Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Installation and Conduction of the Experiment
2.2. Growth Condition
2.3. Experimental Design
2.4. Determination of Dry Matter
2.5. Carbon, Nitrogen, Phosphorus, and Silicon Analysis
2.6. Statistical Analysis
3. Results
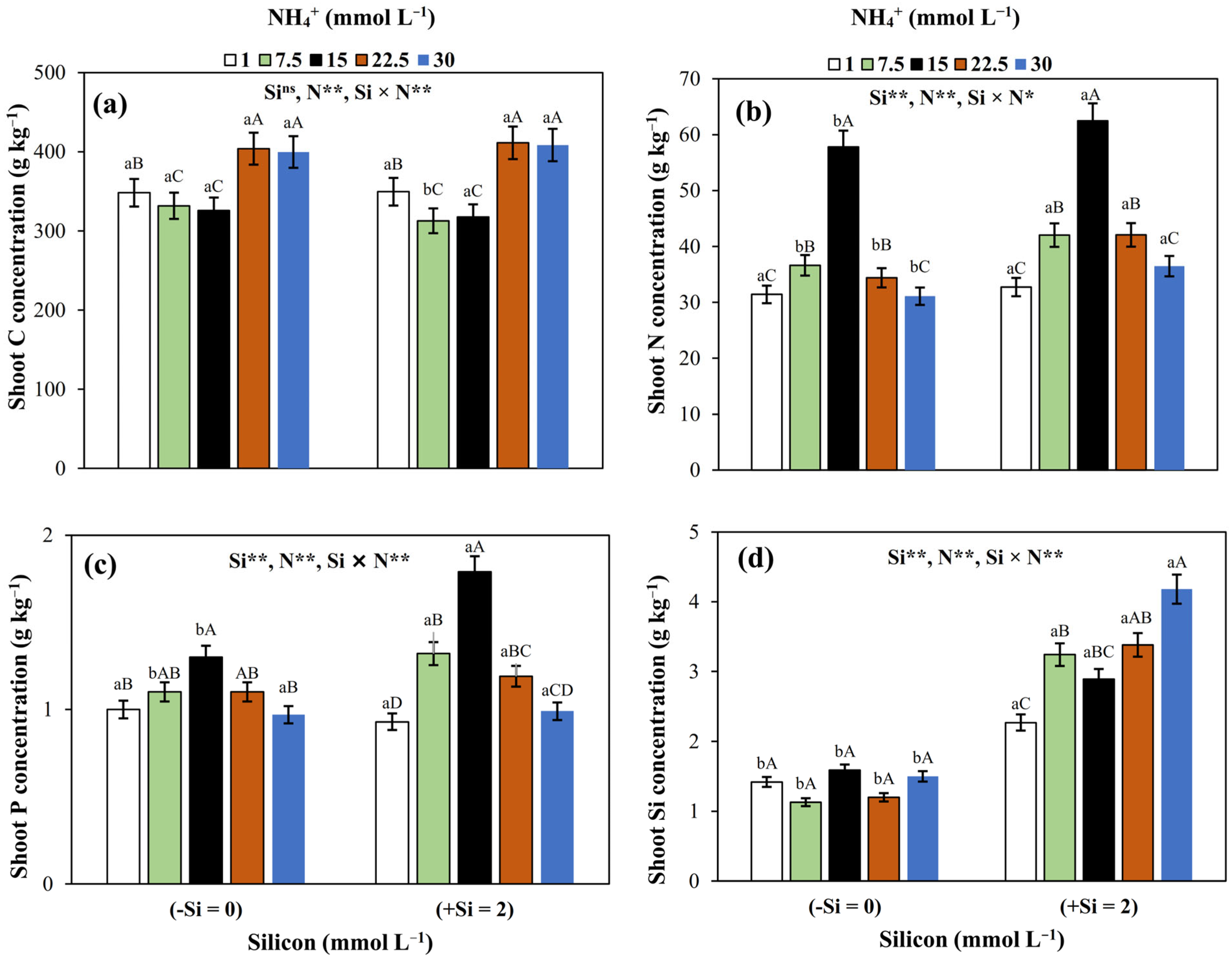
3.1. Nutrient (C, N, P, and Si) Concentrations in Shoots
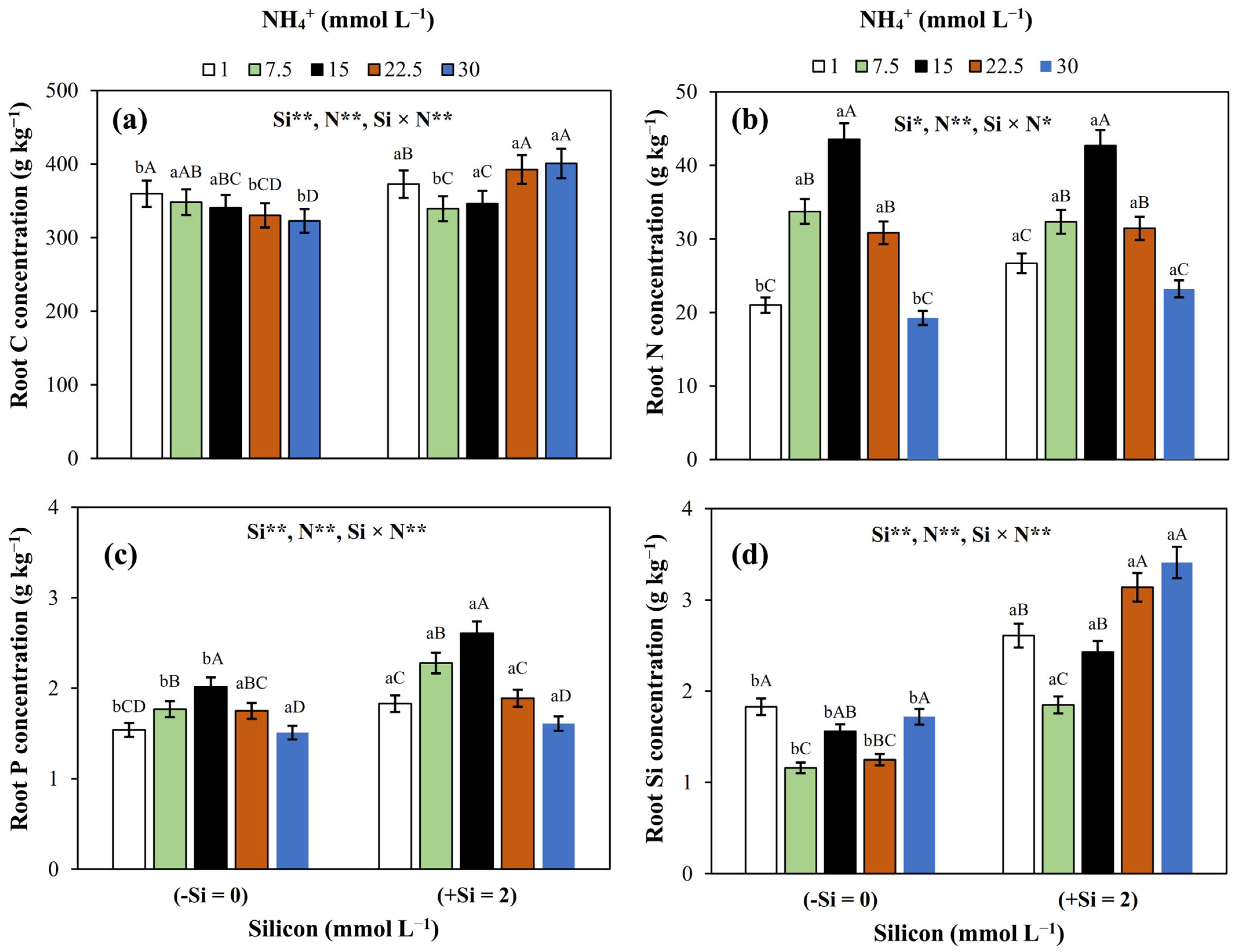
3.2. Nutrient (C, N, P, and Si) Concentrations in Roots
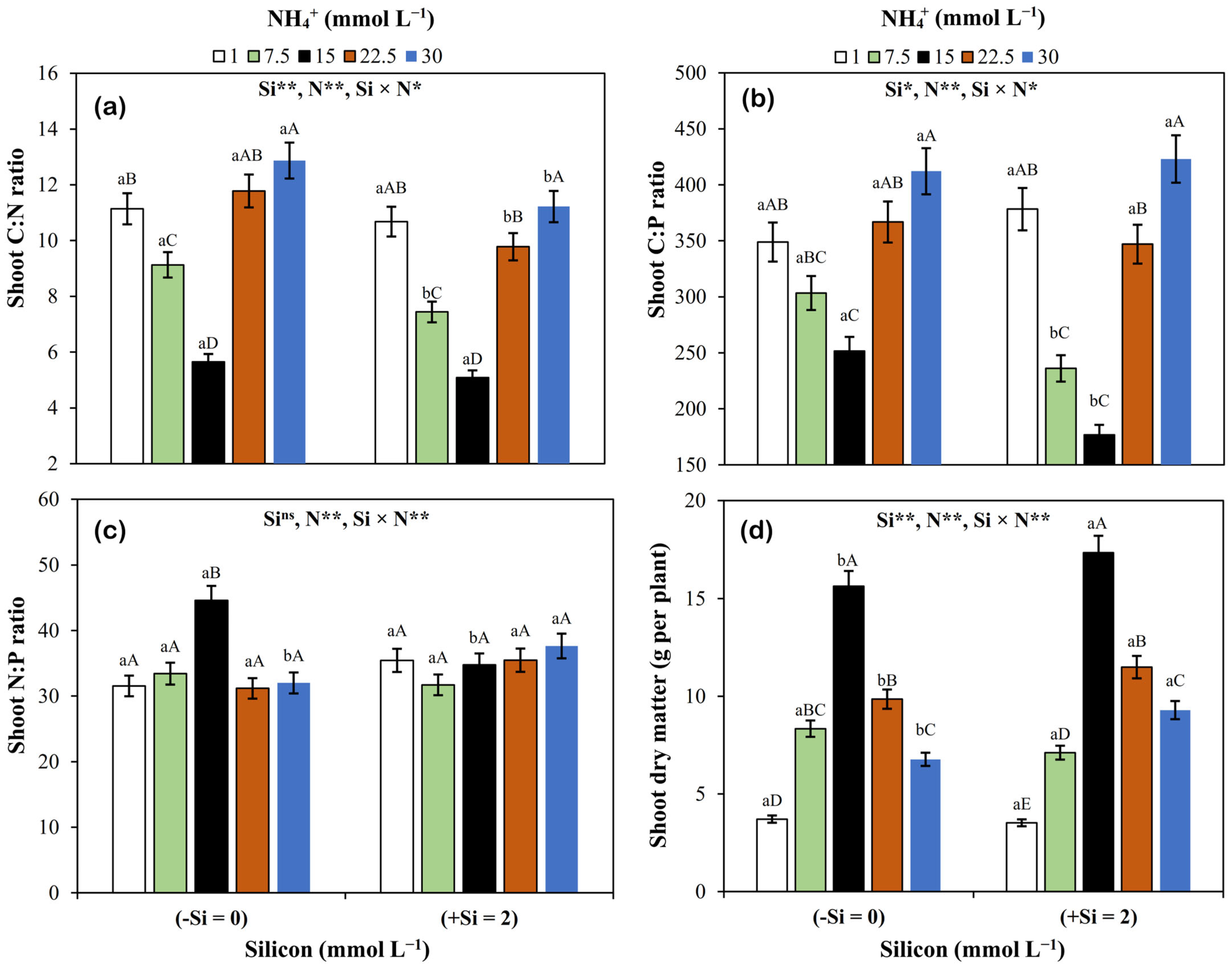
3.3. Dry Matter and C:N, C:P, and N:P Ratios in Shoots
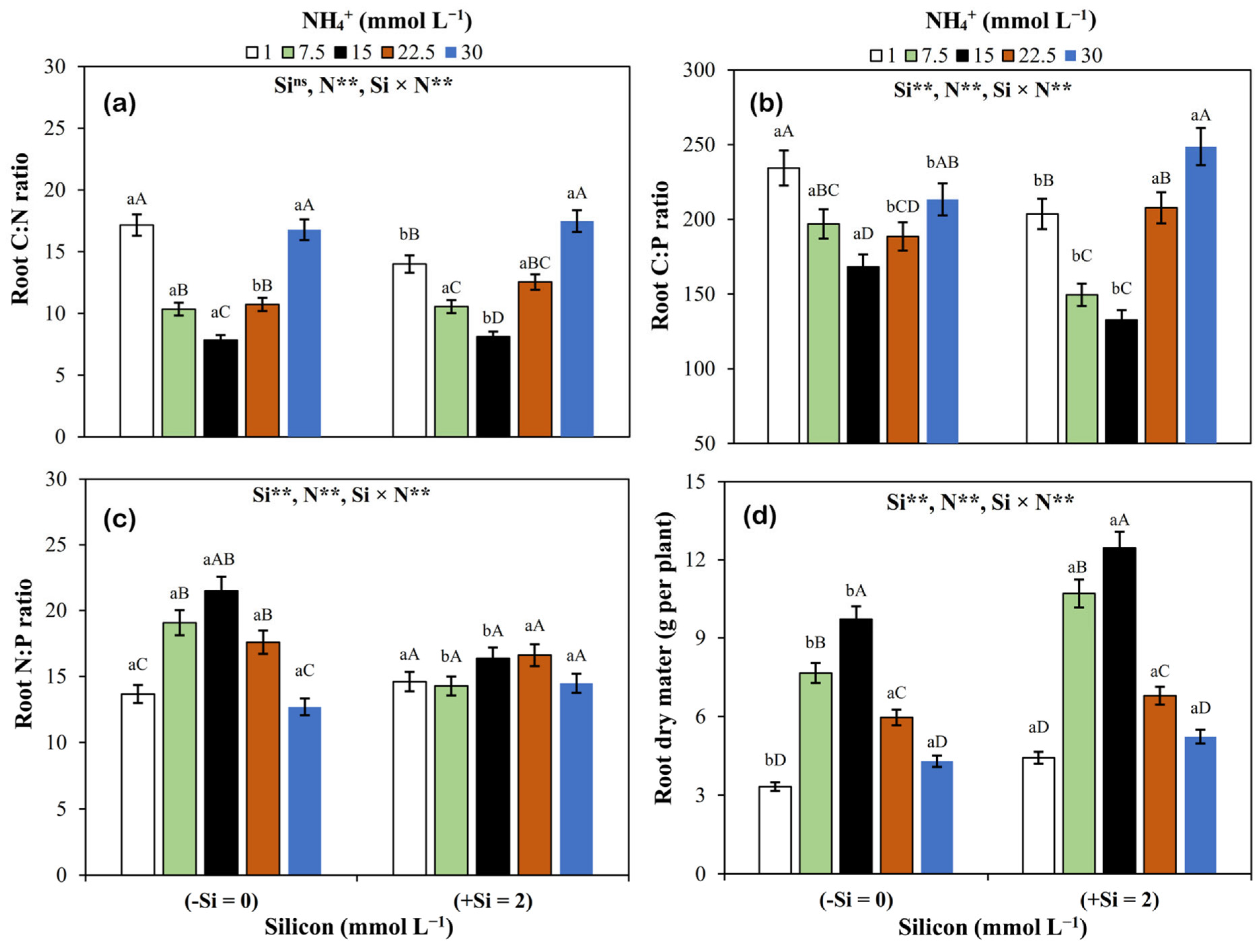
3.4. Dry Matter and C:N, C:P, and N:P Ratios in Roots
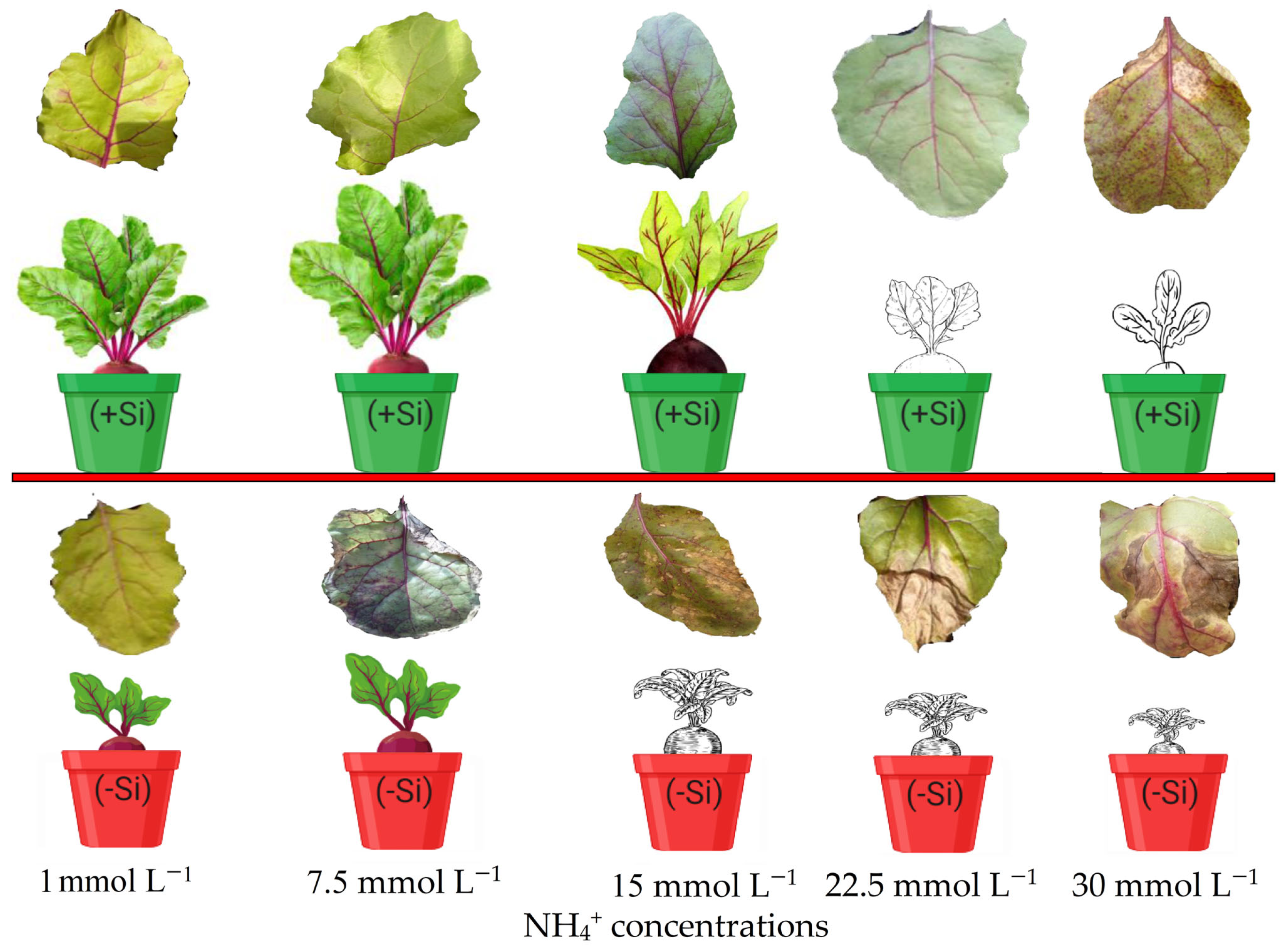
3.5. Visual Evidence of Si-Mediated Suppression of NH4+ Toxicity in Beetroot
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prado, R. Nutrição Mineral de Plantas Tropicais; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-71262-4. [Google Scholar]
- FAO FAOSTAT. Available online: https://fenix.fao.org/faostat/internal/en/#data/RFN (accessed on 6 April 2024).
- de Mello Prado, R. Introduction to Plant Nutrition. In Mineral Nutrition of Tropical Plants; Springer: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Olivera-Viciedo, D.; Mello Prado, R.; Lizcano Toledo, R.; Salas Aguilar, D.; Claudio Nascimento dos Santos, L.; Calero Hurtado, A.; Peña Calzada, K.; Betancourt Aguilar, C. Physiological Role of Silicon in Radish Seedlings under Ammonium Toxicity. J. Sci. Food Agric. 2020, 100, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Noguchi, K. Integrative Response of Plant Mitochondrial Electron Transport Chain to Nitrogen Source. Plant Cell Rep. 2011, 30, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming Ammonium Toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Viciedo, D.; de Mello Prado, R.; Lizcano Toledo, R.; dos Santos, L.; Calero Hurtado, A.; Nedd, L.; Castellanos, L. Silicon Supplementation Alleviates Ammonium Toxicity in Sugar Beet (Beta vulgaris L.). J. Soil Sci. Plant Nutr. 2019, 19, 413–419. [Google Scholar] [CrossRef]
- Olivera-Viciedo, D.; de Mello Prado, R.; Lizcano Toledo, R.; Nascimento dos Santos, L.C.; Peña Calzada, K. Respuesta de Las Plántulas Del Rábano (Raphanus sativus L.) a Diferentes Concentraciones de Nitrógeno Amoniacal En Ausencia y Presencia de Silicio. Agron. Colomb. 2017, 35, 198–204. [Google Scholar] [CrossRef]
- Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Cruz, C.; Martins-Loução, M.A.; Moran, J.F. Nitrogen Nutrition and Antioxidant Metabolism in Ammonium-Tolerant and -Sensitive Plants. Physiol. Plant. 2008, 132, 359–369. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological Significance and Complexity of N-Source Preference in Plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium Phytotoxicity and Tolerance: An Insight into Ammonium Nutrition to Improve Crop Productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The Challenge of Improving Nitrogen Use Efficiency in Crop Plants: Towards a More Central Role for Genetic Variability and Quantitative Genetics within Integrated Approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- de Souza Junior, J.P.; de Mello Prado, R.; chagas Barros de Morais, T.; Frazão, J.J.; dos Santos Sarah, M.M.; de Oliveira, K.R.; de Paula, R.C. Silicon Fertigation and Salicylic Acid Foliar Spraying Mitigate Ammonium Deficiency and Toxicity in Eucalyptus spp. Clonal Seedlings. PLoS ONE 2021, 16, e0250436. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon With Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.F.; Schiavon Júnior, A.A.; Maggio, M.A.; de Mello Prado, R. Silicon Alleviates Ammonium Toxicity in Cauliflower and in Broccoli. Sci. Hortic. 2017, 225, 743–750. [Google Scholar] [CrossRef]
- Campos, C.N.S.; da Silva Júnior, G.B.; de Mello Prado, R.; de David, C.H.O.; de Souza Junior, J.P.; Teodoro, P.E. Silicon Mitigates Ammonium Toxicity in Plants. Agron. J. 2020, 112, 635–647. [Google Scholar] [CrossRef]
- Xiao, C.; Fang, Y.; Wang, S.; He, K. The Alleviation of Ammonium Toxicity in Plants. J. Integr. Plant Biol. 2023, 65, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Nikolic, M.; Ye, M.; Xiao, Z.; Liang, Y. Silicon Acquisition and Accumulation in Plant and Its Significance for Agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon Mitigates Biotic Stresses in Crop Plants: A Review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Rocha, J.R.; de Mello Prado, R.; de Cássia Piccolo, M. New Outcomes on How Silicon Enables the Cultivation of Panicum Maximum in Soil with Water Restriction. Sci. Rep. 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Calero-Hurtado, A.; Chiconato, D.A.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Gratão, P.L.; Felisberto, G.; Olivera Viciedo, D.; Mathias dos Santos, D.M. Different Methods of Silicon Application Attenuate Salt Stress in Sorghum and Sunflower by Modifying the Antioxidative Defense Mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef] [PubMed]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon Improves the Growth of Cucumber under Excess Nitrate Stress by Enhancing Nitrogen Assimilation and Chlorophyll Synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.C.M.; de Mello Prado, R.; Rocha, A.M.S.; de Cássia Piccolo, M. Root- and Foliar-Applied Silicon Modifies C: N: P Ratio and Increases the Nutritional Efficiency of Pre-Sprouted Sugarcane Seedlings under Water Deficit. PLoS ONE 2020, 15, e0240847. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Gessner, M.O.; Bäuker, E.; Dudel, E.G. Silicon Supply Modifies C:N:P Stoichiometry and Growth of Phragmites Australis. Plant Biol. 2012, 14, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Calero-Hurtado, A.; Aparecida Chiconato, D.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Felisberto, G. Silicon Attenuates Sodium Toxicity by Improving Nutritional Efficiency in Sorghum and Sunflower Plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yu, Y.; Baerson, S.R.; Song, Y.; Liang, G.; Ding, C.; Niu, J.; Pan, Z.; Zeng, R. Interactions between Nitrogen and Silicon in Rice and Their Effects on Resistance toward the Brown Planthopper Nilaparvata Lugens. Front. Plant Sci. 2017, 8, 216375. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; Damatta, F.M. Silicon Nutrition Increases Grain Yield, Which, in Turn, Exerts a Feed-Forward Stimulation of Photosynthetic Rates via Enhanced Mesophyll Conductance and Alters Primary Metabolism in Rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Sediyama, M.A.N.; Santos, M.R.; Vidigal, S.M.; Salgado, L.T. Produtividade e Exportação de Nutrientes Em Beterraba Cultivada Com Cobertura Morta e Adubação Orgânica. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 883–889. [Google Scholar] [CrossRef]
- Biondo, P.B.F.; Boeing, J.S.; Barizão, É.O.; de Souza, N.E.; Matsushita, M.; de Oliveira, C.C.; Boroski, M.; Visentainer, J.V. Evaluation of Beetroot (Beta vulgaris L.) Leaves during Its Developmental Stages: A Chemical Composition Study. Food Sci. Technol. 2014, 34, 94–101. [Google Scholar] [CrossRef]
- Sinta, Z.; Garo, G. Influence of Plant Density and Nitrogen Fertilizer Rates on Yield and Yield Components of Beetroot (Beta vulgaris L.). Int. J. Agron. 2021, 2021, 6670243. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 0691074909. [Google Scholar]
- Huang, J.; Wang, P.; Niu, Y.; Yu, H.; Ma, F.; Xiao, G.; Xu, X. Changes in C:N:P Stoichiometry Modify N and P Conservation Strategies of a Desert Steppe Species Glycyrrhiza Uralensis. Sci. Rep. 2018, 8, 12668. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Viciedo, D.; de Mello Prado, R.; Martinez, C.A.; Habermann, E.; de Cássia Piccolo, M.; Calero Hurtado, A.; Barreto, R.F.; Peña Calzada, K. Changes in Soil Water Availability and Air-Temperature Impact Biomass Allocation and C:N:P Stoichiometry in Different Organs of Stylosanthes Capitata Vogel. J. Environ. Manag. 2021, 278, 111540. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological Stoichiometry of Plant Production: Metabolism, Scaling and Ecological Response to Global Change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Olivera-Viciedo, D.; de Mello Prado, R.; Martínez, C.A.; Habermann, E.; de Cássia Piccolo, M. Short-Term Warming and Water Stress Affect Panicum Maximum Jacq. Stoichiometric Homeostasis and Biomass Production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef]
- Calero-Hurtado, A.; Olivera-Viciedo, D.; Mello Prado, R. Beneficial Role of Silicon in Plant Nutrition Under Salinity Conditions. In Benefits of Silicon in the Nutrition of Plants; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 253–274. ISBN 9783031266737. [Google Scholar]
- Olivera-Viciedo, D.; Oliveira, K.S.; de Mello Prado, R.; Habermann, E.; Martínez, C.A.; de Moura Zanine, A. Silicon Uptake and Utilization on Panicum Maximum Grass Modifies C:N:P Stoichiometry under Warming and Soil Water Deficit. Soil Tillage Res. 2024, 235, 105884. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon Improves Water Use Efficiency in Maize Plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Pereira, H.S.; Nolla, A. Análise de Silício No Solo, Planta e Fertilizantes, 2nd ed.; UFU: Uberlândia, Brazil, 2004. [Google Scholar]
- Bataglia, O.; Teixeira, J.; Furlani, P.; Furlani, A. Métodos de Análise Química de Plantas; IAC: Campina, Romania, 1983. [Google Scholar]
- Barbosa, J.; Maldonado Junior, W. Experimentação Agronômica e AgroEstat: Sistema Para Análises Estatísticas de Ensaios Agronômicos; Universidade Estadual Paulista “Júlio de Mesquita Filho”: Jaboticabal, Brazil, 2015. [Google Scholar]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate Ammonium: NItrate Alleviates Low Light Intensity Stress in Mini Chinese Cabbage Seedling by Regulating Root Architecture and Photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Di, D.W.; Sun, L.; Zhang, X.; Li, G.; Kronzucker, H.J.; Shi, W. Involvement of Auxin in the Regulation of Ammonium Tolerance in Rice (Oryza sativa L.). Plant Soil 2018, 432, 373–387. [Google Scholar] [CrossRef]
- Calero-Hurtado, A.; Chiconato, D.A.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Olivera Viciedo, D.; de Cássia Piccolo, M. Silicon Application Induces Changes C:N:P Stoichiometry and Enhances Stoichiometric Homeostasis of Sorghum and Sunflower Plants under Salt Stress. Saudi J. Biol. Sci. 2020, 27, 3711–3719. [Google Scholar] [CrossRef]
- Calero-Hurtado, A.; Chiconato, D.A.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Viciedo, D.O.; Díaz, Y.P.; Calzada, K.P.; Gratão, P.L. Silicon Alleviates Sodium Toxicity in Sorghum and Sunflower Plants by Enhancing Ionic Homeostasis in Roots and Shoots and Increasing Dry Matter Accumulation. Silicon 2021, 13, 475–486. [Google Scholar] [CrossRef]
- Cruz, C.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Bio, A.; Martins-Loução, M.A.; Moran, J.F. Intra-Specific Variation in Pea Responses to Ammonium Nutrition Leads to Different Degrees of Tolerance. Environ. Exp. Bot. 2011, 70, 233–243. [Google Scholar] [CrossRef]
- Peña-Calzada, K.; Olivera-Viciedo, D.; Calero-Hurtado, A.; De, R.; Prado, M.; Habermann, E.; Felipe, L.; Tenesaca, L.; Ajila, G.; De Oliveira, R.; et al. Silicon Mitigates the Negative Impacts of Salt Stress in Soybean Plants. J. Sci. Food Agric. 2023, 103, 4360–4370. [Google Scholar] [CrossRef]
- Peña-Calzada, K.; Calero Hurtado, A.; Olivera Viciedo, D.; Habermann, E.; de Mello Prado, R.; de Oliveira, R.; Ajila, G.; Tenesaca, L.F.L.; Rodríguez, J.C.; Gratão, P.L. Regulatory Role of Silicon on Growth, Potassium Uptake, Ionic Homeostasis, Proline Accumulation, and Antioxidant Capacity of Soybean Plants Under Salt Stress. J. Plant Growth Regul. 2023, 42, 4528–4540. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera-Viciedo, D.; Salas Aguilar, D.; de Mello Prado, R.; Peña Calzada, K.; Calero Hurtado, A.; de Cássia Piccolo, M.; Bomfim Soares, M.; Lizcano Toledo, R.; Alves, G.R.; Ferreira, D.; et al. Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress. Agronomy 2024, 14, 1104. https://doi.org/10.3390/agronomy14061104
Olivera-Viciedo D, Salas Aguilar D, de Mello Prado R, Peña Calzada K, Calero Hurtado A, de Cássia Piccolo M, Bomfim Soares M, Lizcano Toledo R, Alves GR, Ferreira D, et al. Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress. Agronomy. 2024; 14(6):1104. https://doi.org/10.3390/agronomy14061104
Chicago/Turabian StyleOlivera-Viciedo, Dilier, Daimy Salas Aguilar, Renato de Mello Prado, Kolima Peña Calzada, Alexander Calero Hurtado, Marisa de Cássia Piccolo, Mariana Bomfim Soares, Rodolfo Lizcano Toledo, Guilherme Ribeiro Alves, Daniele Ferreira, and et al. 2024. "Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress" Agronomy 14, no. 6: 1104. https://doi.org/10.3390/agronomy14061104
APA StyleOlivera-Viciedo, D., Salas Aguilar, D., de Mello Prado, R., Peña Calzada, K., Calero Hurtado, A., de Cássia Piccolo, M., Bomfim Soares, M., Lizcano Toledo, R., Alves, G. R., Ferreira, D., Rodrigues, R., & de Moura Zanine, A. (2024). Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress. Agronomy, 14(6), 1104. https://doi.org/10.3390/agronomy14061104











