and the relative importance of site, crop, crop rotation, and nitrogen. Weed Sci. 1998, 46, 30–38. [Google Scholar] [CrossRef]
- Travlos, I.; Gazoulis, I.; Kanatas, P.; Tsekoura, A.; Zannopoulos, S.; Papastylianou, P. Key factors affecting weed seeds’ germination, weed emergence, and their possible role for the efficacy of false seedbed technique as weed management practice. Front. Agron. 2020, 2, 1. [Google Scholar] [CrossRef]
- KÖVIZIG (Körös Valley District Environment and Water Directorate). Available online: http://www.kovizig.hu/ (accessed on 16 December 2023). (In Hungarian).
- Dorner, Z.; Nam, P.Q.; Szalai, M.; Zalai, M.; Keresztes, Z. Weed composition and diversity of organic and conventional maize in Jászság region, in Hungary. Herbologia 2012, 13, 75–85. [Google Scholar]
- Nam, P.Q.; Dorner, Z.; Szalai, M.; Zalai, M. Weed flora of organic and conventional maize fields in the Jászság Region, Hungary. In Proceedings of the International EU-SEA Scientific Symposium on Agricultural Research for Development (ARD) with Special Regards to Ecological Farming Systems, Can Tho, Vietnam, 26–30 August 2012; p. 42. [Google Scholar]
- Menalled, U.D.; Adeux, G.; Cordeau, S.; Smith, R.G.; Mirsky, S.B.; Ryan, M.R. Cereal rye mulch biomass and crop density affect weed suppression and community assembly in no-till planted soybean. Ecosphere 2022, 13, e4147. [Google Scholar] [CrossRef]
- Mas, M.T.; Verdu, A.M.C.; Kruk, B.C.; De Abelleyra, D.; Guglielmini, A.C.; Satorre, E.H. Weed communities of transgenic glyphosate-tolerant soyabean crops in expasture land in the southern Mesopotamic Pampas of Argentina. Weed Res. 2010, 50, 320–330. [Google Scholar] [CrossRef]
- Hanzlik, K.; Gerowitt, B. The importance of climate, site and management on weed vegetation in oilseed rape in Germany. Agric. Ecosyst. Environ. 2011, 141, 323–331. [Google Scholar] [CrossRef]
- de Mol, F.; von Redwitz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Srivastava, T.K.; Chauhan, R.S.; Lal, H. Weed dynamics and their management in sugarcane under different preceding crops and tillage systems. Indian J. Agric. Sci. 2005, 75, 256–260. [Google Scholar]
- Pinke, G.; Pál, R.W.; Tóth, K.; Karácsony, P.; Czucz, B.; Botta-Dukát, Z. Weed vegetation of poppy (Papaver somniferum) fields in Hungary: Effects of management and environmental factors on species composition. Weed Res. 2011, 51, 621–630. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- Chavan, Y.R.; Thite, S.V.; Aparadh, V.T.; Kore, B.A. Comparative mineral uptake potential of some exotic weeds from family Asteraceae. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1013–1021. [Google Scholar]
- Alda, L.M.; Gogoasa, I.; Alda, S.; Bordean, M.D.; Cristea, T.; Danci, M.; Gergen, I. Analysis of Magnesium contents in Zea mays, Beta vulgaris, Medicago sativa, Cirsium arvense and Agropyron repens. J. Hortic. For. Biotechnol. 2014, 18, 30–32. [Google Scholar]
- Steyn, N.P.; Olivier, J.; Winter, P.; Burger, S.; Nesamvuni, C. A survey of wild, green, leafy vegetables and their potential in combating micronutrient deficiencies in rural populations: Research in action. S. Afr. J. Sci. 2001, 97, 276–278. [Google Scholar]
- Lehoczky, É.; Kamuti, M.; Mazsu, N.; Tamás, J.; Sáringer-Kenyeres, D.; Gólya, G. Influence of NPK fertilization on weed flora in maize field. Agrokémia És Talajt.—Agrochem. Soil Sci. 2014, 63, 139–148. [Google Scholar] [CrossRef]
- Tóth, D.M.; Puskás, S.G.; Rohr, R.; Balázsy, S. Cadmium, copper, nickel and zinc content of ragweed (Ambrosia elatior L.) on ruderal sites. Agrokémia És Talajt.—Agrochem. Soil Sci. 2005, 54, 403–416. [Google Scholar]
- White, P.J.; Greenwood, D.J. Properties and management of cationic elements for crop growth. In Soil Conditions and Plant Growth; Gregory, P.J., Nortcliff, S., Eds.; Wiley-Blackwell: Oxford, UK, 2013; pp. 160–194. [Google Scholar]
- Barber, S.A. Soil chemistry and the availability of plant nutrients. In Chemistry in the Soil Environment; Dowdy, R.H., Ryan, J.A., Volk, V.V., Baker, D.E., Eds.; American Society of Agronomy & Soil Science Society of America: Madison, WI, USA, 1981; pp. 1–12. [Google Scholar] [CrossRef]
- Andreasen, C.; Skovgaard, I.M. Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agric. Ecosyst. Environ. 2009, 133, 61–67. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z.; Lengyel, A. The influence of environment, management and site context on species composition of summer arable weed vegetation in Hungary. Appl. Veg. Sci. 2012, 15, 136–144. [Google Scholar] [CrossRef]


| Variable (Unit) | Range/Recorded or Calculated Values |
|---|---|
| Soil factors | – |
| Soil texture (KArany) B | 53–66 |
| Soil pH (KCl) B | 4.15–7.33 |
| Soil properties (m/m %) | |
| Salt A | 0.066–0.7 |
| Humus B | 1.45–6.64 |
| CaCO3 B | 0.04–1.8 |
| Soil properties (mg kg−1) | |
| N B | 1.4–29.1 |
| P2O5 B | 31–2020 |
| K2O B | 228–880 |
| Na B | 26–252 |
| Mg | 355–1420 |
| S | 15–42 |
| Cu | 3.1–18.1 |
| Mn | 82–475 |
| Zn | 0.8–8.53 |
| Seasonality factors | |
| Year | 2017–2020 |
| Sampling date (Julian day) A | 149–202 |
| Management factors | |
| Farming B | Conventional, organic |
| Preceding crops a | |
| Wintering crops b | 0–0.7 |
| Spring row crops c | 0.1–0.7 |
| Spring dense crops A d | 0–0.7 |
| Tillage system B | Ploughing, loosening, shallow tillage |
| Tillage depth (cm) A | 15–35 |
| Sowing date (Julian day) B | 58–110 |
| Nr. of mechanical weed control applications A | 0–1 |
| Nr. of herbicide applications A | 0–1 |
| Amount of Nitrogen fertilizer (kg a.i. ha−1) A | 0–54 |
| Factor | Total Weed Coverage [%] | Species Richness | Shannon Diversity | Yield [kg ha−1] |
|---|---|---|---|---|
| p-value of ANCOVAs (Pearson correlations)/ [mean and sign. classes of Tukey tests] | ||||
| Soil texture | 0.007 (−0.11) | <0.001 (−0.49) | 0.032 (−0.20) | <0.001 (−0.18) |
| Soil reaction | 0.002 (−0.14) | <0.001 (+0.58) | 0.003 (+0.34) | <0.001 (−0.14) |
| Soil properties | ||||
| Humus | <0.001 (−0.08) | ns | 0.018 (+0.01) | <0.001 (+0.28) |
| N | <0.001 (+0.27) | 0.004 (−0.68) | <0.001 (−0.63) | <0.001 (−0.42) |
| P2O5 | <0.001 (−0.11) | ns | ns | ns |
| K2O | ns | ns | 0.018 (−0.14) | <0.001 (−0.09) |
| CaCO3 | 0.009 (−0.16) | ns | Ns | <0.001 (−0.15) |
| Na | <0.001 (−0.11) | ns | ns | <0.001 (−0.07) |
| Mg | <0.001 (−0.18) | ns | 0.036 (−0.02) | <0.001 (+0.09) |
| S | 0.001 (+0.03) | ns | ns | ns |
| Cu | <0.001 (+0.06) | ns | 0.003 (−0.12) | 0.001 (+0.06) |
| Mn | <0.001 (−0.10) | ns | ns | ns |
| Zn | ns | 0.004 (−0.14) | ns | <0.001 (+0.02) |
| Year | <0.001 [2017—6.1 a 2018—10.9 b 2019—2.4 a 2020—23.8 b] | ns | ns | <0.001 [2017—1861 b 2018—1348 a 2019—1822 b 2020—1304 a] |
| Farming conv (conventional) org (organic) | <0.001 [conv.—4.53 a org.—17.45 b] | ns | 0.031 [conv.—1.68 b org.—1.24 a] | 0.007 [conv.—1565 a org.—1620 b] |
| Preceding crops a | ||||
| Wintering crops b | 0.016 (+0.34) | ns | 0.036 (−0.29) | 0.003 (−0.35) |
| Spring row crops c | ns | ns | ns | <0.001 (−0.27) |
| Tillage system MT (minimum tillage) LO (loosening) PL (ploughing) | 0.003 [MT—0.9 a LO—11.7 b PL—10.6 b] | 0.017 [MT—8.5 b LO—7.8 a PL—8.0 ab] | 0.004 [MT—1.8 b LO—1.4 a PL—1.6 ab] | <0.001 [MT—2037 b LO—1453 a PL—1827 b] |
| Sowing date | 0.022 (+0.21) | ns | ns | ns |
| Factors | Df | Gross Effect | Net Effect | ||||
|---|---|---|---|---|---|---|---|
| Explained Variation (%) | R2adj | Explained Variation (%) | R2adj | F | p-Value | ||
| Soil S content | 1 | 13.43 | 0.104 | 5.96 | 0.060 | 3.387 | 0.030 |
| Soil Cu content | 1 | 6.38 | 0.031 | 4.86 | 0.044 | 2.761 | 0.002 |
| Soil Mg content | 1 | 4.94 | 0.017 | 3.52 | 0.025 | 1.996 | 0.032 |
| Soil Mn content | 1 | 6.98 | 0.038 | 3.87 | 0.030 | 2.195 | 0.026 |
| Soil Zn content | 1 | 5.02 | 0.017 | 3.41 | 0.024 | 1.937 | 0.045 |
| Year | 3 | 23.70 | 0.152 | 21.06 | 0.206 | 3.987 | 0.001 |
| Winter preceding crop | 1 | 6.88 | 0.037 | 5.93 | 0.060 | 3.370 | 0.003 |
| Spring row preceding crop | 1 | 4.73 | 0.014 | 3.33 | 0.022 | 1.893 | 0.042 |
| Species | Ax 1 Score | Fit | Species | Ax 1 Score | Fit |
|---|---|---|---|---|---|
| Soil S content (+ high; − low) | Soil Mn content (+ high; − low) | ||||
| Xanthium strumarium | 0.321 | 0.275 | Cirsium arvense | 0.227 | 0.095 |
| Convolvulus arvensis | 0.112 | 0.057 | Hibiscus trionum | 0.098 | 0.033 |
| Ambrosia artemisiifolia | 0.104 | 0.096 | Sinapis arvensis | 0.086 | 0.144 |
| Persicaria lapathifolia | 0.101 | 0.092 | Abutilon theophrasti | 0.076 | 0.142 |
| Calystegia sepium | 0.094 | 0.084 | Datura stramonium | 0.050 | 0.057 |
| Amaranthus retroflexus | 0.082 | 0.069 | Avena fatua | 0.037 | 0.025 |
| Sonchus asper | 0.059 | 0.101 | Sonchus asper | −0.067 | 0.130 |
| Sinapis arvensis | −0.050 | 0.048 | Ambrosia artemisiifolia | −0.107 | 0.103 |
| Setaria viridis | −0.166 | 0.077 | Calystegia sepium | −0.109 | 0.112 |
| Echinochloa crus-galli | −0.185 | 0.063 | Xanthium strumarium | −0.159 | 0.067 |
| Soil Cu content (+ high; − low) | Soil Zn content (+ high; − low) | ||||
| Xanthium strumarium | 0.146 | 0.057 | Xanthium strumarium | 0.168 | 0.075 |
| Triticum spelta | 0.043 | 0.134 | Convolvulus arvensis | 0.147 | 0.098 |
| Sonchus asper | 0.034 | 0.032 | Sonchus asper | 0.060 | 0.103 |
| Avena fatua | −0.028 | 0.015 | Ambrosia artemisiifolia | 0.045 | 0.018 |
| Datura stramonium | −0.045 | 0.046 | Calystegia sepium | 0.042 | 0.017 |
| Calystegia sepium | −0.049 | 0.023 | Triticum spelta | 0.029 | 0.062 |
| Abutilon theophrasti | −0.071 | 0.125 | Sinapis arvensis | −0.045 | 0.040 |
| Amaranthus retroflexus | −0.082 | 0.068 | Abutilon theophrasti | −0.047 | 0.056 |
| Hibiscus trionum | −0.241 | 0.203 | Helianthus annuus | −0.118 | 0.040 |
| Helianthus annuus | −0.264 | 0.199 | Hibiscus trionum | −0.194 | 0.131 |
| Soil Mg content (+ high; − low) | |||||
| Hibiscus trionum | 0.241 | 0.202 | |||
| Helianthus annuus | 0.121 | 0.042 | |||
| Chenopodium album | 0.077 | 0.019 | |||
| Avena fatua | 0.059 | 0.065 | |||
| Setaria viridis | 0.055 | 0.008 | |||
| Calystegia sepium | 0.049 | 0.023 | |||
| Sinapis arvensis | 0.024 | 0.011 | |||
| Triticum spelta | −0.029 | 0.060 | |||
| Xanthium strumarium | −0.068 | 0.012 | |||
| Echinochloa crus-galli | −0.187 | 0.065 | |||
| Species | Ax 1 Score | Fit | Species | Ax 1 Score | Fit |
|---|---|---|---|---|---|
| 2017 (+ high; − low) | 2018 (+ high; − low) | ||||
| Trifolium repens | 0.208 | 0.365 | Persicaria lapathifolia | 0.186 | 0.317 |
| Calystegia sepium | 0.136 | 0.174 | Chenopodium album | 0.155 | 0.079 |
| Sinapis arvensis | 0.097 | 0.186 | Echinochloa crus-galli | 0.147 | 0.040 |
| Sonchus asper | 0.073 | 0.153 | Setaria viridis | 0.130 | 0.047 |
| Datura stramonium | −0.054 | 0.065 | Amaranthus retroflexus | 0.053 | 0.029 |
| Avena fatua | −0.075 | 0.105 | Abutilon theophrasti | 0.045 | 0.051 |
| Persicaria lapathifolia | −0.080 | 0.058 | Datura stramonium | −0.045 | 0.045 |
| Amaranthus retroflexus | −0.096 | 0.094 | Trifolium repens | −0.080 | 0.054 |
| Chenopodium album | −0.137 | 0.062 | Helianthus annuus | −0.183 | 0.095 |
| Echinochloa crus-galli | −0.450 | 0.376 | Cirsium arvense | −0.273 | 0.138 |
| 2019 (+ high; − low) | 2020 (+ high; − low) | ||||
| Cirsium arvense | 0.256 | 0.121 | Echinochloa crus-galli | 0.438 | 0.357 |
| Hibiscus trionum | 0.235 | 0.191 | Avena fatua | 0.149 | 0.421 |
| Helianthus annuus | 0.202 | 0.117 | Datura stramonium | 0.063 | 0.089 |
| Chenopodium album | 0.136 | 0.061 | Triticum spelta | −0.029 | 0.062 |
| Amaranthus retroflexus | 0.134 | 0.182 | Trifolium repens | −0.087 | 0.064 |
| Triticum spelta | 0.038 | 0.106 | Amaranthus retroflexus | −0.089 | 0.080 |
| Persicaria lapathifolia | −0.055 | 0.027 | Hibiscus trionum | −0.121 | 0.051 |
| Calystegia sepium | −0.058 | 0.032 | Chenopodium album | −0.146 | 0.070 |
| Echinochloa crus-galli | −0.129 | 0.031 | Helianthus annuus | −0.147 | 0.062 |
| Setaria viridis | −0.174 | 0.084 | Cirsium arvense | −0.156 | 0.045 |
| Species | Ax 1 Score | Fit | Species | Ax 1 Score | Fit |
|---|---|---|---|---|---|
| Wintering preceding crop (+ high; − low) | Spring row preceding crop (+ high; − low) | ||||
| Cirsium arvense | 0.265 | 0.130 | Cirsium arvense | 0.157 | 0.045 |
| Echinochloa crus-galli | 0.120 | 0.027 | Hibiscus trionum | 0.144 | 0.072 |
| Hibiscus trionum | 0.109 | 0.041 | Convolvulus arvensis | 0.108 | 0.053 |
| Sinapis arvensis | 0.072 | 0.101 | Xanthium strumarium | 0.078 | 0.016 |
| Triticum spelta | 0.023 | 0.040 | Ambrosia artemisiifolia | 0.073 | 0.048 |
| Calystegia sepium | −0.087 | 0.072 | Avena fatua | 0.073 | 0.100 |
| Helianthus annuus | −0.089 | 0.023 | Persicaria lapathifolia | 0.029 | 0.008 |
| Ambrosia artemisiifolia | −0.125 | 0.138 | Triticum spelta | 0.023 | 0.039 |
| Xanthium strumarium | −0.126 | 0.042 | Setaria viridis | −0.070 | 0.014 |
| Setaria viridis | −0.265 | 0.197 | Echinochloa crus-galli | −0.214 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorner, Z.; Kovács, E.B.; Iványi, D.; Zalai, M. How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields. Agronomy 2024, 14, 1169. https://doi.org/10.3390/agronomy14061169
Dorner Z, Kovács EB, Iványi D, Zalai M. How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields. Agronomy. 2024; 14(6):1169. https://doi.org/10.3390/agronomy14061169
Chicago/Turabian StyleDorner, Zita, Endre Béla Kovács, Dóra Iványi, and Mihály Zalai. 2024. "How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields" Agronomy 14, no. 6: 1169. https://doi.org/10.3390/agronomy14061169
APA StyleDorner, Z., Kovács, E. B., Iványi, D., & Zalai, M. (2024). How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields. Agronomy, 14(6), 1169. https://doi.org/10.3390/agronomy14061169





