Light Intensity Affects Growth and Nutrient Value of Hydroponic Barley Fodder
Abstract
1. Introduction
2. Materials and Methods
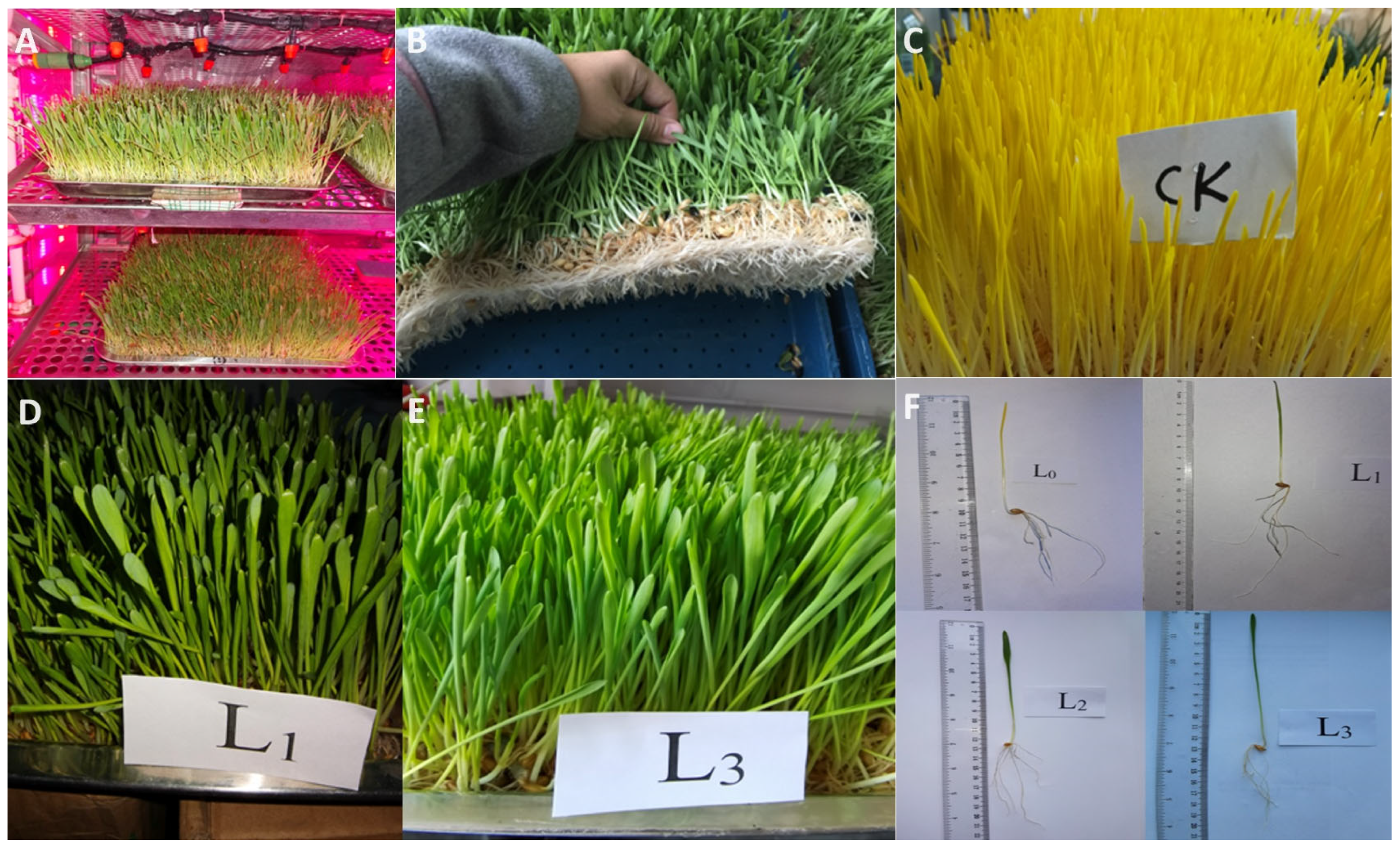
2.1. The Design of the Controllable Environmental Cultivation System with Different Light Conditions
2.2. The Seed Germination
2.3. Measurement of Biomass and Nutritional Quality
2.4. Photosynthetic Characteristics
2.5. Statistical Analysis
3. Results
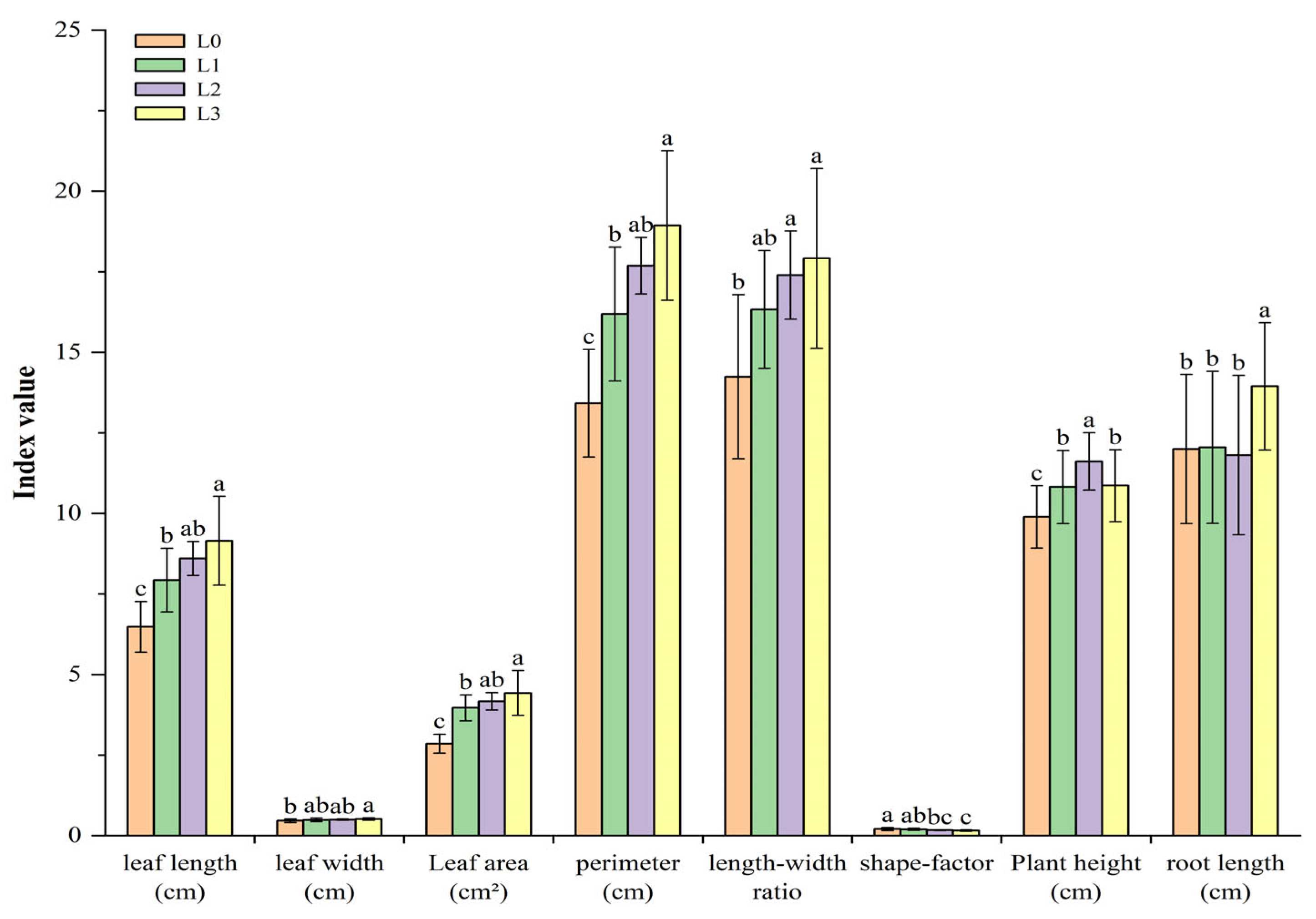
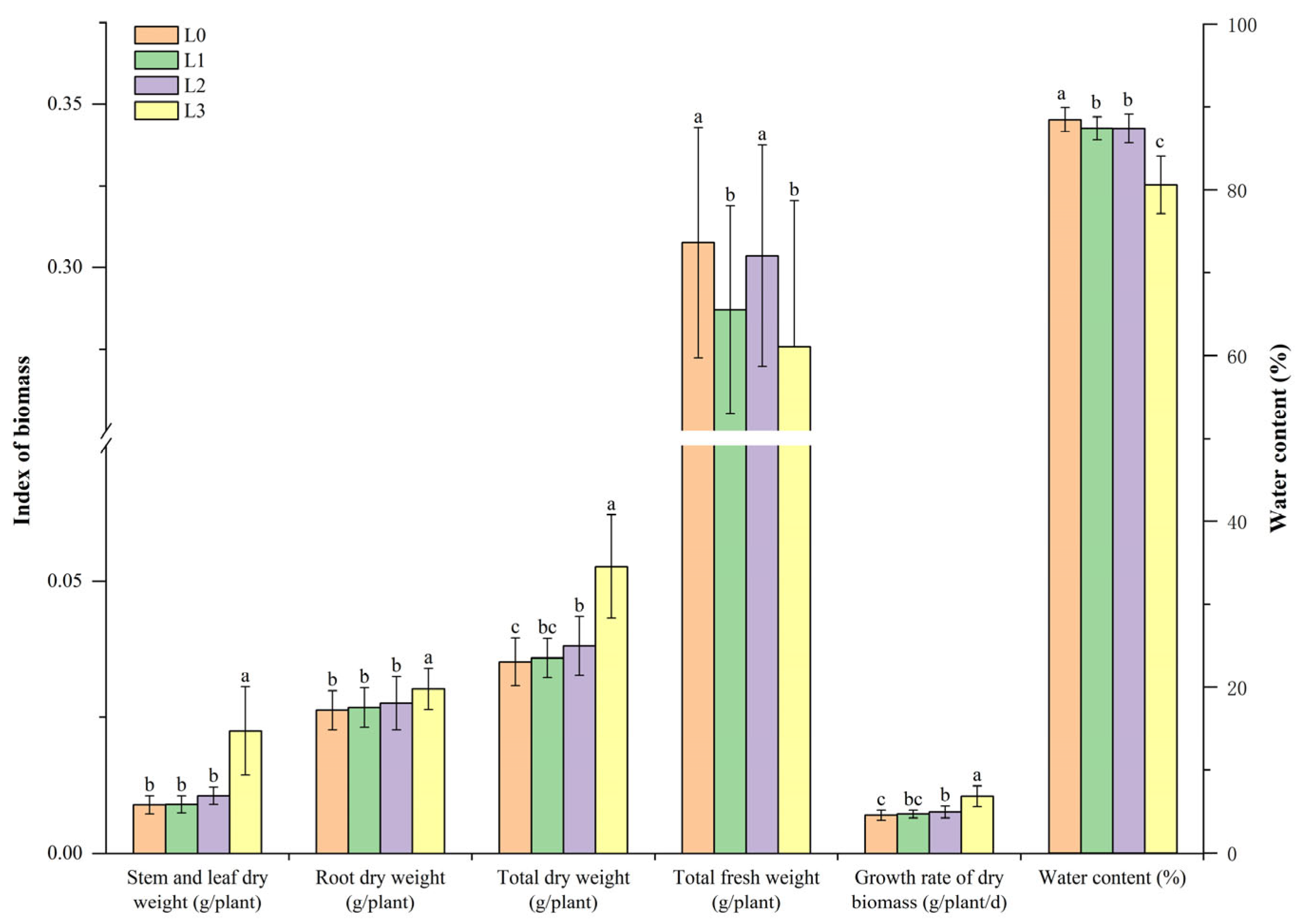
3.1. The Effects of Different Light Intensities on the Growth of Hydroponic Barley Fodder
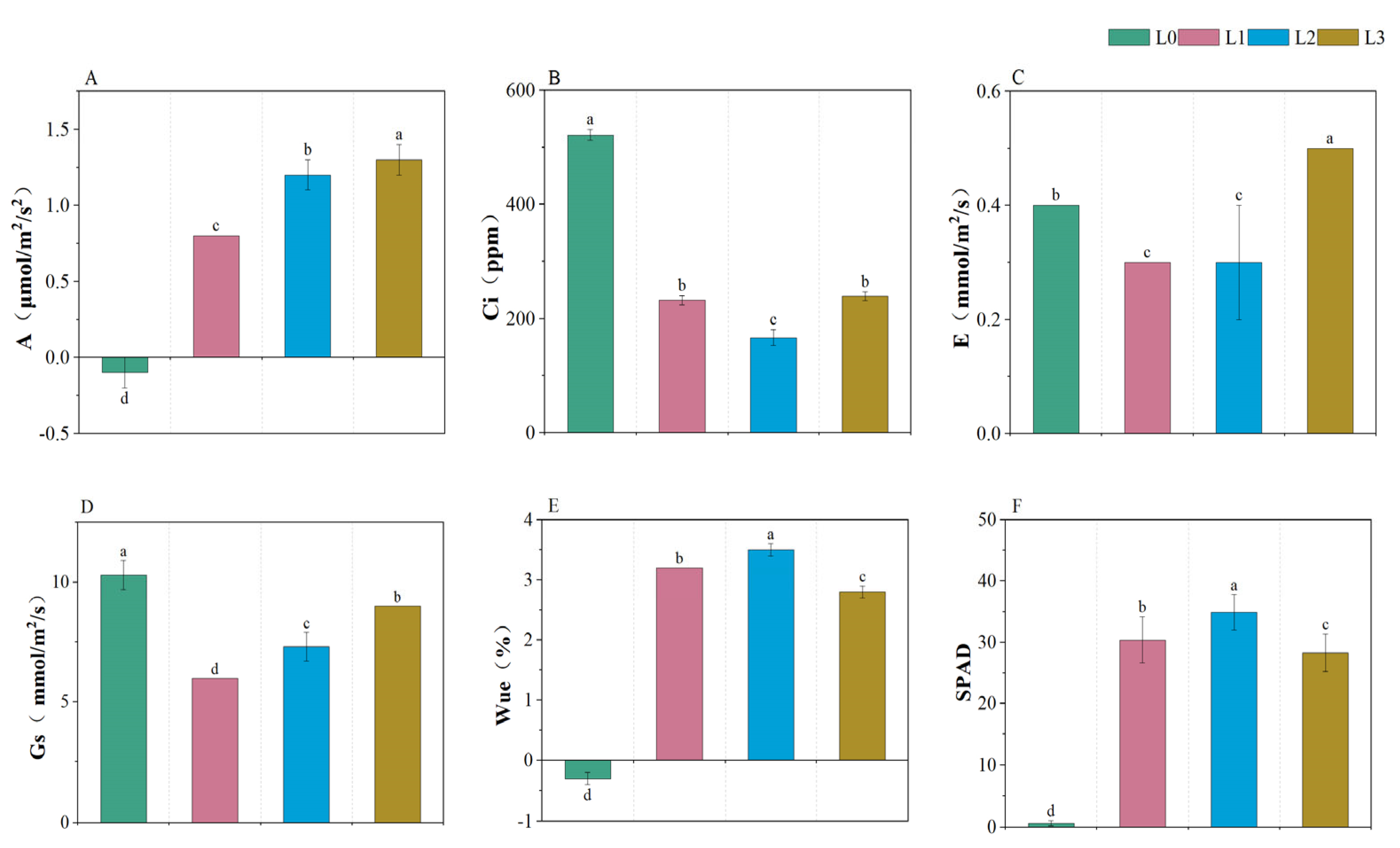
3.2. Comparison of Photosynthetic Parameters in Leaves of Hydroponic Barley Fodder under Different Light Intensities
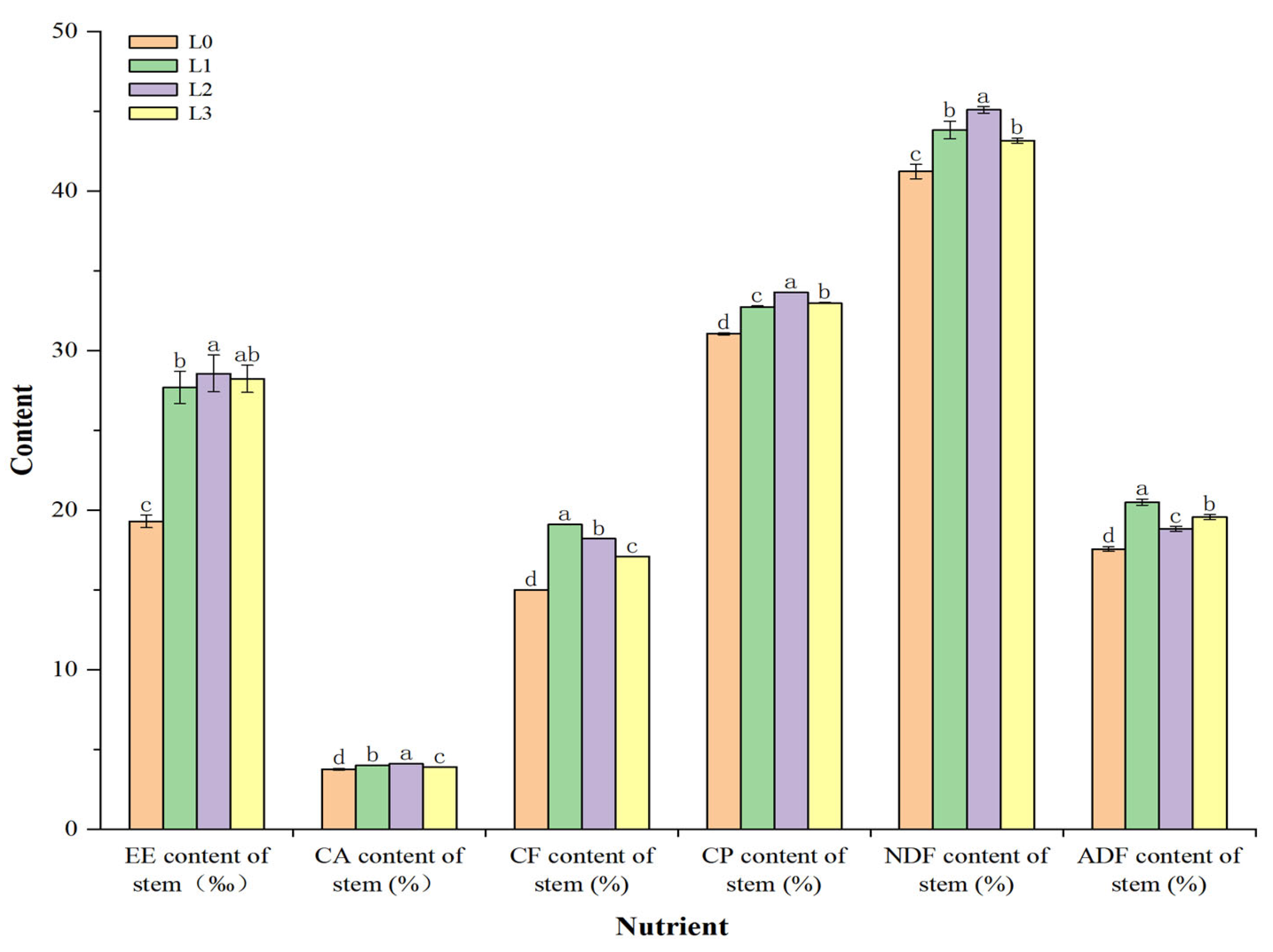
3.3. Feeding Quality of Stems and Leaves of Hydroponic Barley Fodder under Different Light Intensities
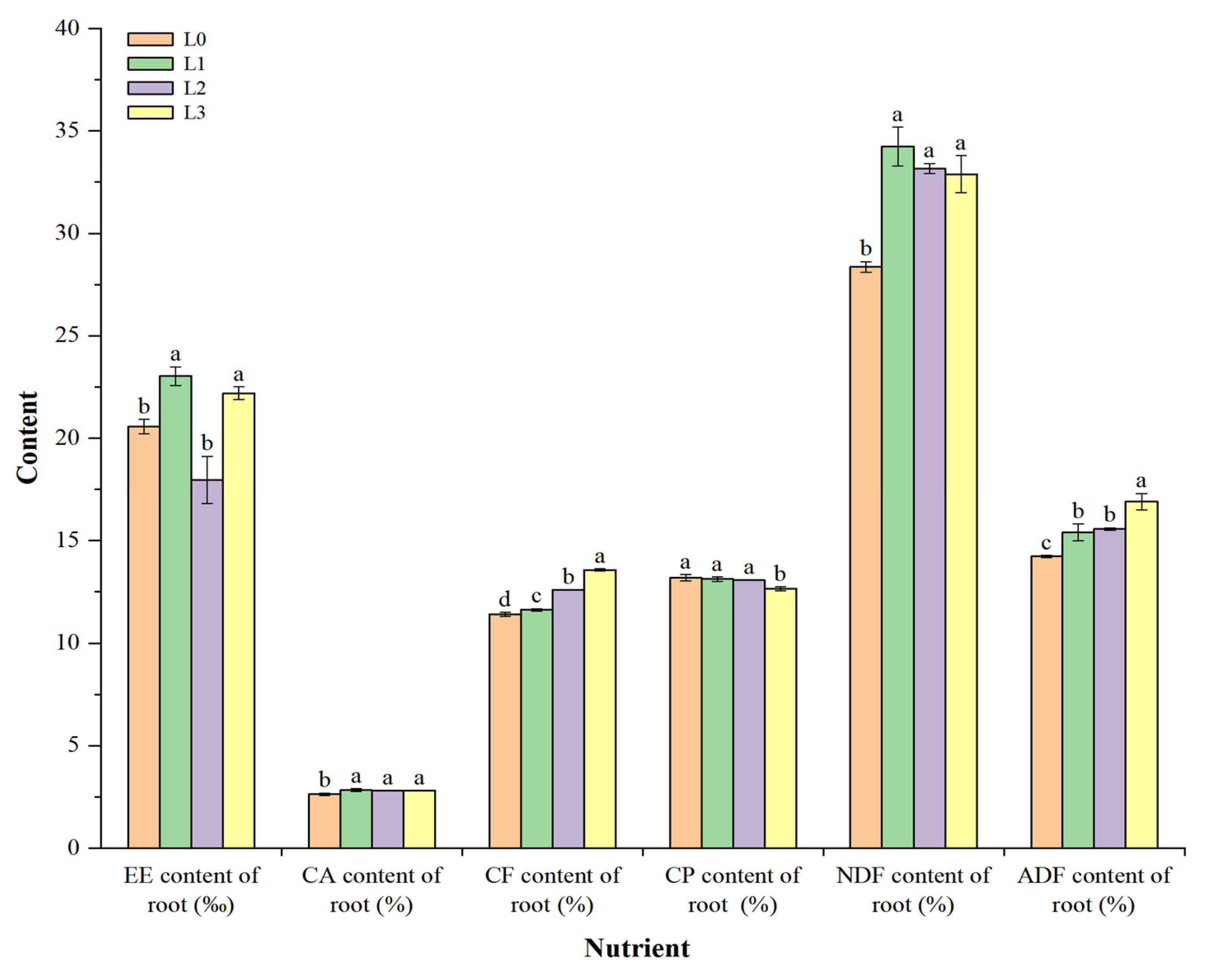
3.4. Feeding Quality of Barley Fodder Roots under Different Light Intensities
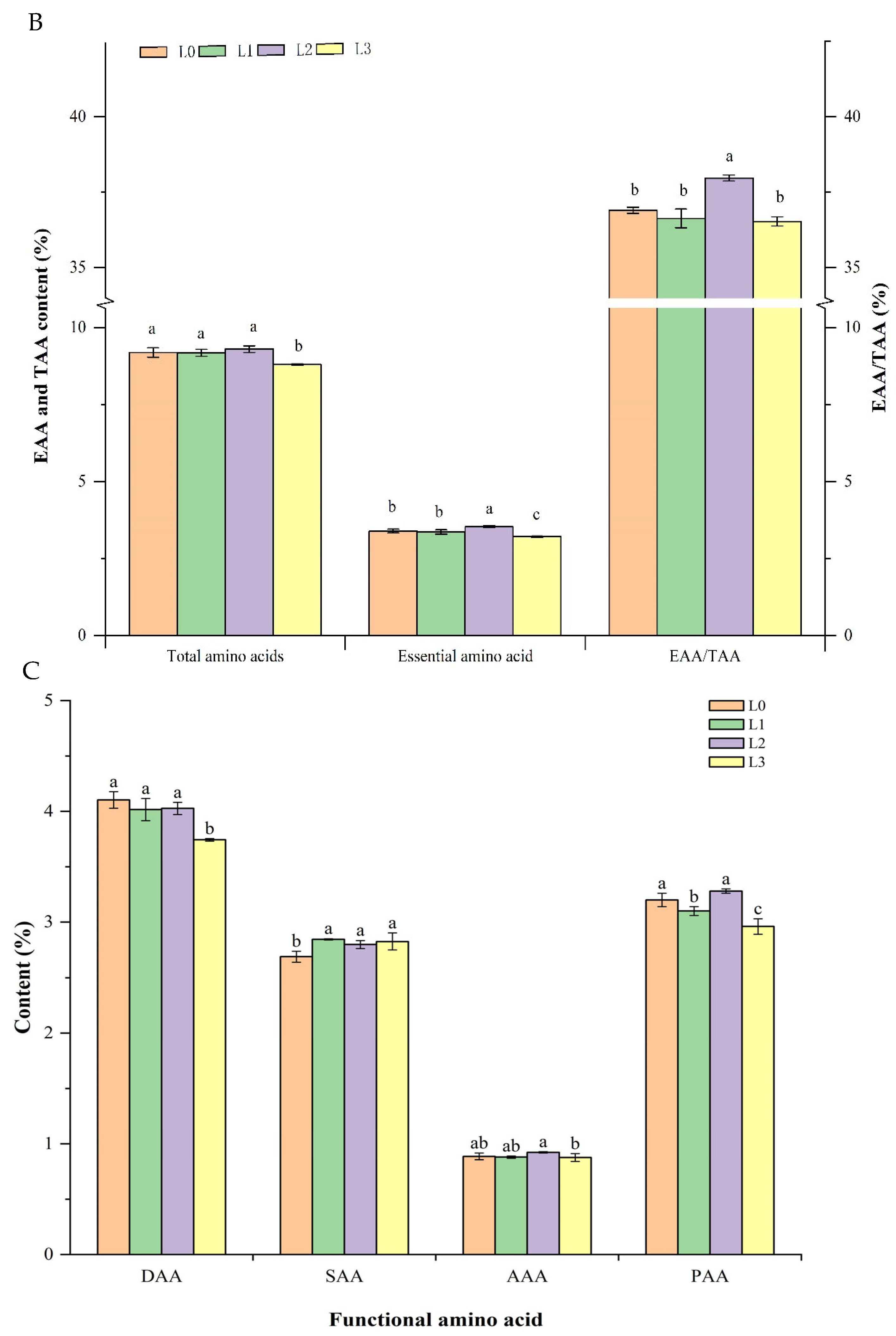
3.5. Analysis of Amino Acid Composition in Hydroponic Barley Fodder under Different Light Intensities
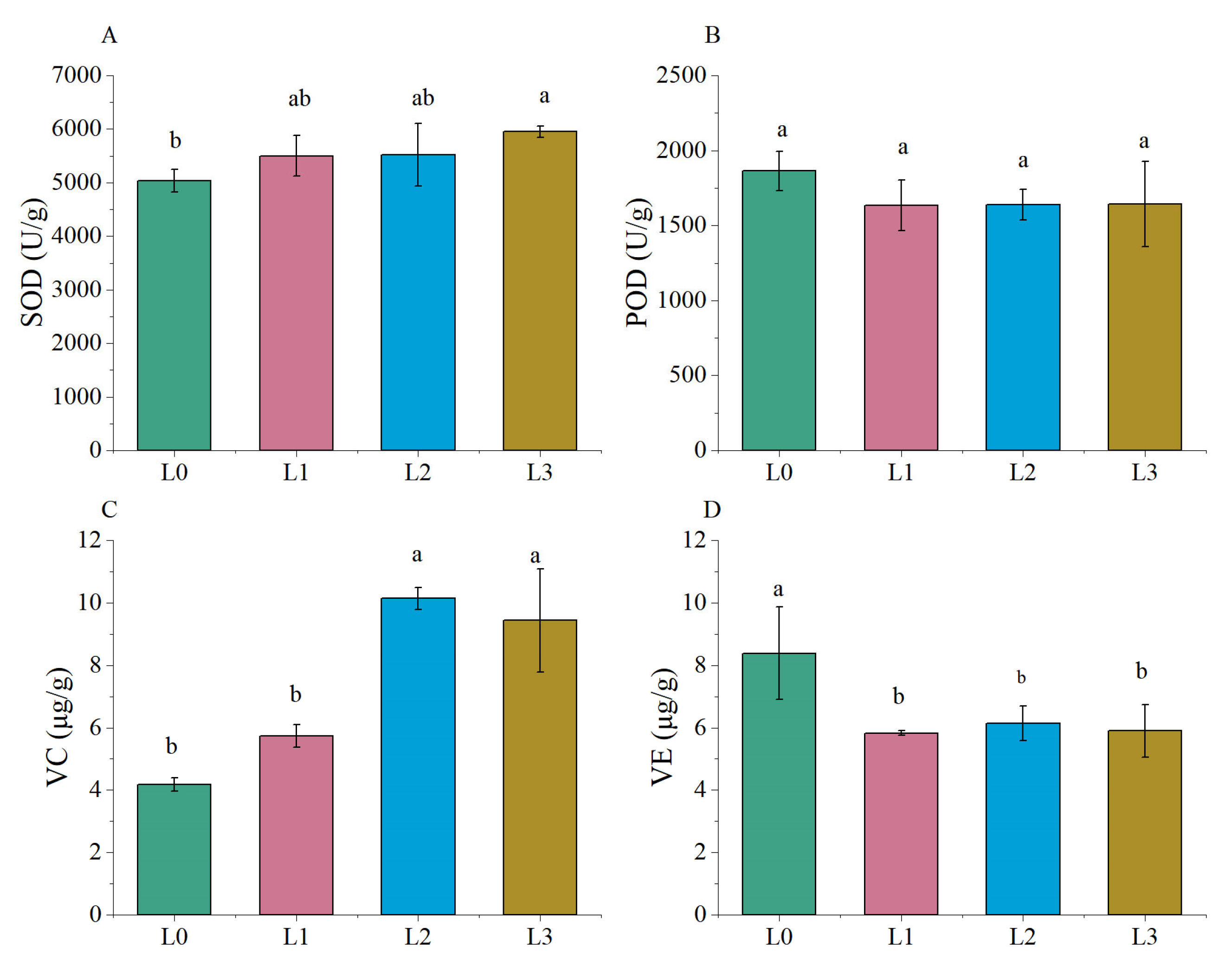
3.6. Analysis of Antioxidant Activity in Hydroponic Barley Seedlings under Different Light Intensities
3.7. Principal Component Analysis of Growth and Quality Indicators of Hydroponic Barley Seedlings under Different Light Intensities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Wen, Z.; Ghanizadeh, H.; Fan, Y.; Zheng, C.; Yang, X.; Yan, X.; Li, W. Stochastic processes dominate assembly of soil fungal community in grazing excluded grasslands in northwestern China. J. Soils Sed. 2023, 23, 156–171. [Google Scholar] [CrossRef]
- Yang, X.; Yan, X.; Guo, Q.; Ghanizadeh, H.; Li, M.; Tuo, H.; Wen, Z.; Li, W. Effects of different management practices on plant community and soil properties in a restored grassland. J. Soil Sci. Plant Nutr. 2022, 22, 3811–3821. [Google Scholar] [CrossRef]
- Tian, Z.; Ma, W.; Yang, Q.; Duan, F. Application status and challenges of machine vision in plant factory—A review. Inf. Proc. Agric. 2022, 9, 195–211. [Google Scholar] [CrossRef]
- Sneath, R.; McIntosh, F. Review of Hydroponic Fodder Production for Beef Cattle; Meat & Livestock Australia Limited: Sydney, Australia, 2003; Volume 84, p. 54. [Google Scholar]
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. Future Foods; Academic Press: Cambridge, UK, 2022; pp. 555–567. [Google Scholar]
- Nikkhah, A. Barley forages for modern global ruminant agriculture: A review. Russ. Agric. Sci. 2013, 39, 206–213. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Meng, T.; Nakamura, E.; Irino, N.; Joshi, K.; Devkota, H.; Yahara, S.; Kondo, R. Effects of irradiation with light of different photon densities on the growth of young green barley plants. Agric. Sci. 2015, 6, 208–216. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Xu, Y.; Chang, Y.; Chen, G.; Lin, H. The research on LED supplementary lighting system for plants. Optik 2016, 127, 7193–7201. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, A.; Cheng, Z.-M. Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Yao, X.; Liu, X.; Xu, Z.; Jiao, X. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Mohsen, A.F.; Nasr, A.H.; Metwalli, A.M. Effect of different light intensities on growth, reproduction, amino acid synthesis, fat and sugar contents in ulva fasciata delile. Hydrobiologia 1973, 43, 125–135. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of light intensity and temperature on the photosynthesis characteristics and yield of lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-l. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Chen, H.; Wang, L.; Wang, S.; Guo, X.; Cheng, X. Effect of photoperiod on vitamin E and carotenoid biosynthesis in mung bean (Vigna radiata) sprouts. Food Chem. 2021, 358, 129915. [Google Scholar] [CrossRef]
- Pan, J.; Guo, B. Effects of light intensity on the growth, photosynthetic characteristics, and flavonoid content of Epimedium pseudowushanense B.L.Guo. Molecules 2016, 21, 1475. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- He, J.; Jawahir, N.K.B.; Qin, L. Quantity of supplementary LED lightings regulates photosynthetic apparatus, improves photosynthetic capacity and enhances productivity of Cos lettuce grown in a tropical greenhouse. Photosynth. Res. 2021, 149, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.S.; Sultan, M.; Shamshiri, R.R.; Rahman, M.M.; Aleem, M.; Balasundram, S.K. Present status and challenges of fodder production in controlled environments: A review. Smart Agric. Technol. 2023, 3, 100080. [Google Scholar] [CrossRef]
- Ren, P.; Deng, M.; Feng, J.; Li, R.; Ma, X.; Liu, J.; Wang, D. Partial replacement of oat hay with whole-plant hydroponic barley seedlings modulates ruminal microbiota and affects growth performance of Holstein heifers. Microorganisms 2022, 10, 2000. [Google Scholar] [CrossRef]
- Grigas, A.; Kemzūraitė, A.; Steponavičius, D.; Steponavičienė, A.; Domeika, R. Impact of slope of growing trays on productivity of wheat green fodder by a nutrient film technique system. Water 2020, 12, 3009. [Google Scholar] [CrossRef]
- Girma, F.; Gebremariam, B. Review on hydroponic feed value to livestock production. J. Sci. Innov. Res. 2018, 7, 106–109. [Google Scholar] [CrossRef]
- Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT6433-2006 (accessed on 16 May 2024).
- Available online: https://www.chinesestandard.net/Related.aspx/GBT6434-2022?Redirect=YES (accessed on 16 May 2024).
- Available online: https://www.chinesestandard.net/Related.aspx/GBT20806-2022?Redirect=YES (accessed on 16 May 2024).
- Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT18246-2019 (accessed on 16 May 2024).
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino acid profiles and nutritional evaluation of fresh sweet–waxy corn from three different regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, S.L.; Gujral, S.K. Plant Physiology: Theory and Applications, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 201–252. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Li, Q.; Deng, M.; Xiong, Y.; Coombes, A.; Zhao, W. Morphological and photosynthetic response to high and low irradiance of Aeschynanthus longicaulis. Sci. World J. 2014, 2014, 347461. [Google Scholar] [CrossRef]
- Timergalina, L.N.; Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G.R. Effect of increased irradiance on the hormone content, water relations, and leaf elongation in wheat seedlings. Russ. J. Plant Physiol. 2007, 54, 633–638. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Li, X.; Ghanizadeh, H.; Han, Z.; Wang, Q.; Li, F.; Qiu, Y.; Zhang, Y.; Chen, X.; Liu, J.; Wang, A. Metabolic profile and physiological mechanisms underlying the promoting effects of TiO2NPs on the photosynthesis and growth of tomato. Sci. Hortic. 2023, 322, 112394. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; Van Santen, E.; Gómez, C. Growth, water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red light. Horticulturae 2018, 4, 16. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, H.; Guo, H.; Zhou, A.; Huang, Y.; Sun, Y.; Li, M. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS ONE 2014, 9, e85996. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Zhou, F. Effects of light intensity and photoperiod on the fresh locking and quality of Hydroponic arugula in the harvesting period. Agronomy 2023, 13, 1667. [Google Scholar] [CrossRef]
- Abeysekara, S.; Christensen, D.A.; Niu, Z.; Theodoridou, K.; Yu, P. Molecular structure, chemical and nutrient profiles, and metabolic characteristics of the proteins and energy in new cool-season corn varieties harvested as fresh forage for dairy cattle. J. Dairy Sci. 2013, 96, 6631–6643. [Google Scholar] [CrossRef] [PubMed]
- Tlahig, S.; Neji, M.; Atoui, A.; Seddik, M.; Dbara, M.; Yahia, H.; Nagaz, K.; Najari, S.; Khorchani, T.; Loumerem, M. Genetic and seasonal variation in forage quality of lucerne (Medicago sativa L.) for resilience to climate change in arid environments. J. Agric. Food Res. 2024, 15, 100986. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, D.W.; Huang, C.F.; Liu, L.; Piao, X.S.; Wang, F.L. Determination and prediction of digestible and metabolizable energy from the chemical composition of chinese corn gluten feed fed to finishing pigs. Asian-Australas J. Anim. Sci. 2014, 27, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Gyugos, M.; Ahres, M.; Gulyás, Z.; Szalai, G.; Darkó, É.; Végh, B.; Boldizsár, Á.; Mednyánszky, Z.; Kar, R.K.; Dey, N.; et al. Role of light-intensity-dependent changes in thiol and amino acid metabolism in the adaptation of wheat to drought. J. Agron. Crop Sci. 2019, 205, 562–570. [Google Scholar] [CrossRef]
- Ma, Q.; Cao, X.; Wu, L.; Mi, W.; Feng, Y. Light intensity affects the uptake and metabolism of glycine by pakchoi (Brassica chinensis L.). Sci. Rep. 2016, 6, 21200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Feng, X.; Huang, W.; Liu, Q.; Ibrahim, S.A.; Liu, Y. Effects of light intensity on the biosynthesis of glucosinolate in Chinese cabbage plantlets. Sci. Hortic. 2023, 316, 112036. [Google Scholar] [CrossRef]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H. Light intensity and photoperiod affect growth and nutritional quality of brassica microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Liu, W. LED red and blue light intensity affected productivity and nitrogen metabolisms of ryegrass. J. Plant Nut. 2024, 47, 705–717. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Ghanizadeh, H.; Kerckhoffs, H.; Sofkova-Bobcheva, S.; Wu, W.; Wang, X.; Liu, Y.; Li, X.; Zhao, H.; et al. Comparative genomic and physiological analyses of a superoxide dismutase mimetic (SODm-123) for its ability to respond to oxidative stress in tomato plants. J. Agri. Food Chem. 2020, 68, 13608–13619. [Google Scholar] [CrossRef]
- Dai, A.-H.; Nie, Y.-X.; Yu, B.; Li, Q.; Lu, L.-Y.; Bai, J.-G. Cinnamic acid pretreatment enhances heat tolerance of cucumber leaves through modulating antioxidant enzyme activity. Environ. Exp. Bot. 2012, 79, 1–10. [Google Scholar] [CrossRef]
- Yan, N.; Xu, X.F.; Wang, Z.D.; Huang, J.Z.; Guo, D.P. Interactive effects of temperature and light intensity on photosynthesis and antioxidant enzyme activity in Zizania latifolia Turcz. plants. Photosynthetica 2013, 51, 127–138. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, F.; Zhang, H.; Zhu, Z. Responses of the growth, photosynthetic characteristics, endogenous hormones and antioxidant activity of Carpinus betulus L. seedlings to different light intensities. Front. Plant Sci. 2022, 13, 1055984. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ. Exp. Bot. 2005, 54, 109–120. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, X. The effect of light in vtamin C metabolism regulation and accumulation in mung bean (Vigna radiata) germination. Plant Foods Hum. Nutr. 2020, 75, 24–29. [Google Scholar] [CrossRef]

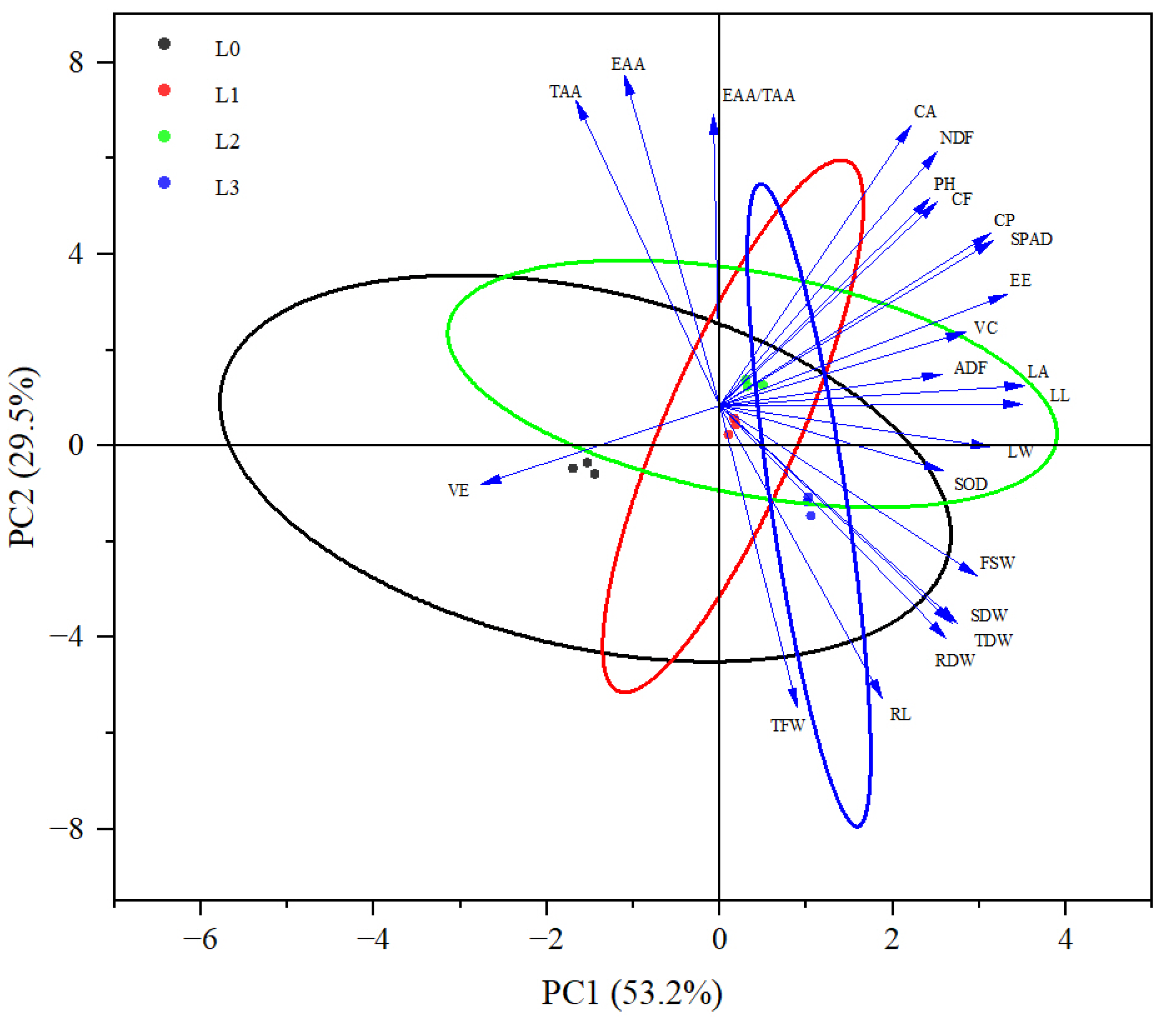
| Treatments | Score of Extracted Reference Factors for Each Treatment | ||
|---|---|---|---|
| Y (i,1) | Y (i,2) | Overall Score | |
| L0 | −5.4413 | −1.2266 | −3.2550 |
| L1 | 0.5290 | 1.0749 | 0.5984 |
| L2 | 1.3089 | 3.3787 | 1.6926 |
| L3 | 3.6035 | −3.2271 | 0.9640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, J.; Wang, X.; Zhang, B. Light Intensity Affects Growth and Nutrient Value of Hydroponic Barley Fodder. Agronomy 2024, 14, 1099. https://doi.org/10.3390/agronomy14061099
Yang J, Sun J, Wang X, Zhang B. Light Intensity Affects Growth and Nutrient Value of Hydroponic Barley Fodder. Agronomy. 2024; 14(6):1099. https://doi.org/10.3390/agronomy14061099
Chicago/Turabian StyleYang, Jinyu, Jiusheng Sun, Xihe Wang, and Bo Zhang. 2024. "Light Intensity Affects Growth and Nutrient Value of Hydroponic Barley Fodder" Agronomy 14, no. 6: 1099. https://doi.org/10.3390/agronomy14061099
APA StyleYang, J., Sun, J., Wang, X., & Zhang, B. (2024). Light Intensity Affects Growth and Nutrient Value of Hydroponic Barley Fodder. Agronomy, 14(6), 1099. https://doi.org/10.3390/agronomy14061099





